Associations between Vertebral Localized Contrast Changes and Adjacent Annular Fissures in Patients with Low Back Pain: A Radiomics Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diagnostic Procedures and Imaging Protocols
2.2. IVD and Vertebrae Tissue Grading
2.3. Image Segmentation
2.4. Radiomics
2.4.1. Image Preprocessing
- Interpolation—To ensure rotationally invariant features, the MR images were interpolated to isotropic voxels of size 1 × 1 × 1 mm3 [38].
- ∙ Intensity discretization—Discretization of the image intensities inside the ROI was performed to reduce the image noise level [41,42] and allow for feature calculation [43]. Even though the Image Biomarker Standardization Initiative (IBSI) recommends intensity discretization using a fixed bin number for MR images [44], studies have shown that a fixed bin width approach produces more reproducible features [45,46] when the number of bins is kept between 32 and 128 [47,48]. As such, with regard to the intensity range present inside the ROIs of the current images, intensity discretization was performed using an appropriate fixed bin width of three.
2.4.2. Feature Calculation and Standardization
2.4.3. Feature Reduction
- The features’ robustness to the variability of ROI segmentation was investigated by calculating future values using the initial vertebral segmentation and segmentation contracted by one pixel in all directions. The Intraclass Correlation Coefficient (ICC) using one-way random effects with absolute agreement, ICC(1, 1), was calculated using individual feature values as subjects and the two ROI perturbations as the raters. Features indicating poor reliability (ICC(1, 1) < 0.5) [55] were excluded from further analysis (see Supplementary Table S1). A similar methodology has been applied in recent studies [56].
- Similarly, features robustness to image acquisition and reconstruction was evaluated by interpolating the MR images into voxels of size 1 × 1 × 1 mm3 and 1.1 × 1.1 × 1.1 mm3 before feature calculation. ICC(1, 1) was calculated using individual feature values as subjects and the two voxel volume perturbations as the raters. Features with ICC < 0.5 were excluded from further analysis (Table S1).
- Using the person correlation metric, features were pairwise tested for linear correlation. Pairs of features that displayed a very high linear correlation (R > 0.9) [57] were individually tested for correlation to the presence of fissure. The feature with the lowest correlation to the presence of a fissure was excluded from further analysis.
- The remaining features were further reduced through sequential backward feature selection algorithms to select the most meaningful features that reflect an association between feature and fissure. Three separate algorithms were used to predict an annular fissure in an adjacent IVD (Figure 1):
- A fully connected neural network with one hidden layer with 100 nodes (Multilayer perceptron);
- A random forest ensemble of 100 trees built with bootstrap samples and balanced class weight;
- K-nearest neighbor classifier using five neighbors with uniform weights, i.e., all points in each neighborhood were weighted equally.
- 5.
- A binary logistic regression using backward elimination was applied to the top-performing features. The procedure establishes the importance of each feature to model fit, which reflects the association between features and fissures. Features that did not significantly contribute to the model fit (p > 0.05) were eliminated from further analysis.

2.5. IVD Fissure Association to Radiomic Features and Fissure Classification
2.6. Comparison between Radiomics and Radiological Markers of Vertebral Change
2.7. Statistical Analysis
3. Results
3.1. Feature Dimensionality Reduction
- gldm_LargeDependenceHighGrayLevelEmphasis_t1w (LDHGLE);
- glszm_LargeAreaHighGrayLevelEmphasis_t1w (LAHGLE);
- glszm_SmallAreaLowGrayLevelEmphasis_t1w (SALGLE).
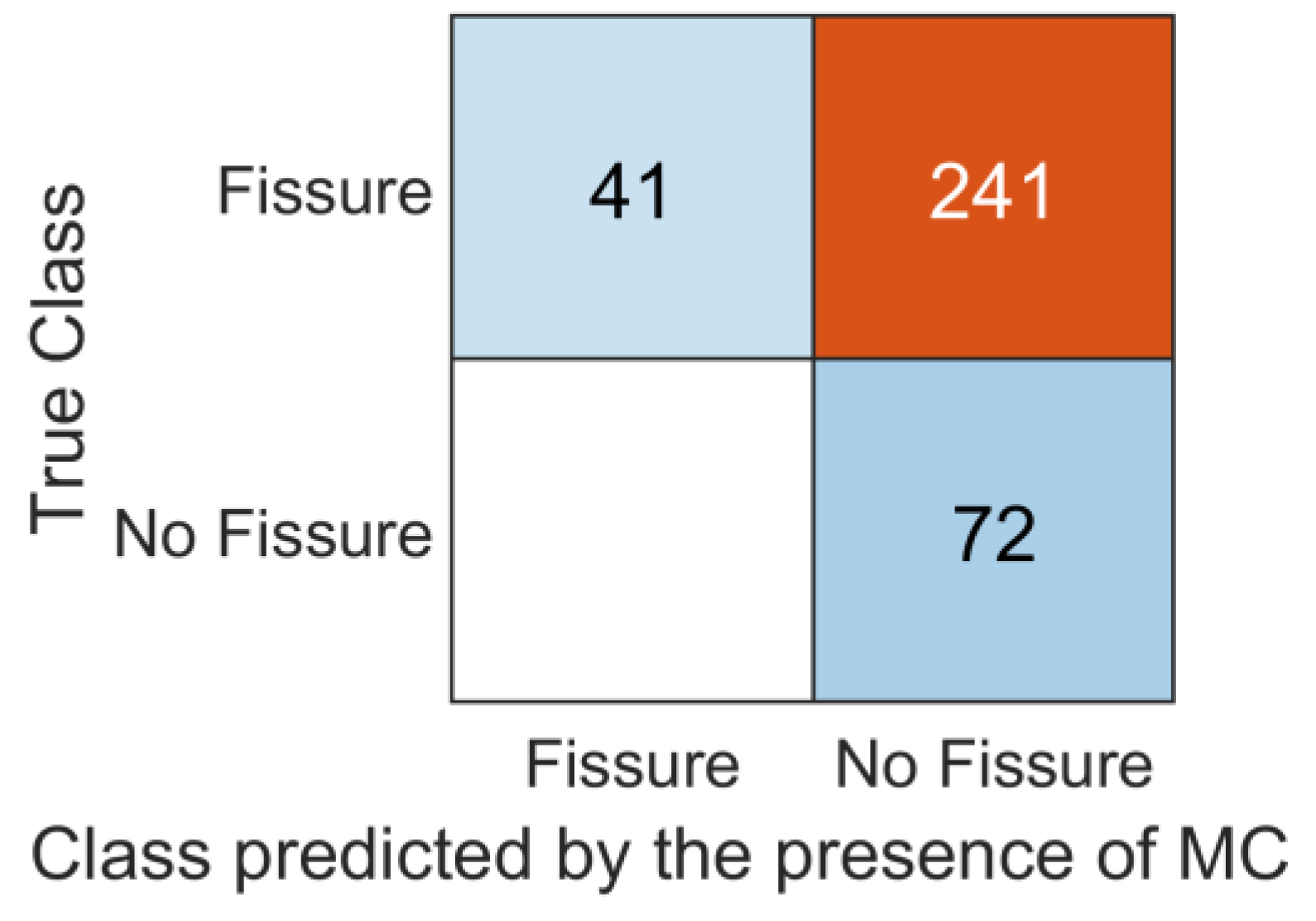
3.2. Association between Vertebra and Adjacent IVDs
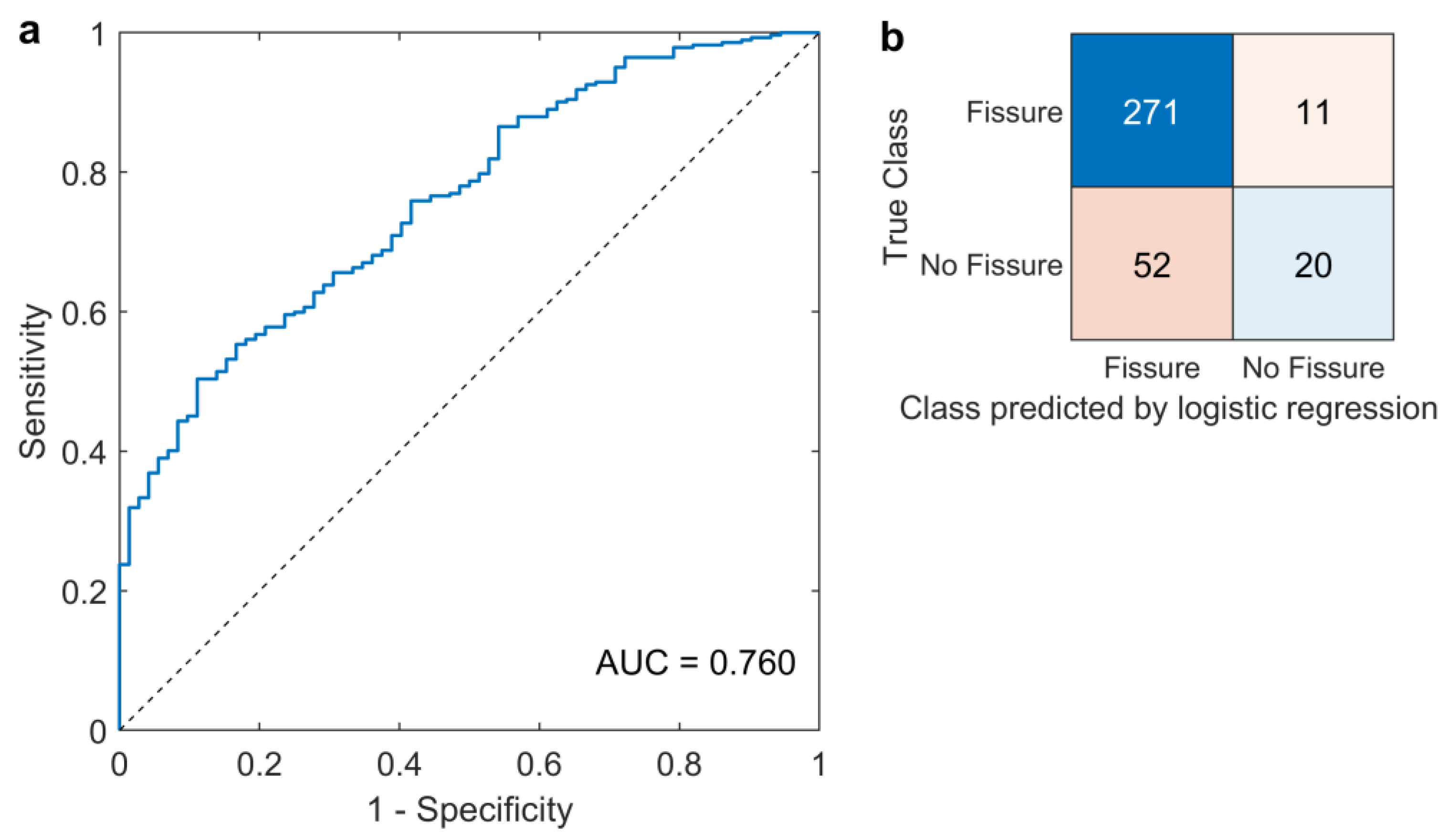
3.3. Fissure Classification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoy, D.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Bain, C.; Williams, G.; Smith, E.; Vos, T.; Barendregt, J.; et al. The global burden of low back pain: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 968–974. [Google Scholar] [CrossRef]
- Hansson, E.K.; Hansson, T.H. The costs for persons sick-listed more than one month because of low back or neck problems. A two-year prospective study of Swedish patients. Eur. Spine J. 2005, 14, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Videman, T.; Nurminen, M. The occurrence of anular tears and their relation to lifetime back pain history: A cadaveric study using barium sulfate discography. Spine 2004, 29, 2668–2676. [Google Scholar] [CrossRef]
- Lim, C.-H.; Jee, W.-H.; Son, B.C.; Kim, D.-H.; Ha, K.-Y.; Park, C.-K. Discogenic lumbar pain: Association with MR imaging and CT discography. Eur. J. Radiol. 2005, 54, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Mok, F.P.S.; Samartzis, D.; Karppinen, J.; Fong, D.Y.T.; Luk, K.D.K.; Cheung, K.M.C. Modic changes of the lumbar spine: Prevalence, risk factors, and association with disc degeneration and low back pain in a large-scale population-based cohort. Spine J. 2016, 16, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.P.G.; Smith, S.; Fairbank, J.C.T. Nutrition of the Intervertebral Disc. Spine 2004, 29, 2700–2709. [Google Scholar] [CrossRef] [PubMed]
- Conger, A.; Smuck, M.; Truumees, E.; Lotz, J.C.; DePalma, M.J.; McCormick, Z.L. Vertebrogenic Pain: A Paradigm Shift in Diagnosis and Treatment of Axial Low Back Pain. Pain Med. 2022, 23, S63–S71. [Google Scholar] [CrossRef]
- Peng, B.; Hou, S.; Wu, W.; Zhang, C.; Yang, Y. The pathogenesis and clinical significance of a high-intensity zone (HIZ) of lumbar intervertebral disc on MR imaging in the patient with discogenic low back pain. Eur. Spine J. 2006, 15, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Freemont, A.J.; Watkins, A.; Le Maitre, C.; Baird, P.; Jeziorska, M.; Knight, M.T.N.; Ross, E.R.S.; O’Brien, J.P.; Hoyland, J.A. Nerve growth factor expression and innervation of the painful intervertebral disc. J. Pathol. 2002, 197, 286–292. [Google Scholar] [CrossRef]
- Bailey, J.F.; Liebenberg, E.; Degmetich, S.; Lotz, J.C. Innervation patterns of PGP 9.5-positive nerve fibers within the human lumbar vertebra. J. Anat. 2011, 218, 263–270. [Google Scholar] [CrossRef]
- Antonacci, M.D.; Mody, D.R.; Heggeness, M.H. Innervation of the human vertebral body: A histologic study. Clin. Spine Surg. 1998, 11, 526–531. [Google Scholar] [CrossRef]
- Fields, A.J.; Liebenberg, E.C.; Lotz, J.C. Innervation of pathologies in the lumbar vertebral end plate and intervertebral disc. Spine J. 2014, 14, 513–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, J.; Yamamoto, I.; Kitamura, N.; Sone, T.; Itoh, H.; Torizuka, K.; Takasu, K. End plate of the discovertebral joint: Degenerative change in the elderly adult. Radiology 1987, 164, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Benneker, L.M.; Heini, P.F.; Alini, M.; Anderson, S.E.; Ito, K. 2004 Young Investigator Award Winner: Vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine 2005, 30, 167–173. [Google Scholar] [CrossRef]
- Holm, S.; Holm, A.K.; Ekström, L.; Karladani, A.; Hansson, T. Experimental disc degeneration due to endplate injury. J. Spinal Disord. Tech. 2004, 17, 64–71. [Google Scholar] [CrossRef]
- Dudli, S.; Fields, A.J.; Samartzis, D.; Karppinen, J.; Lotz, J.C. Pathobiology of Modic changes. Eur. Spine J. 2016, 25, 3723–3734. [Google Scholar] [CrossRef] [Green Version]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef]
- Dudli, S.; Sing, D.C.; Hu, S.S.; Berven, S.H.; Burch, S.; Deviren, V.; Cheng, I.; Tay, B.K.B.; Alamin, T.F.; Ith, M.A.M.; et al. ISSLS PRIZE IN BASIC SCIENCE 2017: Intervertebral disc/bone marrow cross-talk with Modic changes. Eur. Spine J. 2017, 26, 1362–1373. [Google Scholar] [CrossRef] [Green Version]
- Marshman, L.A.; Metcalfe, A.V.; Krishna, M.; Friesem, T. Are high-intensity zones and Modic changes mutually exclusive in symptomatic lumbar degenerative discs? J. Neurosurg. Spine 2010, 12, 351–356. [Google Scholar] [CrossRef]
- Teraguchi, M.; Samartzis, D.; Hashizume, H.; Yamada, H.; Muraki, S.; Oka, H.; Cheung, J.P.Y.; Kagotani, R.; Iwahashi, H.; Tanaka, S. Classification of high intensity zones of the lumbar spine and their association with other spinal MRI phenotypes: The Wakayama Spine Study. PLoS ONE 2016, 11, e0160111. [Google Scholar] [CrossRef] [Green Version]
- Lagerstrand, K.; Brisby, H.; Hebelka, H. Associations between high-intensity zones, endplate, and Modic changes and their effect on T2-mapping with and without spinal load. J. Orthop. Res. 2021, 39, 2703–2710. [Google Scholar] [CrossRef] [PubMed]
- Pfirrmann, C.W.; Metzdorf, A.; Zanetti, M.; Hodler, J.; Boos, N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 2001, 26, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Waldenberg, C.; Hebelka, H.; Brisby, H.; Lagerstrand, K.M. MRI histogram analysis enables objective and continuous classification of intervertebral disc degeneration. Eur. Spine J. 2018, 27, 1042–1048. [Google Scholar] [CrossRef] [Green Version]
- Waldenberg, C.; Eriksson, S.; Brisby, H.; Hebelka, H.; Lagerstrand, K.M. Detection of Imperceptible Intervertebral Disc Fissures in Conventional MRI—An AI Strategy for Improved Diagnostics. J. Clin. Med. 2023, 12, 11. [Google Scholar] [CrossRef]
- Martín-Noguerol, T.; Oñate Miranda, M.; Amrhein, T.J.; Paulano-Godino, F.; Xiberta, P.; Vilanova, J.C.; Luna, A. The role of Artificial intelligence in the assessment of the spine and spinal cord. Eur. J. Radiol. 2023, 161, 110726. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [Green Version]
- Torén, L.; Lagerstrand, K.; Waldenberg, C.; Brisby, H.; Hebelka, H. MRI During Spinal Loading Reveals Intervertebral Disc Behavior Corresponding to Discogram Findings of Annular Fissures and Pain Provocation. Spine 2020, 45, E1500–E1506. [Google Scholar] [CrossRef]
- Hebelka, H.; Nilsson, A.; Hansson, T. Pressure Increase in Adjacent Discs During Clinical Discography Questions the Methods Validity. Spine 2014, 39, 893–899. [Google Scholar] [CrossRef]
- Hebelka, H.; Hansson, T. HIZ’s relation to axial load and low back pain: Investigated with axial loaded MRI and pressure controlled discography. Eur. Spine J. 2013, 22, 734–739. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, S.; Waldenberg, C.; Torén, L.; Grimby-Ekman, A.; Brisby, H.; Hebelka, H.; Lagerstrand, K. Texture Analysis of Magnetic Resonance Images Enables Phenotyping of Potentially Painful Annular Fissures. Spine 2021. [Google Scholar] [CrossRef] [PubMed]
- Sachs, B.L.; Vanharanta, H.; Spivey, M.A.; Guyer, R.D.; Videman, T.; Rashbaum, R.F.; Johnson, R.G.; Hochschuler, S.H.; Mooney, V. Dallas discogram description. A new classification of CT/discography in low-back disorders. Spine 1987, 12, 287–294. [Google Scholar] [CrossRef]
- Derby, R.; Kim, B.-J.; Chen, Y.; Seo, K.-S.; Lee, S.-H. The Relation Between Annular Disruption on Computed Tomography Scan and Pressure-Controlled Diskography. Arch. Phys. Med. Rehabil. 2005, 86, 1534–1538. [Google Scholar] [CrossRef] [PubMed]
- Modic, M.T.; Steinberg, P.M.; Ross, J.S.; Masaryk, T.J.; Carter, J.R. Degenerative disk disease: Assessment of changes in vertebral body marrow with MR imaging. Radiology 1988, 166, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hebelka, H.; Khalil, M.; Brisby, H.; Lagerstrand, K. Lumbar vertebral T2-relaxation time investigated with T2-mapping at multiple time points in a day demonstrate large individual variations. Diagn. Interv. Radiol. 2022, 28, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Lagerstrand, K.; Hebelka, H.; Brisby, H. Low back pain patients and controls display functional differences in endplates and vertebrae measured with T2-mapping. Eur. Spine J. 2019, 28, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Van Griethuysen, J.J.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.; Fillion-Robin, J.-C.; Pieper, S.; Aerts, H.J. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [Green Version]
- Zwanenburg, A.; Leger, S.; Vallières, M.; Löck, S. Image biomarker standardisation initiative. arXiv 2016, arXiv:1612.07003. [Google Scholar]
- Hu, P.; Wang, J.; Zhong, H.; Zhou, Z.; Shen, L.; Hu, W.; Zhang, Z. Reproducibility with repeat CT in radiomics study for rectal cancer. Oncotarget 2016, 7, 71440–71446. [Google Scholar] [CrossRef] [Green Version]
- Scalco, E.; Belfatto, A.; Mastropietro, A.; Rancati, T.; Avuzzi, B.; Messina, A.; Valdagni, R.; Rizzo, G. T2w-MRI signal normalization affects radiomics features reproducibility. Med. Phys. 2020, 47, 1680–1691. [Google Scholar] [CrossRef]
- Zhao, B. Understanding sources of variation to improve the reproducibility of radiomics. Front. Oncol. 2021, 826. [Google Scholar] [CrossRef]
- Leijenaar, R.T.; Nalbantov, G.; Carvalho, S.; van Elmpt, W.J.; Troost, E.G.; Boellaard, R.; Aerts, H.J.; Gillies, R.J.; Lambin, P. The effect of SUV discretization in quantitative FDG-PET Radiomics: The need for standardized methodology in tumor texture analysis. Sci. Rep. 2015, 5, 11075. [Google Scholar] [CrossRef] [Green Version]
- Yip, S.S.; Aerts, H.J. Applications and limitations of radiomics. Phys. Med. Biol. 2016, 61, R150–R166. [Google Scholar] [CrossRef] [Green Version]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R. The image biomarker standardization initiative: Standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [Green Version]
- Duron, L.; Balvay, D.; Vande Perre, S.; Bouchouicha, A.; Savatovsky, J.; Sadik, J.C.; Thomassin-Naggara, I.; Fournier, L.; Lecler, A. Gray-level discretization impacts reproducible MRI radiomics texture features. PLoS ONE 2019, 14, e0213459. [Google Scholar] [CrossRef]
- Stamoulou, E.; Spanakis, C.; Manikis, G.C.; Karanasiou, G.; Grigoriadis, G.; Foukakis, T.; Tsiknakis, M.; Fotiadis, D.I.; Marias, K. Harmonization Strategies in Multicenter MRI-Based Radiomics. J. Imaging 2022, 8, 303. [Google Scholar] [CrossRef] [PubMed]
- Wichtmann, B.D.; Harder, F.N.; Weiss, K.; Schönberg, S.O.; Attenberger, U.I.; Alkadhi, H.; Pinto Dos Santos, D.; Baeßler, B. Influence of Image Processing on Radiomic Features From Magnetic Resonance Imaging. Invest. Radiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Carré, A.; Klausner, G.; Edjlali, M.; Lerousseau, M.; Briend-Diop, J.; Sun, R.; Ammari, S.; Reuzé, S.; Alvarez Andres, E.; Estienne, T.; et al. Standardization of brain MR images across machines and protocols: Bridging the gap for MRI-based radiomics. Sci. Rep. 2020, 10, 12340. [Google Scholar] [CrossRef]
- Haga, A.; Takahashi, W.; Aoki, S.; Nawa, K.; Yamashita, H.; Abe, O.; Nakagawa, K. Standardization of imaging features for radiomics analysis. J. Med. Invest. 2019, 66, 35–37. [Google Scholar] [CrossRef]
- Park, S.-H.; Lim, H.; Bae, B.K.; Hahm, M.H.; Chong, G.O.; Jeong, S.Y.; Kim, J.-C. Robustness of magnetic resonance radiomic features to pixel size resampling and interpolation in patients with cervical cancer. Cancer Imaging 2021, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Milligan, G.W.; Cooper, M.C. A study of standardization of variables in cluster analysis. J. Classif. 1988, 5, 181–204. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef]
- Baeßler, B.; Weiss, K.; Pinto Dos Santos, D. Robustness and Reproducibility of Radiomics in Magnetic Resonance Imaging: A Phantom Study. Invest. Radiol. 2019, 54, 221–228. [Google Scholar] [CrossRef]
- Van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging—“how-to” guide and critical reflection. Insights Imaging 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Zwanenburg, A.; Leger, S.; Agolli, L.; Pilz, K.; Troost, E.G.C.; Richter, C.; Löck, S. Assessing robustness of radiomic features by image perturbation. Sci. Rep. 2019, 9, 614. [Google Scholar] [CrossRef] [Green Version]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar] [PubMed]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, T.S.; Bendix, T.; Sorensen, J.S.; Manniche, C.; Korsholm, L.; Kjaer, P. Characteristics and natural course of vertebral endplate signal (Modic) changes in the Danish general population. BMC Musculoskelet. Disord. 2009, 10, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carragee, E.J.; Don, A.S.; Hurwitz, E.L.; Cuellar, J.M.; Carrino, J.A.; Herzog, R. 2009 ISSLS Prize Winner: Does discography cause accelerated progression of degeneration changes in the lumbar disc: A ten-year matched cohort study. Spine 2009, 34, 2338–2345. [Google Scholar] [CrossRef] [PubMed]

| Parameter | T1W MRI (TSE) a | T1W MRI (SE) a | T2W MRI (TSE) a | T2W MRI (TSE) a | CT b |
|---|---|---|---|---|---|
| Imaging plane | Sagittal | Axial | Sagittal | Axial | Sagittal, Axial |
| Repetition time (ms) | 448 | 500 | 4862 | 5000 | |
| Echo time (ms) | 11 | 15 | 97 | 119 | |
| Echo train length | 9 | 1 | 21 | 25 | |
| Slice thickness (mm) | 4.0 | 4.0 | 4.0 | 4.0 | 0.75 (reconstructed) |
| Slice gap (mm) | 0.4 | 0.4 | 0.4 | 0.4 | |
| Number of averages | 4 | 2 | 2 | 4 | |
| Pixel bandwidth (Hz) | 200 | 100 | 190 | 190 | |
| Flip angle (degree) | 149 | 90 | 150 | 150 | |
| Acquisition matrix | 512 × 256 | 256 × 135 | 512 × 256 | 256 × 126 | |
| Reconstruction matrix | 512 × 512 | 384 × 512 | 512 × 512 | 360 × 512 | 512 × 512 |
| Field of view (mm2) | 300 × 300 | 135 × 180 | 300 × 300 | 127 × 180 | 162 × 162 |
| Convolution kernel | B45s |
| Patient and IVD Characteristics | ||
|---|---|---|
| Age (years) | 45 ± 9 a | |
| No. of patients | 61 | |
| No. of female | 32 (52%) | |
| No. of Modic Changes | 41 (12%) b | |
| No. of IVDs | 177 | |
| IVD segment | L1–L2 | 2 (1%) |
| L2–L3 | 21 (12%) | |
| L3–L4 | 57 (32%) | |
| L4–L5 | 57 (32%) | |
| L5–S1 | 40 (22%) | |
| Dallas Discogram Description | Grade 0–1 | 36 (20%) |
| Grade 2–3 | 141 (80%) |
| Feature Name | Random Forest | K-Nearest Neighbors | Multilayer Perceptron |
|---|---|---|---|
| gldm_LargeDependenceHighGrayLevelEmphasis [t1w] | ● | ● | ● |
| glszm_LargeAreaHighGrayLevelEmphasis [t1w] | ● | ● | |
| glszm_SizeZoneNonUniformity [t1w] | ● | ● | |
| glszm_SmallAreaLowGrayLevelEmphasis [t1w] | ● | ||
| glszm_LargeAreaHighGrayLevelEmphasis [t2w] | ● | ||
| glszm_ZonePercentage [t2w] | ● | ● | |
| glszm_ZoneVariance [t2w] | ● | ● | |
| ngtdm_Coarseness [t2w] | ● | ||
| ngtdm_Strength [t2w] | ● |
| Selected Features | B | Significance | Exp(B), (Odds Ratio) |
|---|---|---|---|
| gldm_LargeDependenceHighGrayLevelEmphasis_t1w | −0.98 | <0.001 | 0.38 (0.26 0.56) a |
| glszm_LargeAreaHighGrayLevelEmphasis_t1w | −0.66 | <0.001 | 0.52 (0.38 0.69) a |
| glszm_SmallAreaLowGrayLevelEmphasis_t1w | −0.62 | 0.002 | 0.54 (0.36 0.80) a |
| Constant (intercept) | 1.63 | <0.001 | 5.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waldenberg, C.; Brisby, H.; Hebelka, H.; Lagerstrand, K.M. Associations between Vertebral Localized Contrast Changes and Adjacent Annular Fissures in Patients with Low Back Pain: A Radiomics Approach. J. Clin. Med. 2023, 12, 4891. https://doi.org/10.3390/jcm12154891
Waldenberg C, Brisby H, Hebelka H, Lagerstrand KM. Associations between Vertebral Localized Contrast Changes and Adjacent Annular Fissures in Patients with Low Back Pain: A Radiomics Approach. Journal of Clinical Medicine. 2023; 12(15):4891. https://doi.org/10.3390/jcm12154891
Chicago/Turabian StyleWaldenberg, Christian, Helena Brisby, Hanna Hebelka, and Kerstin Magdalena Lagerstrand. 2023. "Associations between Vertebral Localized Contrast Changes and Adjacent Annular Fissures in Patients with Low Back Pain: A Radiomics Approach" Journal of Clinical Medicine 12, no. 15: 4891. https://doi.org/10.3390/jcm12154891





