Predictors and Long-Term Prognostic Significance of Acute Renal Function Change in Patients Who Underwent Surgical Aortic Valve Replacement
Abstract
:1. Introduction
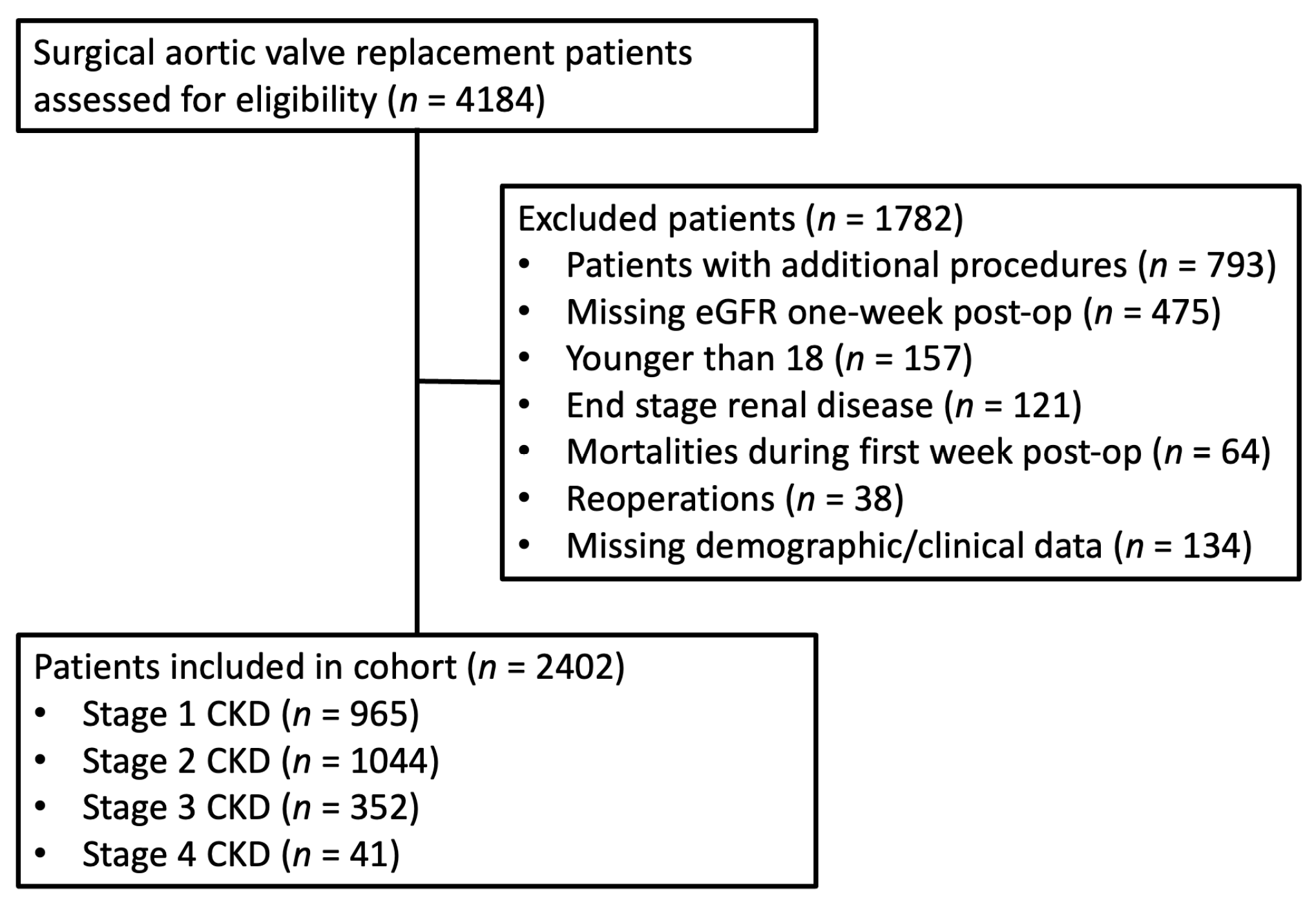
2. Materials and Methods
2.1. Demographics and Outcome Measures
2.2. Statistical Analysis
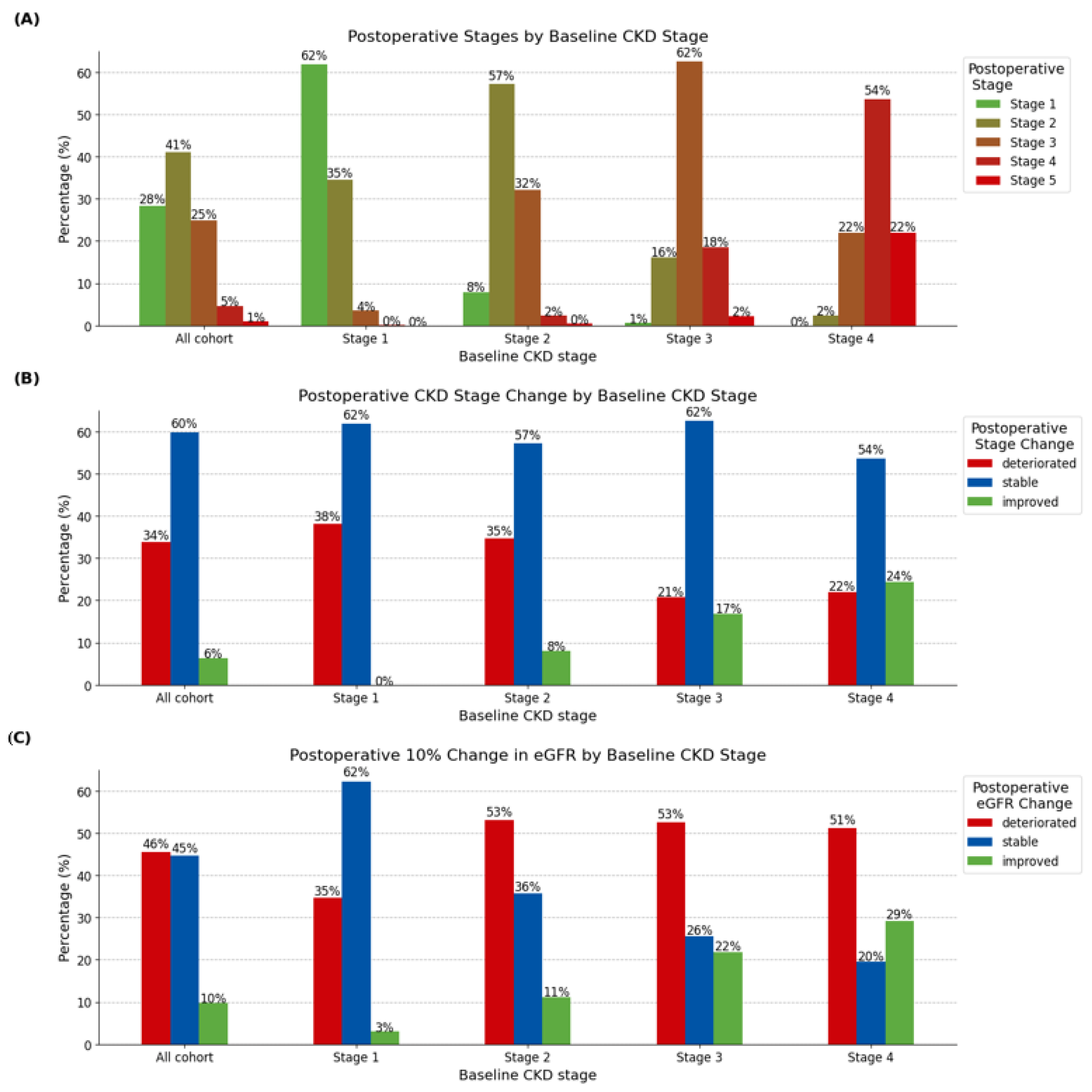
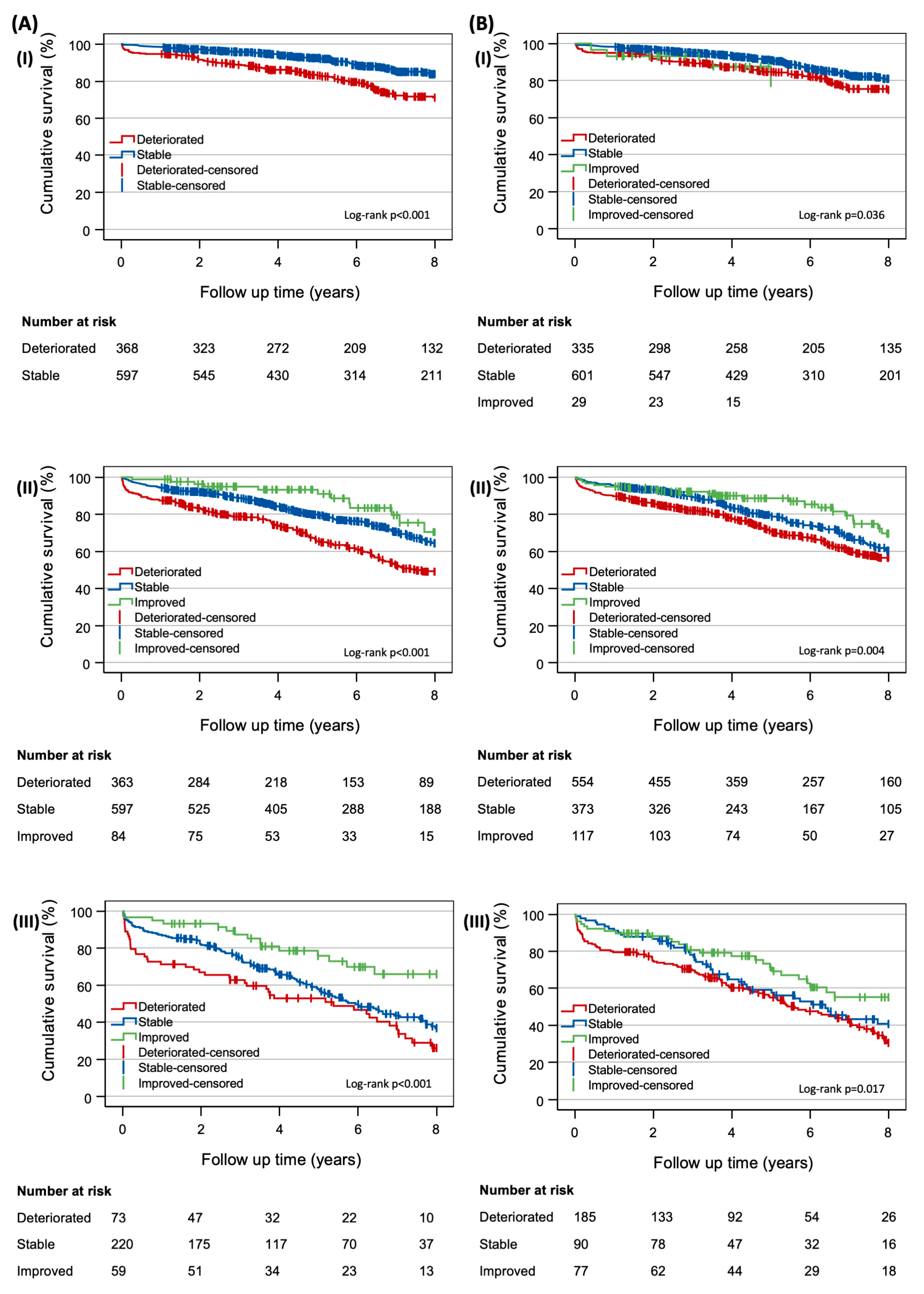
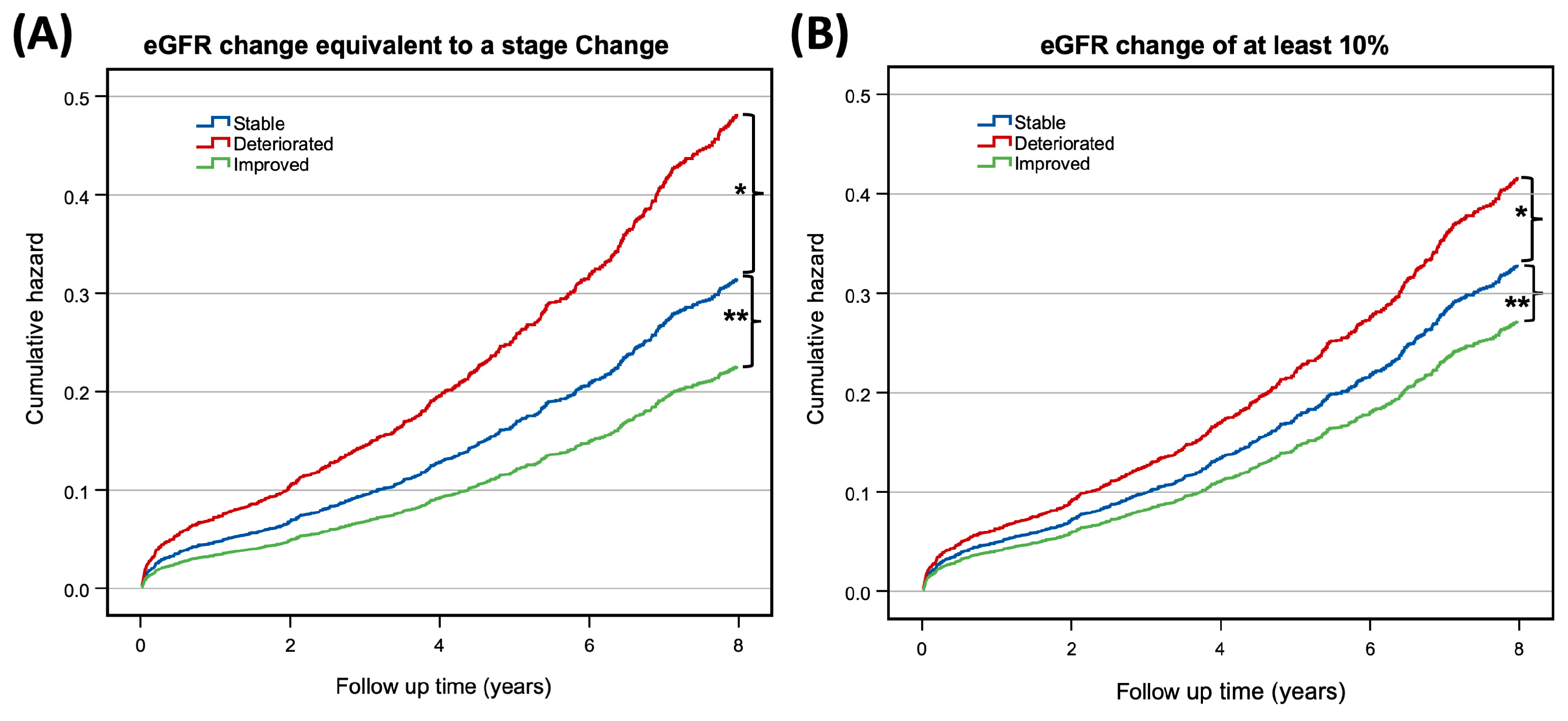
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: Executive summary: A report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. J. Am. Coll. Cardiol. 2021, 77, 450–500. [Google Scholar] [CrossRef]
- Thourani, V.H.; Keeling, W.B.; Sarin, E.L.; Guyton, R.A.; Kilgo, P.D.; Dara, A.B.; Puskas, J.D.; Chen, E.P.; Cooper, W.A.; Vega, J.D.; et al. Impact of preoperative renal dysfunction on long-term survival for patients undergoing aortic valve replacement. Ann. Thorac. Surg. 2011, 91, 1798–1806; discussion 1806. [Google Scholar] [CrossRef] [PubMed]
- Thourani, V.H.; Chowdhury, R.; Gunter, R.L.; Kilgo, P.D.; Chen, E.P.; Puskas, J.D.; Halkos, M.E.; Lattouf, O.M.; Cooper, W.A.; Guyton, R.A. The impact of specific preoperative organ dysfunction in patients undergoing aortic valve replacement. Ann. Thorac. Surg. 2013, 95, 838–845. [Google Scholar] [CrossRef]
- Rosner, M.H.; Okusa, M.D. Acute kidney injury associated with cardiac surgery. Clin. J. Am. Soc. Nephrol. 2006, 1, 19–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witberg, G.; Steinmetz, T.; Landes, U.; Hanit, R.P.; Green, H.; Goldman, S.; Vaknin-Assa, H.; Codner, P.; Perl, L.; Rozen-Zvi, B.; et al. Change in Kidney Function and 2-Year Mortality After Transcatheter Aortic Valve Replacement. JAMA Netw. Open 2021, 4, e213296. [Google Scholar] [CrossRef] [PubMed]
- Azarbal, A.; Leadholm, K.L.; Ashikaga, T.; Solomon, R.J.; Dauerman, H.L. Frequency and prognostic significance of acute kidney recovery in patients who underwent transcatheter aortic valve implantation. Am. J. Cardiol. 2018, 121, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Lahoud, R.; Butzel, D.W.; Parsee, A.; Huang, Y.-L.; Solomon, R.J.; DeVries, J.T.; Flynn, J.M.; Iribarne, A.; Lee, P.V.; Ross, C.S.; et al. Acute kidney recovery in patients who underwent transcatheter versus surgical aortic valve replacement (from the northern new england cardiovascular disease study group). Am. J. Cardiol. 2020, 125, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Azarbal, A.; Malenka, D.J.; Huang, Y.-L.; Ross, C.S.; Solomon, R.J.; DeVries, J.T.; Flynn, J.M.; Butzel, D.; McKay, M.; Dauerman, H.L. Recovery of kidney dysfunction after transcatheter aortic valve implantation (from the Northern New England cardiovascular disease study group). Am. J. Cardiol. 2019, 123, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Haapio, M.; House, A.A.; Anavekar, N.; Bellomo, R. Cardiorenal syndrome. J. Am. Coll. Cardiol. 2008, 52, 1527–1539. [Google Scholar] [CrossRef] [Green Version]
- Najjar, M.; Yerebakan, H.; Sorabella, R.A.; Guglielmetti, L.; Vandenberge, J.; Kurlansky, P.; Williams, M.R.; Argenziano, M.; Smith, C.R.; George, I. Reversibility of chronic kidney disease and outcomes following aortic valve replacement. Interact. Cardiovasc. Thorac. Surg. 2015, 21, 499–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leibowitz, M.; Karpati, T.; Cohen-Stavi, C.J.; Feldman, B.S.; Hoshen, M.; Bitterman, H.; Suissa, S.; Balicer, R.D. Association Between Achieved Low-Density Lipoprotein Levels and Major Adverse Cardiac Events in Patients with Stable Ischemic Heart Disease Taking Statin Treatment. JAMA Intern. Med. 2016, 176, 1105–1113. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39 (Suppl. 1), S1–S266. [Google Scholar]
- Mas-Peiro, S.; Faerber, G.; Bon, D.; Herrmann, E.; Bauer, T.; Bleiziffer, S.; Bekeredjian, R.; Böning, A.; Frerker, C.; Beckmann, A.; et al. Impact of chronic kidney disease in 29,893 patients undergoing transcatheter or surgical aortic valve replacement from the German Aortic Valve Registry. Eur. J. Cardiothorac. Surg. 2021, 59, 532–544. [Google Scholar] [CrossRef]
- Bohbot, Y.; Candellier, A.; Diouf, M.; Rusinaru, D.; Altes, A.; Pasquet, A.; Maréchaux, S.; Vanoverschelde, J.; Tribouilloy, C. Severe aortic stenosis and chronic kidney disease: Outcomes and impact of aortic valve replacement. J. Am. Heart Assoc. 2020, 9, e017190. [Google Scholar] [CrossRef]
- Cubeddu, R.J.; Asher, C.R.; Lowry, A.M.; Blackstone, E.H.; Kapadia, S.R.; Alu, M.C.; Thourani, V.H.; Mack, M.J.; Kodali, S.K.; Herrmann, H.C.; et al. Impact of transcatheter aortic valve replacement on severity of chronic kidney disease. J. Am. Coll. Cardiol. 2020, 76, 1410–1421. [Google Scholar] [CrossRef]
- Whitlock, R.P.; Devereaux, P.J.; Teoh, K.H.; Lamy, A.; Vincent, J.; Pogue, J.; Paparella, D.; Sessler, D.I.; Karthikeyan, G.; Villar, J.C.; et al. Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): A randomised, double-blind, placebo-controlled trial. Lancet 2015, 386, 1243–1253. [Google Scholar] [CrossRef]
- Lee, E.-H.; Kim, W.-J.; Kim, J.-Y.; Chin, J.-H.; Choi, D.-K.; Sim, J.-Y.; Choo, S.-J.; Chung, C.-H.; Lee, J.-W.; Choi, I.-C. Effect of Exogenous Albumin on the Incidence of Postoperative Acute Kidney Injury in Patients Undergoing Off-pump Coronary Artery Bypass Surgery with a Preoperative Albumin Level of Less Than 4.0 g/dL. Anesthesiology 2016, 124, 1001–1011. [Google Scholar] [CrossRef]
- Zheng, Z.; Jayaram, R.; Jiang, L.; Emberson, J.; Zhao, Y.; Li, Q.; Du, J.; Guarguagli, S.; Hill, M.; Chen, Z.; et al. Perioperative rosuvastatin in cardiac surgery. N. Engl. J. Med. 2016, 374, 1744–1753. [Google Scholar] [CrossRef]
- Shin, H.J.; Ko, E.; Jun, I.; Kim, H.J.; Lim, C.H. Effects of perioperative erythropoietin administration on acute kidney injury and red blood cell transfusion in patients undergoing cardiac surgery: A systematic review and meta-analysis. Medicine 2022, 101, e28920. [Google Scholar] [CrossRef]
- Chew, S.T.H.; Ng, R.R.G.; Liu, W.; Goh, S.G.; Caleb, M.G.; Ti, L.K. Miniaturized versus conventional cardiopulmonary bypass and acute kidney injury after cardiac surgery. Perfusion 2016, 31, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Mazer, C.D.; Whitlock, R.P.; Fergusson, D.A.; Hall, J.; Belley-Cote, E.; Connolly, K.; Khanykin, B.; Gregory, A.J.; de Médicis, É.; McGuinness, S.; et al. Restrictive or Liberal Red-Cell Transfusion for Cardiac Surgery. N. Engl. J. Med. 2017, 377, 2133–2144. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.N.A.; Best, D.; Sheppard, S.V.; Smith, D.C. The effect of mannitol on renal function after cardiopulmonary bypass in patients with established renal dysfunction. Anaesthesia 2008, 63, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Fakhari, S.; Bavil, F.M.; Bilehjani, E.; Abolhasani, S.; Mirinazhad, M.; Naghipour, B. Prophylactic furosemide infusion decreasing early major postoperative renal dysfunction in on-pump adult cardiac surgery: A randomized clinical trial. Res. Rep. Urol. 2017, 9, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Zarbock, A.; Küllmar, M.; Ostermann, M.; Lucchese, G.; Baig, K.; Cennamo, A.; Rajani, R.; McCorkell, S.; Arndt, C.; Wulf, H.; et al. Prevention of Cardiac Surgery-Associated Acute Kidney Injury by Implementing the KDIGO Guidelines in High-Risk Patients Identified by Biomarkers: The PrevAKI-Multicenter Randomized Controlled Trial. Anesth. Analg. 2021, 133, 292–302. [Google Scholar] [CrossRef]
- Beohar, N.; Doshi, D.; Thourani, V.; Jensen, H.; Kodali, S.; Zhang, F.; Zhang, Y.; Davidson, C.; McCarthy, P.; Mack, M.; et al. Association of Transcatheter Aortic Valve Replacement With 30-Day Renal Function and 1-Year Outcomes Among Patients Presenting with Compromised Baseline Renal Function: Experience from the PARTNER 1 Trial and Registry. JAMA Cardiol. 2017, 2, 742–749. [Google Scholar] [CrossRef]
| All Cohort (n = 2402) | Stable Stage (n = 1436) | Improved Stage (n = 153) | Deteriorated Stage (n = 813) | Multiple Comp. | Stable vs. Imp. | Stable vs. Det. | Imp. vs. Det. | |
|---|---|---|---|---|---|---|---|---|
| n (%) or Mean ± SD | n (%) or Mean ± SD | n (%) or Mean ± SD | n (%) or Mean ± SD | p-Value a | Post Hoc p-Value | Post Hoc p-Value | Post Hoc p-Value | |
| Demographics | ||||||||
| Age, years | 69.31 (11.39) | 67.94 (12.52) | 66.91 (10.24) | 72.18 (8.61) | <0.001 b | 0.532 d | <0.001 d | <0.001 d |
| Women | 916 (38.1) | 543 (37.8) | 44 (28.8) | 329 (40.5) | 0.022 | |||
| BMI, kg/m2 | 29.40 (5.86) | 29.33 (5.76) | 30.37 (8.34) | 29.33 (5.45) | 0.150 b | |||
| [BMI missing values] | [290] | [161] | [24] | [105] | ||||
| Pre-op Renal Function | ||||||||
| Creatinine, mg/dL | 0.943 (0.365) | 0.912 (0.356) | 1.143 (0.406) | 0.948 (0.359) | <0.001 b | <0.001 d | 0.067 d | <0.001 d |
| eGFR, ml/min/1.73 m2 | 82.28 (20.79) | 84.67 (21.70) | 67.22 (18.47) | 80.91 (18.10) | <0.001 b | <0.001 d | <0.001 d | <0.001 d |
| Baseline CKD | <0.001 c | |||||||
| Stage 1 | 965 (40.2) | 597 (41.6) | 0 | 368 (45.3) | ||||
| Stage 2 | 1044 (43.5) | 597 (41.6) | 84 (54.9) | 363 (44.6) | ||||
| Stage 3 | 352 (14.7) | 220 (15.3) | 59 (38.6) | 73 (9.0) | ||||
| Stage 4 | 41 (1.7) | 22 (1.5) | 10 (6.5) | 9 (1.1) | ||||
| Valve type | <0.001 | |||||||
| Biological | 1487 (61.9) | 838 (58.4) | 91 (59.5) | 558 (68.6) | ||||
| Mechanical | 915 (38.1) | 598 (41.6) | 62 (40.5) | 255 (31.4) | ||||
| Comorbidity | ||||||||
| Smoking | 1099 (45.8) | 662 (46.1) | 87 (56.9) | 350 (43.1) | 0.007 | |||
| CAD | 245 (10.2) | 136 (9.5) | 15 (9.8) | 94 (11.6) | 0.286 | |||
| AF | 769 (32.0) | 419 (29.2) | 44 (28.8) | 306 (37.6) | <0.001 | |||
| CVA | 321 (13.4) | 177 (12.3) | 22 (14.4) | 122 (15.0) | 0.186 | |||
| COPD | 263 (10.9) | 148 (10.3) | 21 (13.7) | 94 (11.6) | 0.345 | |||
| Diabetes | 997 (41.5) | 589 (41.0) | 57 (37.3) | 351 (43.2) | 0.331 | |||
| Liver disease | 53 (2.2) | 27 (1.9) | 10 (6.5) | 16 (2.0) | 0.004c | |||
| Cancer | 154 (6.4) | 95 (6.6) | 6 (3.9) | 53 (6.5) | 0.438 | |||
| Hyperlipidemia | 209 (8.7) | 125 (9.2) | 16 (10.5) | 68 (8.4) | 0.686 | |||
| Hypertension | 1764 (73.4) | 1030 (71.7) | 112 (73.2) | 622 (76.5) | 0.045 | |||
| PVD | 247 (10.3) | 127 (8.8) | 17 (11.1) | 103 (12.7) | 0.015 | |||
| Pre-operative serum values | ||||||||
| Hgb (g/dL) | 11.47 (1.35) | 11.57 (1.34) | 11.69 (1.59) | 11.25 (1.29) | <0.001 b | 0.503 d | <0.001 d | 0.001 d |
| [missing] | [4] | [3] | [0] | [1] | ||||
| Glucose (mg/dL) | 148.92 (39.87) | 147.30 (39.02) | 151.50 (38.77) | 151.30 (41.44) | 0.052 b | |||
| Platelets (103/µL) | 192.06 (61.96) | 192.80 (61.91) | 202.47 (58.37) | 188.81 (62.51) | 0.035 b | 0.161 d | 0.307 d | 0.034 d |
| [missing] | [5] | [3] | [1] | [1] |
| Predictors of Renal Improvement | Adjusted Odds Ratios (95% CI) | p-Value | Predictors of Renal Deterioration | Adjusted Odds Ratios (95% CI) | p-Value |
|---|---|---|---|---|---|
| Preoperative stage 3 CKD a | 3.013 (1.973, 4.600) | <0.001 | Preoperative stage 2 CKD b | 0.540 (0.433, 0.672) | <0.001 |
| Preoperative stage 4 CKD a | 5.243 (2.081, 13.211) | <0.001 | Preoperative stage 3 CKD b | 0.263 (0.189, 0.366) | <0.001 |
| Biological valve | 0.976 (0.656, 1.453) | 0.905 | Preoperative stage 4 CKD b | 0.295 (0.130, 0.671) | 0.004 |
| Diabetes | 0.515 (0.330, 0.805) | 0.004 | Biological valve | 0.720 (0.595, 0.873) | <0.001 |
| Previous malignancy | 0.456 (0.181, 1.144) | 0.094 | Diabetes | 0.943 (0.763, 1.167) | 0.591 |
| Hyperlipidemia | 1.004 (0.525, 1.919) | 0.991 | Previous malignancy | 0.939 (0.653, 1.351) | 0.736 |
| Active smoking | 1.736 (1.123, 2.682) | 0.013 | Hyperlipidemia | 1.131 (0.815, 1.568) | 0.462 |
| Atrial fibrillation | 0.892 (0.585, 1.359) | 0.594 | Current smoker | 0.902 (0.736, 1.106) | 0.323 |
| Hypertension | 0.752 (0.454, 1.245) | 0.268 | Atrial fibrillation | 1.350 (1.112, 1.638) | 0.002 |
| Cerebral vascular accident | 1.047 (0.604, 1.812) | 0.871 | Hypertension | 0.973 (0.771, 1.228) | 0.818 |
| Peripheral vascular disease | 0.863 (0.465, 1.602) | 0.640 | Cerebral vascular accident | 1.071 (0.820, 1.398) | 0.617 |
| COPD | 1.171 (0.654, 2.096) | 0.596 | Peripheral vascular disease | 1.437 (1.067, 1.935) | 0.017 |
| Liver disease | 3.079 (1.211, 7.826) | 0.018 | COPD | 1.072 (0.798, 1.440) | 0.643 |
| Female | 1.059 (0.68, 1.650) | 0.798 | Liver disease | 1.009 (0.526, 1.934) | 0.979 |
| Age (years) | 0.933 (0.914, 0.952) | <0.001 | Female | 0.890 (0.727, 1.090) | 0.260 |
| Preoperative glucose (mg/dL) | 1.003 (0.998, 1.007) | 0.284 | Age (years) | 1.048 (1.037, 1.060) | <0.001 |
| Preoperative hemoglobin (g/dL) | 1.225 (1.068, 1.405) | 0.004 | Preoperative glucose (mg/dL) | 1.002 (0.999, 1.004) | 0.139 |
| Preoperative platelets (103/µL) | 1.002 (0.999, 1.005) | 0.104 | Preoperative hemoglobin (g/dL) | 0.842 (0.781, 0.907) | <0.001 |
| Preoperative platelets (103/µL) | 0.999 (0.998, (1.001) | 0.296 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leviner, D.B.; Erez, E.; Lavi, I.; Saliba, W.; Sharoni, E. Predictors and Long-Term Prognostic Significance of Acute Renal Function Change in Patients Who Underwent Surgical Aortic Valve Replacement. J. Clin. Med. 2023, 12, 4952. https://doi.org/10.3390/jcm12154952
Leviner DB, Erez E, Lavi I, Saliba W, Sharoni E. Predictors and Long-Term Prognostic Significance of Acute Renal Function Change in Patients Who Underwent Surgical Aortic Valve Replacement. Journal of Clinical Medicine. 2023; 12(15):4952. https://doi.org/10.3390/jcm12154952
Chicago/Turabian StyleLeviner, Dror B., Ely Erez, Idit Lavi, Walid Saliba, and Erez Sharoni. 2023. "Predictors and Long-Term Prognostic Significance of Acute Renal Function Change in Patients Who Underwent Surgical Aortic Valve Replacement" Journal of Clinical Medicine 12, no. 15: 4952. https://doi.org/10.3390/jcm12154952
APA StyleLeviner, D. B., Erez, E., Lavi, I., Saliba, W., & Sharoni, E. (2023). Predictors and Long-Term Prognostic Significance of Acute Renal Function Change in Patients Who Underwent Surgical Aortic Valve Replacement. Journal of Clinical Medicine, 12(15), 4952. https://doi.org/10.3390/jcm12154952







