Nusinersen Treatment of Children with Later-Onset Spinal Muscular Atrophy and Scoliosis Is Associated with Improvements or Stabilization of Motor Function †
Abstract
:1. Introduction
2. Methods
2.1. Standard Protocol Approvals, Registrations, and Patient Consents
2.2. Study Population and Outcome Measures
2.3. Statistical Analyses
3. Results
3.1. Participants
3.2. Motor Function
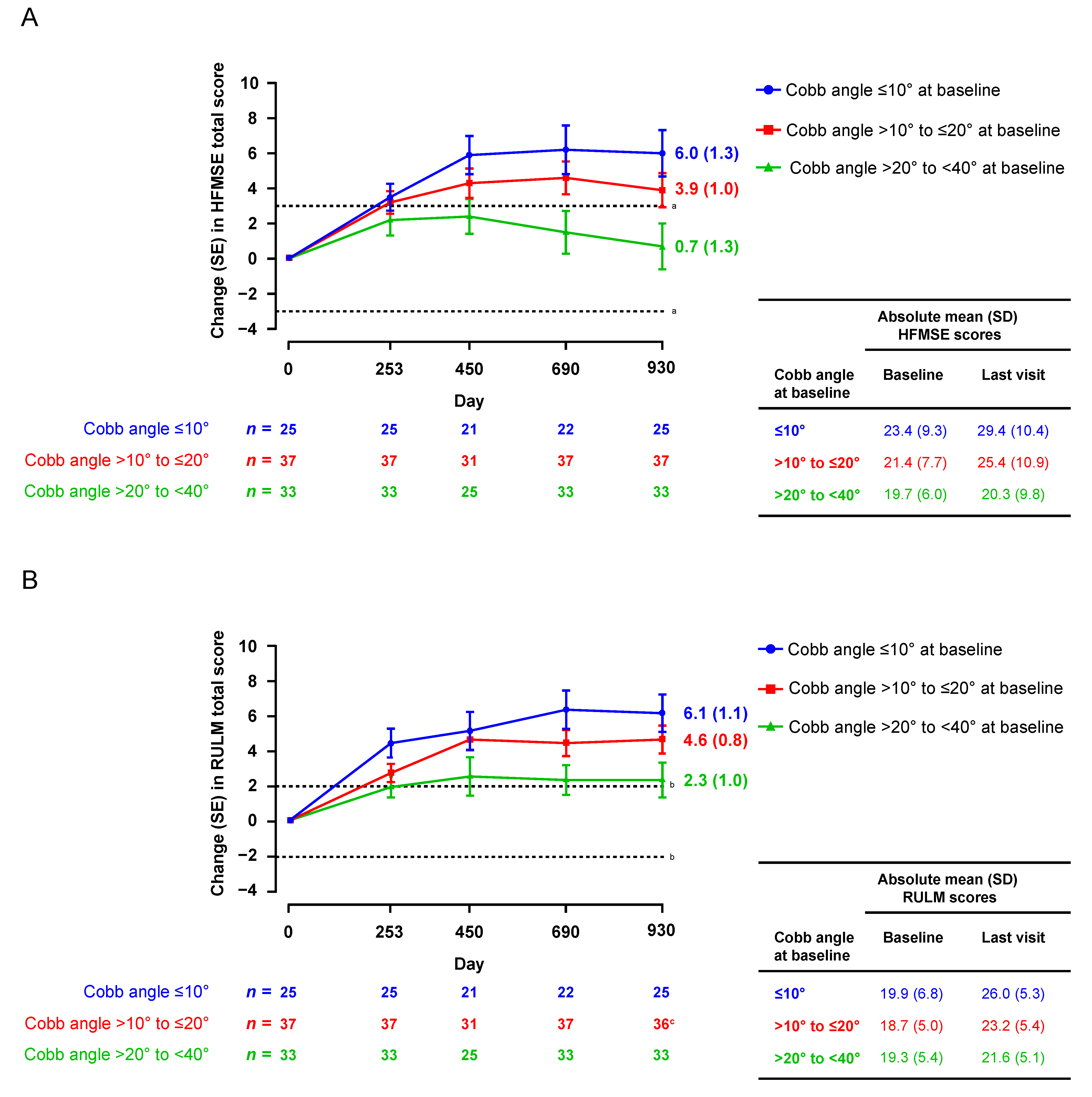
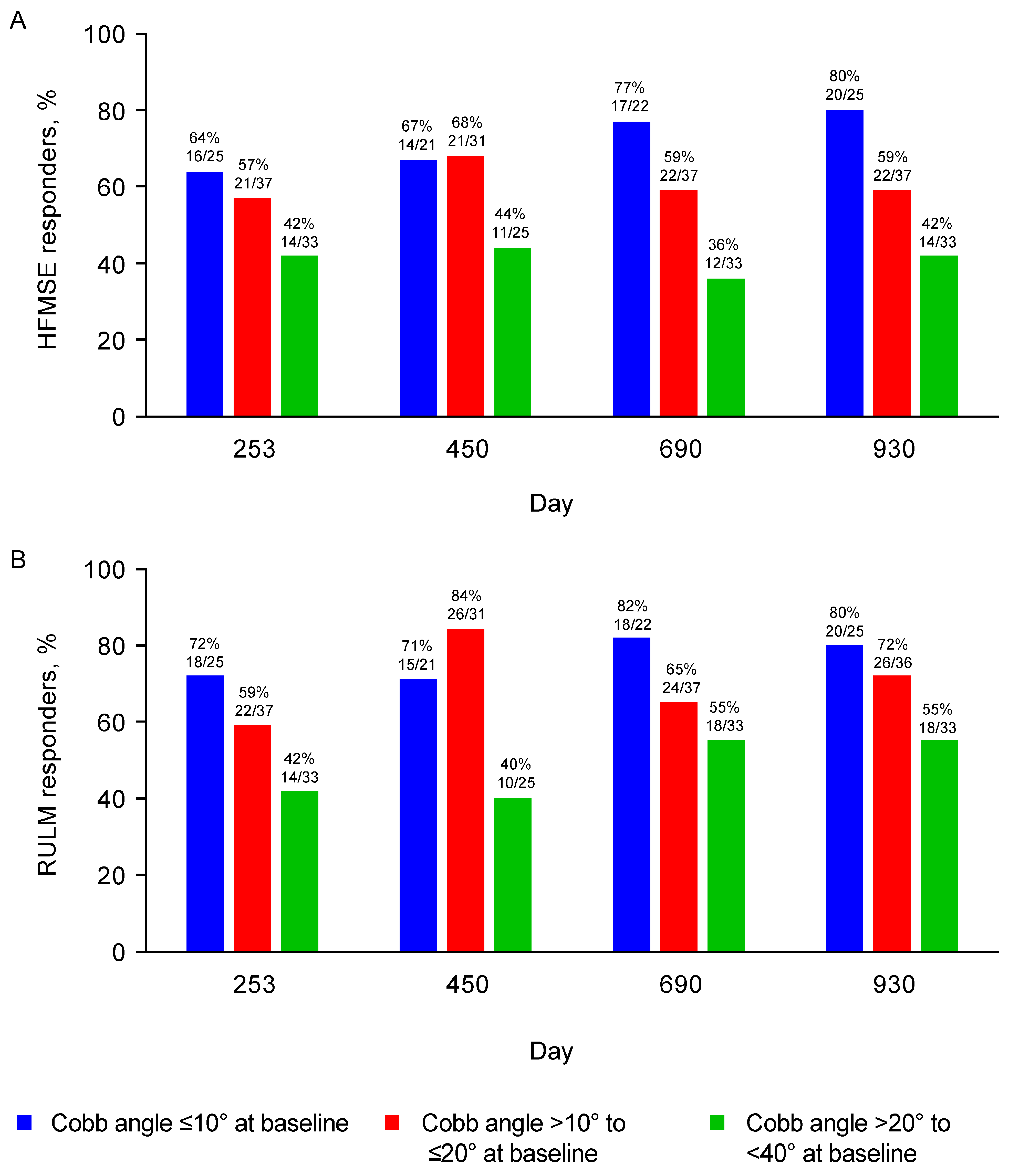
3.2.1. HFMSE and RULM
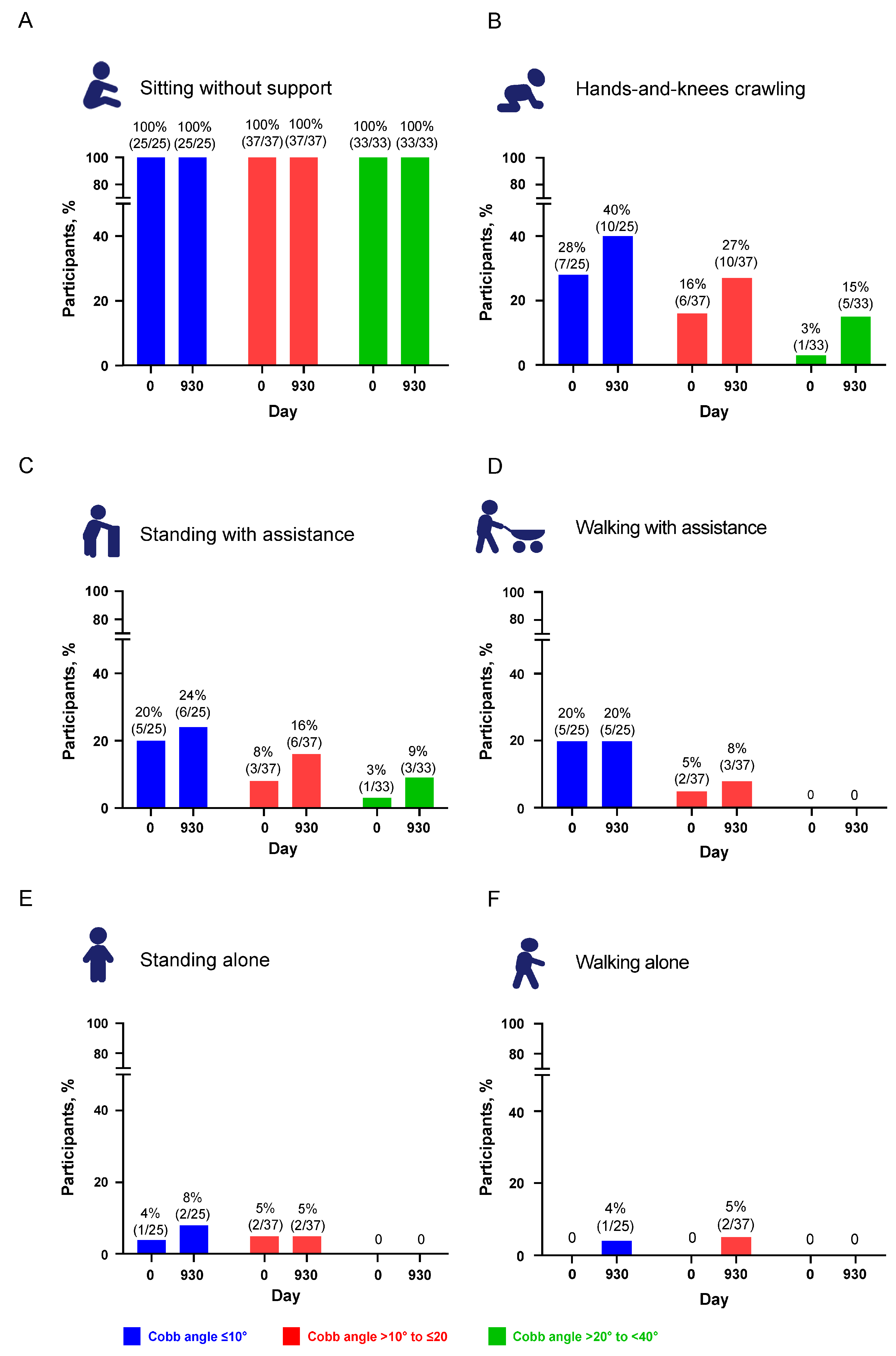
3.2.2. WHO Motor Milestones
3.2.3. Predictors of Motor Function Outcomes
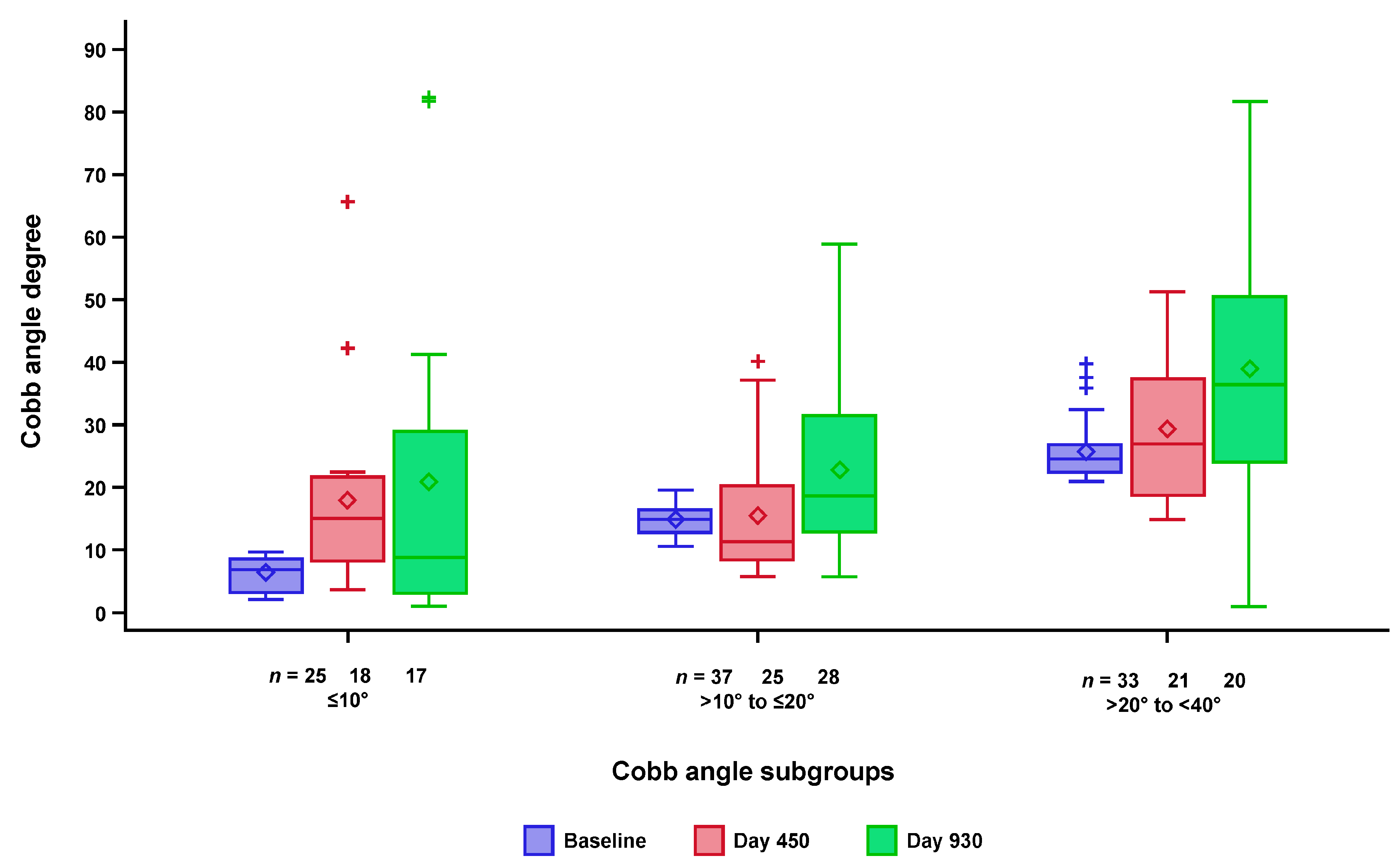
3.3. Changes in Scoliosis over Time
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Darras, B.T.; Monani, U.R.; De Vivo, D.C. Genetic disorders affecting the motor neuron: Spinal muscular atrophy. In Swaiman’s Pediatric Neurology; Swaiman, K.F., Ashwal, S., Ferriero, D.M., Schor, N.F., Finkel, R.S., Gropman, A.L., Pearl, P.L., et al., Eds.; Elsevier: Edinburgh, UK, 2017. [Google Scholar]
- Lunn, M.R.; Wang, C.H. Spinal muscular atrophy. Lancet 2008, 371, 2120–2133. [Google Scholar] [CrossRef] [PubMed]
- Arkblad, E.; Tulinius, M.; Kroksmark, A.K.; Henricsson, M.; Darin, N. A population-based study of genotypic and phenotypic variability in children with spinal muscular atrophy. Acta Paediatr. 2009, 98, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejowska, M.; Milewski, M.; Zimowski, J.; Zagozdzon, P.; Kostera-Pruszczyk, A.; Borkowska, J.; Sielska, D.; Jurek, M.; Hausmanowa-Petrusewicz, I. Incidence of spinal muscular atrophy in Poland–more frequent than predicted? Neuroepidemiology 2010, 34, 152–157. [Google Scholar] [PubMed]
- Prior, T.W.; Snyder, P.J.; Rink, B.D.; Pearl, D.K.; Pyatt, R.E.; Mihal, D.C.; Conlan, T.; Schmalz, B.; Montgomery, L.; Ziegler, K.; et al. Newborn and carrier screening for spinal muscular atrophy. Am. J. Med. Genet. A 2010, 152, 1608–1616. [Google Scholar] [CrossRef]
- Sugarman, E.A.; Nagan, N.; Zhu, H.; Akmaev, V.R.; Zhou, Z.; Rohlfs, E.M.; Flynn, K.; Hendrickson, B.C.; Scholl, T.; Sirko-Osadsa, D.A.; et al. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: Clinical laboratory analysis of >72,400 specimens. Eur. J. Hum. Genet. 2012, 20, 27–32. [Google Scholar] [CrossRef]
- Haaker, G.; Fujak, A. Proximal spinal muscular atrophy: Current orthopedic perspective. Appl. Clin. Genet. 2013, 6, 113–120. [Google Scholar]
- Zenios, M.; Sampath, J.; Cole, C.; Khan, T.; Galasko, C.S. Operative treatment for hip subluxation in spinal muscular atrophy. J. Bone Jt. Surg. Br. 2005, 87, 1541–1544. [Google Scholar]
- Wijngaarde, C.A.; Brink, R.C.; De Kort, F.A.S.; Stam, M.; Otto, L.A.M.; Asselman, F.-L.; Bartels, B.; Van Eijk, R.P.A.; Sombroek, J.; Cuppen, I.; et al. Natural course of scoliosis and lifetime risk of scoliosis surgery in spinal muscular atrophy. Neurology 2019, 93, e149–e158. [Google Scholar] [CrossRef]
- Rodillo, E.; Marini, M.L.; Heckmatt, J.Z.; Dubowitz, V. Scoliosis in spinal muscular atrophy: Review of 63 cases. J. Child. Neurol. 1989, 4, 118–123. [Google Scholar]
- Negrini, S.; Donzelli, S.; Aulisa, A.G.; Czaprowski, D.; Schreiber, S.; De Mauroy, J.C.; Diers, H.; Grivas, T.B.; Knott, P.; Kotwicki, T.; et al. 2016 SOSORT guidelines: Orthopaedic and rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis Spinal Disord. 2018, 13, 3. [Google Scholar]
- Granata, C.; Merlini, L.; Magni, E.; Marini, M.L.; Stagni, S.B. Spinal muscular atrophy: Natural history and orthopaedic treatment of scoliosis. Spine 1989, 14, 760–762. [Google Scholar] [CrossRef]
- Coratti, G.; Pera, M.C.; D’Amico, A.; Bruno, C.; Bovis, F.; Gullì, C.; Brolatti, N.; Pedemonte, M.; Apicella, M.; Antonaci, L.; et al. Long term follow-up of scoliosis progression in type II SMA patients. Neuromuscul. Disord. 2022, 32, 879–885. [Google Scholar] [CrossRef]
- Young, S.D.; Montes, J.; Salazar, R.; Glanzman, A.M.; Pasternak, A.; Mirek, E.; Martens, W.; Finkel, R.S.; Darras, B.T.; De Vivo, D.C. Scoliosis surgery significantly impacts motor abilities in higher-functioning individuals with spinal muscular atrophy1. J. Neuromuscul. Dis. 2020, 7, 183–192. [Google Scholar] [CrossRef]
- Allam, A.M.; Schwabe, A.L. Neuromuscular scoliosis. PM&R 2013, 5, 957–963. [Google Scholar]
- Fujak, A.; Raab, W.; Schuh, A.; Richter, S.; Forst, R.; Forst, J. Natural course of scoliosis in proximal spinal muscular atrophy type II and IIIa: Descriptive clinical study with retrospective data collection of 126 patients. BMC Musculoskelet. Disord. 2013, 14, 283. [Google Scholar] [CrossRef] [Green Version]
- Mercuri, E.; Lucibello, S.; Pera, M.; Carnicella, S.; Coratti, G.; De Sanctis, R.; Mazzone, E.; Forcina, N.; Fanelli, L.; Norcia, G.; et al. Long-term progression in type II spinal muscular atrophy: A retrospective observational study. Neurology 2019, 29, e1241–e1247. [Google Scholar] [CrossRef]
- Acsadi, G.; Crawford, T.O.; Müller-Felber, W.; Shieh, P.B.; Richardson, R.; Natarajan, N.; Castro, D.; Ramirez-Schrempp, D.; Gambino, G.; Sun, P.; et al. Safety and efficacy of nusinersen in spinal muscular atrophy: The EMBRACE study. Muscle Nerve 2021, 63, 668–677. [Google Scholar] [CrossRef]
- Darras, B.T.; Chiriboga, C.A.; Iannaccone, S.T.; Swoboda, K.J.; Montes, J.; Mignon, L.; Xia, S.; Bennett, C.F.; Bishop, K.M.; Shefner, J.M.; et al. Nusinersen in later-onset spinal muscular atrophy: Long-term results from the phase 1/2 studies. Neurology 2019, 92, e2492–e2506. [Google Scholar] [CrossRef] [Green Version]
- De Vivo, D.C.; Bertini, E.; Swoboda, K.J.; Hwu, W.-L.; Crawford, T.O.; Finkel, R.S.; Kirschner, J.; Kuntz, N.L.; Parsons, J.A.; Ryan, M.M.; et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul. Disord. 2019, 29, 842–856. [Google Scholar] [CrossRef] [Green Version]
- Finkel, R.S.; A Chiriboga, C.; Vajsar, J.; Day, J.W.; Montes, J.; De Vivo, D.C.; Bishop, K.M.; Foster, R.; Liu, Y.; Ramirez-Schrempp, D.; et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: Final report of a phase 2, open-label, multicentre, dose-escalation study. Lancet Child Adolesc. Health 2021, 5, 491–500. [Google Scholar] [CrossRef]
- Finkel, R.S.; Chiriboga, C.A.; Vajsar, J.; Day, J.W.; Montes, J.; De Vivo, D.C.; Yamashita, M.; Rigo, F.; Hung, G.; Schneider, E.; et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: A phase 2, open-label, dose-escalation study. Lancet 2016, 388, 3017–3026. [Google Scholar] [CrossRef] [PubMed]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagenacker, T.; Wurster, C.D.; Günther, R.; Schreiber-Katz, O.; Osmanovic, A.; Petri, S.; Weiler, M.; Ziegler, A.; Kuttler, J.; Koch, J.C.; et al. Nusinersen in adults with 5q spinal muscular atrophy: A non-interventional, multicentre, observational cohort study. Lancet Neurol. 2020, 19, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Darras, B.T.; Chiriboga, C.A.; Day, J.W.; Campbell, C.; Connolly, A.M.; Iannaccone, S.T.; Kirschner, J.; Kuntz, N.L.; Saito, K.; et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N. Engl. J. Med. 2018, 378, 625–635. [Google Scholar] [CrossRef]
- Szabó, L.; Gergely, A.; Jakus, R.; Fogarasi, A.; Grosz, Z.; Molnár, M.J.; Andor, I.; Schulcz, O.; Goschler, Á.; Medveczky, E.; et al. Efficacy of nusinersen in type 1, 2 and 3 spinal muscular atrophy: Real world data from Hungarian patients. Eur. J. Paediatr. Neurol. 2020, 27, 37–42. [Google Scholar] [CrossRef]
- Walter, M.C.; Wenninger, S.; Thiele, S.; Stauber, J.; Hiebeler, M.; Greckl, E.; Stahl, K.; Pechmann, A.; Lochmüller, H.; Kirschner, J.; et al. Safety and treatment effects of nusinersen in longstanding adult 5q-SMA type 3—A prospective observational study. J. Neuromuscul. Dis. 2019, 6, 453–465. [Google Scholar] [CrossRef] [Green Version]
- Darras, B.T.; Day, J.W.; Swoboda, K.J.; Chiriboga, C.A.; Iannaccone, S.T.; De Vivo, D.C.; Deconinck, N.; Finkel, R.S.; Tulinius, M.; Saito, K.; et al. Nusinersen in adolescents and young adults with SMA: Longitudinal experience from an expanded cohort of CS2/CS12 and SHINE participants. In Proceedings of the Cure SMA—2020 Annual Conference (Virtual Presentation), Virtual, 8–12 June 2020. [Google Scholar]
- Glanzman, A.M.; O’Hagen, J.M.; McDermott, M.P.; Martens, W.B.; Flickinger, J.; Riley, S.; Quigley, J.; Montes, J.; Dunaway, S.; Deng, L.; et al. Validation of the Expanded Hammersmith Functional Motor Scale in spinal muscular atrophy type II and III. J. Child. Neurol. 2011, 26, 1499–1507. [Google Scholar] [CrossRef]
- O’Hagen, J.M.; Glanzman, A.M.; McDermott, M.P.; Ryan, P.A.; Flickinger, J.; Quigley, J.; Riley, S.; Sanborn, E.; Irvine, C.; Martens, W.B.; et al. An expanded version of the Hammersmith Functional Motor Scale for SMA II and III patients. Neuromuscul. Disord. 2007, 17, 693–697. [Google Scholar] [CrossRef]
- Carman, D.L.; Browne, R.H.; Birch, J.G. Measurement of scoliosis and kyphosis radiographs. Intraobserver and interobserver variation. J. Bone Jt. Surg. Am. 1990, 72, 328–333. [Google Scholar] [CrossRef]
- Morrissy, R.T.; Goldsmith, G.S.; Hall, E.C.; Kehl, D.; Cowie, G.H. Measurement of the Cobb angle on radiographs of patients who have scoliosis. Evaluation of intrinsic error. J. Bone Jt. Surg. Am. 1990, 72, 320–327. [Google Scholar] [CrossRef]
- Wick, J.M.; Konze, J.; Alexander, K.; Sweeney, C. Infantile and juvenile scoliosis: The crooked path to diagnosis and treatment. Aorn J. 2009, 90, 347–376. [Google Scholar] [CrossRef]
- WHO Motor Development Study: Windows of achievement for six gross motor development milestones. Acta Paediatr. Suppl. 2006, 450, 86–95.
- Mazzone, E.S.; Mayhew, A.; Montes, J.; Ramsey, D.; Fanelli, L.; Young, S.D.; Salazar, R.; De Sanctis, R.; Pasternak, A.; Glanzman, A.; et al. Revised upper limb module for spinal muscular atrophy: Development of a new module. Muscle Nerve 2017, 55, 869–874. [Google Scholar] [CrossRef]
- Horng, M.H.; Kuok, C.P.; Fu, M.J.; Lin, C.J.; Sun, Y.N. Cobb angle measurement of spine from X-ray images using convolutional neural network. Comput. Math. Methods Med. 2019, 2019, 6357171. [Google Scholar] [CrossRef] [Green Version]
- Williams, V.; Stull, D.E.; Houghton, K.; Williams, N.; Teynor, M. Minimal clinically important differences of the Expanded Hammersmith Functional Motor Scale in later-onset spinal muscular atrophy: Results from the phase 3 CHERISH trial. J. Manag. Care Spec. Pharm. 2019, 25 (Suppl. 3a), S54. [Google Scholar]
- Pera, M.C.; Coratti, G.; Mazzone, E.S.; Montes, J.; Scoto, M.; De Sanctis, R.; Main, M.; Mayhew, A.; Muni Lofra, R.; Dunaway Young, S.; et al. Revised upper limb module for spinal muscular atrophy: 12 month changes. Muscle Nerve 2019, 59, 426–430. [Google Scholar] [CrossRef]
- Pera, M.C.; Coratti, G.; Bovis, F.; Pane, M.; Pasternak, A.; Montes, J.; Sansone, V.A.; Dunaway Young, S.; Duong, T.; Messina, S.; et al. Nusinersen in pediatric and adult patients with type III spinal muscular atrophy. Ann. Clin. Transl. Neurol. 2021, 8, 1622–1634. [Google Scholar] [CrossRef]
- Merlini, L.; Granata, C.; Bonfiglioli, S.; Marini, M.L.; Cervellati, S.; Savini, R. Scoliosis in spinal muscular atrophy: Natural history and management. Dev. Med. Child. Neurol. 1989, 31, 501–508. [Google Scholar] [CrossRef]
- Sumner, C.J.; Crawford, T.O. Two breakthrough gene-targeted treatments for spinal muscular atrophy: Challenges remain. J. Clin. Investig. 2018, 128, 3219–3227. [Google Scholar] [CrossRef]
- Finkel, R.S.; Benatar, M. Pre-symptomatic spinal muscular atrophy: A proposed nosology. Brain 2022, 145, 2247–2249. [Google Scholar] [CrossRef]
- Glascock, J.; Sampson, J.; Haidet-Phillips, A.; Connolly, A.; Darras, B.; Day, J.; Finkel, R.; Howell, R.R.; Klinger, K.; Kuntz, N.; et al. Treatment algorithm for infants diagnosed with spinal muscular atrophy through newborn screening. J. Neuromuscul. Dis. 2018, 5, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Giannoglou, V.; Stylianidis, E. Review of advances in Cobb angle calculation and image-based modeling techniques for spinal deformities. ISPRS Ann. Photogramm. Remote Sens. Spat. Inf. Sci. 2016, III-5, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Lam, G.C.; Hill, D.L.; Le, L.H.; Raso, J.V.; Lou, E.H. Vertebral rotation measurement: A summary and comparison of common radiographic and CT methods. Scoliosis 2008, 3, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristic | Cobb Angle Subgroups | Total N = 95 | ||
|---|---|---|---|---|
| ≤10° n = 25 | >10° to ≤20° n = 37 | >20° to <40° n = 33 | ||
| Female, n (%) | 11 (44) | 22 (59) | 18 (55) | 51 (54) |
| SMN2 copy number, n (%) a | ||||
| 2 | 3 (12) | 2 (5) | 1 (3) | 6 (6) |
| 3 | 21 (84) | 33 (89) | 32 (97) | 86 (91) |
| 4 | 0 (0) | 1 (3) | 0 (0) | 1 (1) |
| Median (range) age at first dose, years | 4.0 (2.1–8.1) | 4.0 (2.1–9.2) | 4.4 (2.1–8.9) | 4.2 (2.1–9.2) |
| Median (range) age at symptom onset, months | 13.0 (7.0–18.0) | 11.0 (6.0–18.0) | 10.0 (6.0–20.0) | 11.0 (6.0–20.0) |
| Cobb angle | ||||
| Mean (SD) | 6.3 (2.64) | 14.9 (2.69) | 25.8 (4.83) | 16.5 (8.46) |
| Median (range) | 6.8 (2.1–9.8) | 14.9 (10.4–19.6) | 24.5 (20.9–39.8) | 15.9 (2.1–39.8) |
| Mean (SD) weight, kg | 14.8 (5.24) | 15.3 (4.72) | 15.6 (5.94) | 15.3 (5.26) |
| Mean (SD) ulnar length, cm | 14.8 (2.32) b | 14.8 (2.04) c | 17.5 (14.69) d | 15.8 (8.98) e |
| Mean (SD) HFMSE score | 23.4 (9.32) | 21.4 (7.73) | 19.7 (5.98) | 21.4 (7.70) |
| Mean (SD) RULM score | 19.9 (6.82) | 18.7 (4.97) | 19.3 (5.36) | 19.2 (5.60) |
| Variables | Estimate (95% CI) | p Value |
|---|---|---|
| Change in HFMSE at: | ||
| Intercept | 12.86 (7.8,17.91) | <0.0001 |
| Baseline HFMSE | 0.04 (−0.09, 0.18) | 0.52 |
| Age at first dose of nusinersen | −2.69 (−3.27, −2.12) | <0.0001 |
| Age at SMA onset | 0.34 (0.04, 0.64) | 0.03 |
| Baseline Cobb angle | −0.14 (−0.26, −0.02) | 0.02 |
| Change in RULM at | ||
| Intercept | 16.2 (12.49, 19.91) | <0.0001 |
| Baseline RULM | −0.32 (−0.46, −0.17) | <0.0001 |
| Age at first dose of nusinersen | −1.63 (−2.11, −1.15) | <0.0001 |
| Age at SMA onset | 0.3 (0.08, 0.52) | 0.008 |
| Baseline Cobb angle | −0.12 (−0.21, −0.04) | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunaway Young, S.; Montes, J.; Glanzman, A.M.; Gee, R.; Day, J.W.; Finkel, R.S.; Darras, B.T.; De Vivo, D.C.; Gambino, G.; Foster, R.; et al. Nusinersen Treatment of Children with Later-Onset Spinal Muscular Atrophy and Scoliosis Is Associated with Improvements or Stabilization of Motor Function. J. Clin. Med. 2023, 12, 4901. https://doi.org/10.3390/jcm12154901
Dunaway Young S, Montes J, Glanzman AM, Gee R, Day JW, Finkel RS, Darras BT, De Vivo DC, Gambino G, Foster R, et al. Nusinersen Treatment of Children with Later-Onset Spinal Muscular Atrophy and Scoliosis Is Associated with Improvements or Stabilization of Motor Function. Journal of Clinical Medicine. 2023; 12(15):4901. https://doi.org/10.3390/jcm12154901
Chicago/Turabian StyleDunaway Young, Sally, Jacqueline Montes, Allan M. Glanzman, Richard Gee, John W. Day, Richard S. Finkel, Basil T. Darras, Darryl C. De Vivo, Giulia Gambino, Richard Foster, and et al. 2023. "Nusinersen Treatment of Children with Later-Onset Spinal Muscular Atrophy and Scoliosis Is Associated with Improvements or Stabilization of Motor Function" Journal of Clinical Medicine 12, no. 15: 4901. https://doi.org/10.3390/jcm12154901





