Management of Axillary Contracture in Poland Syndrome: Differentiating Fibrous Band and Skin for Optimal Release
Abstract
:1. Introduction
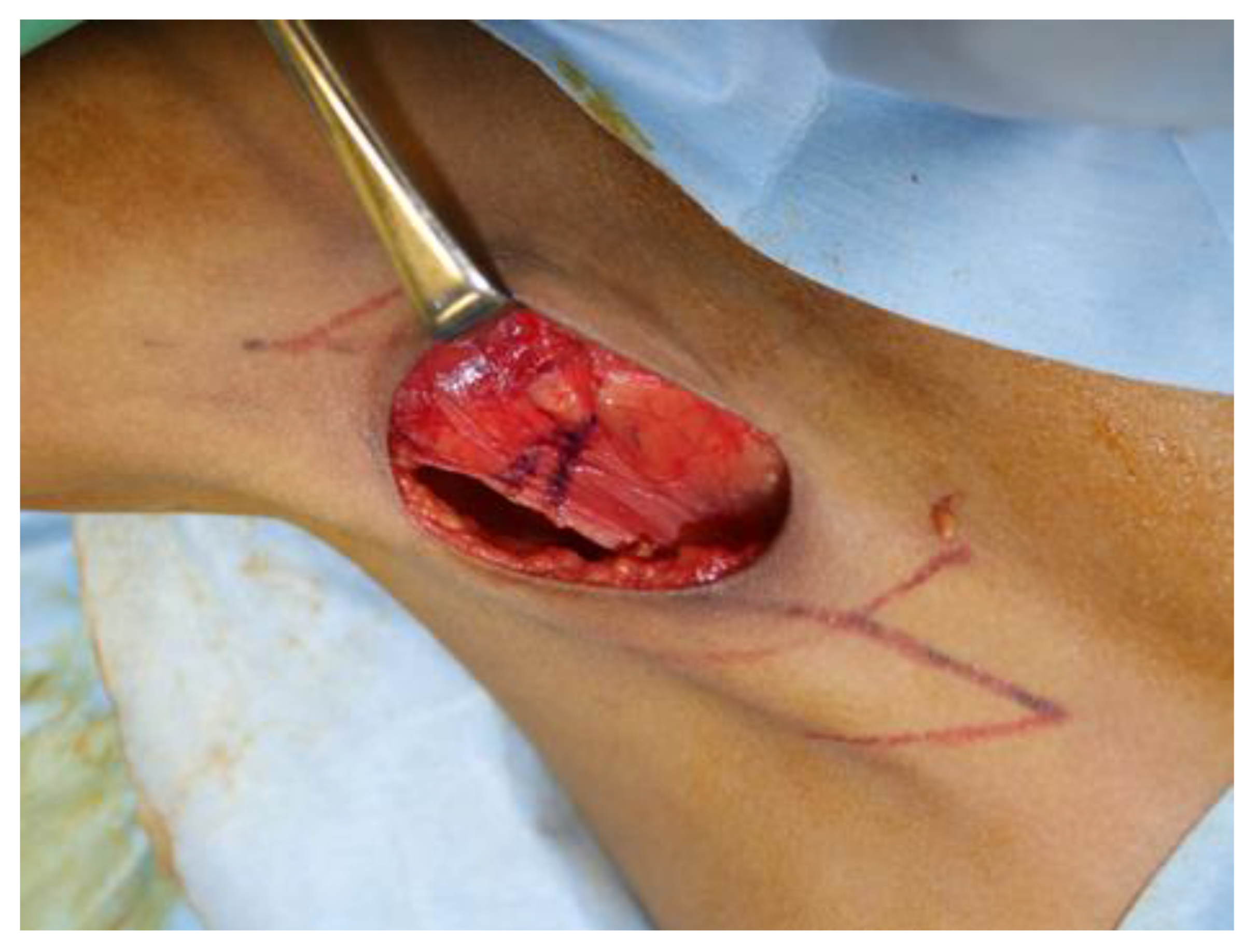
2. Case Description
Literature Review: Literature Search Strategy
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petleshkova, P.; Krasteva, M.; Gencheva, D.; Anesteva Ivanova, N.; Grozdanova, L.; Parahuleva, N. Poland syndrome: Two cases of new-borns with left-sided chest defect and extrocardia. Biomed. Res. 2019, 30, 362–365. [Google Scholar] [CrossRef] [Green Version]
- Cingel, V.; Bohac, M.; Mestanova, V.; Zabojnikova, L.; Varga, I. Poland syndrome: From embryological basis to plastic surgery. Surg. Radiol. Anat. 2013, 35, 639–646. [Google Scholar] [CrossRef]
- Hashim, E.A.A.; Quek, B.H.; Chandran, S. A narrative review of Poland’s syndrome: Theories of its genesis, evolution and its diagnosis and treatment. Transl. Pediatr. 2021, 10, 1008–1019. [Google Scholar] [CrossRef]
- Poland, A. Deficiency of the pectoral muscles. Guys Hosp. Rep. 1841, 6, 191–193. [Google Scholar]
- Clarkson, P. Poland’s syndactyly. Guys Hosp. Rep. 1962, 111, 335–336. [Google Scholar]
- Fokin, A.A.; Robicsek, F. Poland’s syndrome revisited. Ann. Thorac. Surg. 2002, 74, 2218–2225. [Google Scholar] [CrossRef]
- Garreau, M.F. Procès-Verbaux du 12 Frimaire An 12 Jusques au 1er Fructidor an 12; Société anatomique de Paris: Paris, France, 1996; p. 42. [Google Scholar]
- Charlier, P.; Deo, S.; Galassi, F.M.; Benmoussa, N. Poland syndrome before Alfred Poland: The oldest medical description (Paris, France, 1803). Surg. Radiol. Anat. 2019, 41, 1117–1118. [Google Scholar] [CrossRef]
- Lallemand, L.M. Absence de trois côtes simulant un enfoncement accidentel. Éphémérides Med. Montpelier 1826, 1, 144–147. [Google Scholar]
- Froriep, R. Beobachtung eines Falles von Mangel der Brustdrüse. Not. Aus Dem Geb. Der Nat. Und Heilkd. 1839, 10, 9–14. [Google Scholar]
- Brown, J.B.; McDowell, F. Syndacylism with absence of pectoralis major. Surgery 1940, 7, 599–601. [Google Scholar]
- McDowell, F. On the propagation, perpetuation, and parroting of erroneous. eponyms such as “polands syndrome”. Plast. Reconstr. Surg. 1977, 59, 561–563. [Google Scholar] [PubMed]
- Ram, A.N.; Chung, K.C. Poland’s syndrome: Current thoughts in the setting of a controversy. Plast. Reconstr. Surg. 2009, 123, 949–953. [Google Scholar] [CrossRef] [Green Version]
- Al-Qattan, M.M. Classification of hand anomalies in Poland’s syndrome. Br. J. Plast. Surg. 2001, 54, 132–136. [Google Scholar] [CrossRef]
- Van Allen, M.I.; Hoyme, H.E.; Jones, K.L. Vascular pathogenesis of limb defects. Radial artery anatomy in radial aplasia. J. Pediatr. 1982, 101, 832–838. [Google Scholar] [CrossRef]
- Bavinck, J.N.B.; Weaver, D.D. Subclavian artery supply disruption sequence: Hypothesis of a vascular etiology for Poland, Klippel-Feil, and Mobius anomalies. Am. J. Med. Genet. 1986, 23, 903–918. [Google Scholar] [CrossRef] [PubMed]
- Merlob, P.; Schonfeld, A.; Ovadia, Y.; Reisner, S.H. Real-time echo-doppler duplex scanner in the evaluation of patients with Poland sequence. Eur. J. Obstet. Gynecol. Reprod. Biol. 1989, 32, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Van Allen, M.I. Fetal vascular disruptions: Mechanisms and some resulting birth defects. Pediatr. Ann. 1981, 10, 219–233. [Google Scholar] [PubMed]
- Hoyme, H.E.; Jone, K.L.; Van Allen, M.I.; Saunders, B.S.; Benirschke, K. Vascular pathogenesis of transverse limb reduction defects. J. Pediatr. 1982, 101, 839–843. [Google Scholar] [CrossRef]
- Congdon, E.D. Transformation of the aortic-arch system during the development of the human embryo. Carnegie Inst. Wash. Contrib. Embryol. 1922, 14, 47–113. [Google Scholar]
- Puvabanditsin, S.; Garrow, E.; Augustin, G.; Titapiwatanakul, R.; Kuniyoshi, K.M. Poland-Möebius syndrome and cocaine abuse: A relook at vascular etiology. Pediatr. Neurol. 2005, 32, 285–287. [Google Scholar] [CrossRef]
- Pinney, S.E.; Ganapathy, K.; Bradfield, J.; Stokes, D.; Sasson, A.; Mackiewicz, K.; Boodhansingh, K.; Hughes, N.; Becker, S.; Givler, S.; et al. Dominant form of congenital hyperinsulinism maps to HK1 region on 10q. Horm. Res. Paediatr. 2013, 80, 18–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaccari, C.M.; Romanini, M.V.; Musante, I.; Tassano, E.; Gimelli, S.; Divizia, M.T.; Torre, M.; Morovic, C.G.; Lerone, M.; Ravazzolo, R.; et al. De novo deletion of chromosome 11q12.3 in monozygotic twins affected by Poland syndrome. BMC Med. Genet. 2014, 15, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bamforth, J.S.; Fabian, C.; Machin, G.; Honore, L. Poland anomaly with a limb body wall disruption defect: Case report and review. Am. J. Med. Genet. 1992, 43, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Darian, V.B.; Argenta, L.C.; Pasyk, K.A. Familial Poland’s syndrome. Ann. Plast. Surg. 1989, 23, 531–537. [Google Scholar] [CrossRef]
- Fraser, F.C.; Ronen, G.M.; O’Leary, E. Pectoralis major defect and Poland sequence in second cousins: Extension of the Poland sequence spectrum. Am. J. Med. Genet. 1989, 33, 468–470. [Google Scholar] [CrossRef]
- Giri, D.; Patil, P.; Hart, R.; Didi, M.; Senniappan, S. Congenital hyperinsulinism and Poland syndrome in association with 10p13-14 duplication. Endocrinol. Diabetes Metab. Case Rep. 2017, 2017, 16–0125. [Google Scholar] [CrossRef]
- Issaivanan, M.; Virdi, V.S.; Parmar, V.R. Subclavian artery supply disruption sequence-Klippel–Feil and Mobius anomalies. Indian J. Pediatr. 2002, 69, 441–442. [Google Scholar] [CrossRef]
- Foucras, L.; Grolleau-Raoux, J.L.; Chavoin, J.P. Poland’s syndrome: Clinic series and thoraco-mammary reconstruction. Report of 27 cases. Ann. Chir. Plast. Esthet. 2003, 48, 54–66. [Google Scholar] [CrossRef]
- Mojallal, A.; La Marca, S.; Shipkov, C.; Sinna, R.; Braye, F. Poland syndrome and breast tumor: A case report and review of the literature. Aesthet. Surg. J. 2012, 32, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.C., Jr.; Meijer, R.; Aranda, D. Syndactylism with deformity of the pectoralis muscle. Poland’s syndrome. J. Pediatr. Surg. 1969, 4, 569–572. [Google Scholar] [CrossRef]
- Caouette-Laberge, L.; Borsuk, D. Congenital anomalies of the breast. Semin. Plast. Surg. 2013, 27, 36–41. [Google Scholar] [PubMed] [Green Version]
- Chandran, S.; Revanna, K.G.; Ari, D.; Rana, A.A. Lung herniation: An uncommon presentation of Poland’s syndrome in a neonate at birth. BMJ Case Rep. 2013, 2013, bcr2013200106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torre, M.; Baban, A.; Buluggiu, A.; Costanzo, S.; Bricco, L.; Lerone, M.; Bianca, S.; Gatti, G.L.; Sénès, F.M.; Valle, M.; et al. Dextrocardia in patients with Poland syndrome: Phenotypic characterization provides insight into the pathogenesis. J. Thorac. Cardiovasc. Surg. 2010, 139, 1177–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baharin, N.S.; Hashim, E.A.; Huey, Q.B.; Chandran, S. Reinforcing the vascular disruption theory of the genesis of Poland’s syndrome: A rare association of diaphragmatic eventration in a preterm infant with severe musculoskeletal defects. BMJ Case Rep. 2021, 14, e238392. [Google Scholar] [CrossRef] [PubMed]
- Romanini, M.V.; Calevo, M.G.; Puliti, A.; Vaccari, C.; Valle, M.; Senes, F.; Torre, M. Poland syndrome: A proposed classification system and perspectives on diagnosis and treatment. Semin. Pediatr. Surg. 2018, 27, 189–199. [Google Scholar] [CrossRef]
- Romanini, M.V.; Torre, M.; Santi, P.; Dova, L.; Valle, M.; Martinoli, C.; Baldelli, I. Proposal of the TBN Classification of Thoracic Anomalies and Treatment Algorithm for Poland Syndrome. Plast. Reconstr. Surg. 2016, 138, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Seyfer, A.E.; Fox, J.P.; Hamilton, C.G. Poland syndrome: Evaluation and treatment of the chest wall in 63 patients. Plast. Reconstr. Surg. 2010, 126, 902–911. [Google Scholar] [CrossRef]
- Ersen, B.; Kahveci, R.; Saki, C.M.; Sahin, A.; Tunali, O.; Aksu, I. A variant of Poland’s syndrome: Axillary web containing rudimentary pectoralis major muscle. Eur. J. Plast. Surg. 2015, 38, 161–162. [Google Scholar] [CrossRef]
- Mendo, T.D.S.; Almeida, T.; Maria, A.T.; Tuna, M.L. Poland syndrome: Neonatal presentation with axillary pterygium. BMJ Case Rep. 2021, 14, e241395. [Google Scholar] [CrossRef]
- Holler, A.S.; Muensterer, J.; Goedeke, O.J. Pterygium axillae as a rare manifestation of Poland syndrome. J. Pediatr. Surg. Case Rep. 2016, 15, 48–49. [Google Scholar] [CrossRef] [Green Version]
- Hove, C.R.; Williams, E.F., III; Rodgers, B.J. Z-plasty: A concise review. Facial Plast. Surg. 2001, 17, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Hanumadass, M.; Kagan, R.; Matsuda, T.; Jayaram, B. Classification and surgical correction of postburn axillary contractures. J. Trauma 1986, 26, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Karki, D.; Narayan, R.P. Role of square flap in post burn axillary contractures. World J. Plast. Surg. 2017, 6, 285–291. [Google Scholar] [PubMed]
- Fraulin, F.O.; Thomson, H.G. First webspace deepening: Comparing the four-flap and five-flap Z-plasty. Which gives the most gain? Plast Reconstr. Surg. 1999, 104, 120–128. [Google Scholar] [CrossRef]





| Case | Sex | Body Side | Age at Diagnosis | Age at Surgery | Surgical Technique | Reference |
|---|---|---|---|---|---|---|
| 1 | Female | Right | Neonate | Pending | [40] | |
| 2 | Female | Left | 6 years | 6 years | Double-opposing Z-plasty plus step-ladder incision-lengthening | [39] |
| 3 | Female | Right | 3 months | 9 months | Z-plasty | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuura, R.; Shimizu, Y.; Matsuura, N.; Ntege, E.H.; Wada, N. Management of Axillary Contracture in Poland Syndrome: Differentiating Fibrous Band and Skin for Optimal Release. J. Clin. Med. 2023, 12, 4957. https://doi.org/10.3390/jcm12154957
Matsuura R, Shimizu Y, Matsuura N, Ntege EH, Wada N. Management of Axillary Contracture in Poland Syndrome: Differentiating Fibrous Band and Skin for Optimal Release. Journal of Clinical Medicine. 2023; 12(15):4957. https://doi.org/10.3390/jcm12154957
Chicago/Turabian StyleMatsuura, Rikako, Yusuke Shimizu, Naoki Matsuura, Edward Hosea Ntege, and Naoki Wada. 2023. "Management of Axillary Contracture in Poland Syndrome: Differentiating Fibrous Band and Skin for Optimal Release" Journal of Clinical Medicine 12, no. 15: 4957. https://doi.org/10.3390/jcm12154957
APA StyleMatsuura, R., Shimizu, Y., Matsuura, N., Ntege, E. H., & Wada, N. (2023). Management of Axillary Contracture in Poland Syndrome: Differentiating Fibrous Band and Skin for Optimal Release. Journal of Clinical Medicine, 12(15), 4957. https://doi.org/10.3390/jcm12154957






