The Effects of Intraoperative Remifentanil Infusion on Postoperative Opioid Consumption in Patients Who Underwent Total Knee Arthroplasty with Femoral Nerve Block
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Randomization and Blinding Method
2.4. Interventions
2.5. Intraoperative Management
2.6. Postoperative Management
2.7. Outcome Measurement
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.; Lee, H.; Shin, J. Explosive increase in tramadol use in Korea 2003–2013: Analysis of patient trends based on the Korea national health insurance database. J. Psychoact. Drugs 2020, 52, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.; Jeon, Y.; Choi, J. Trends in chronic opioid use and association with five-year survival in South Korea: A population-based cohort study. Br. J. Anaesth. 2019, 123, 655–663. [Google Scholar] [CrossRef]
- Paulozzi, L.J.; Jones, C.M.; Mack, K.A.; Rudd, R.A. Centers for Disease Control and Prevention (CDC). Vital signs: Overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR Morb. Mortal. Wkly. Rep. 2011, 60, 1487–1492. Available online: http://www.cdc.gov/mmwr (accessed on 15 January 2023).
- Daubresse, M.; Chang, H.-Y.; Yu, Y.; Viswanathan, S.; Shah, N.D.; Stafford, R.S.; Kruszewski, S.P.; Alexander, G.C. Ambulatory Diagnosis and Treatment of Nonmalignant Pain in the United States, 2000–2010. Med. Care 2013, 51, 870–878. [Google Scholar] [CrossRef]
- Wright, E.A.; Katz, J.N.; Abrams, S.; Solomon, D.H.; Losina, E. Trends in Prescription of Opioids from 2003–2009 in Persons with Knee Osteoarthritis. Arthritis Care Res. 2014, 66, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, A.S.; Valenstein, M.; Bair, M.J.; Ganoczy, D.; McCarthy, J.F.; Ilgen, M.A.; Blow, F.C. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA 2011, 305, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Gomes, T.; Zheng, H.; Mamdani, M.M.; Juurlink, D.N.; Bell, C.M. Long-term Analgesic Use After Low-Risk Surgery. Arch. Intern. Med. 2012, 172, 425–430. [Google Scholar] [CrossRef]
- Clarke, H.; Soneji, N.; Ko, D.; Yun, L.; Wijeysundera, D.N. Rates and risk factors for prolonged opioid use after major surgery: Population based cohort study. BMJ 2014, 348, g1251. [Google Scholar] [CrossRef] [PubMed]
- Raebel, M.A.; Newcomer, S.R.; Reifler, L.M.; Boudreau, D.; Elliott, T.E.; DeBar, L.; Ahmed, A.; Pawloski, P.A.; Fisher, D.; Donahoo, W.T.; et al. Chronic Use of Opioid Medications Before and After Bariatric Surgery. JAMA 2013, 310, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, C.; Noto, A.; Crimi, C.; Sanfilippo, F. Remifentanil-induced postoperative hyperalgesia: Current perspectives on mechanisms and therapeutic strategies. Local Reg. Anesth. 2018, 11, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lim, H.-S.; Kim, M.-J.; Jeong, W.; Ko, S. High-dose intraoperative remifentanil infusion increases early postoperative analgesic consumption: A prospective, randomized, double-blind controlled study. J. Anesth. 2018, 32, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-W.; Cho, A.-R.; Lee, H.-J.; Kim, H.-J.; Byeon, G.J.; Yoon, J.-W.; Kim, K.-H.; Kwon, J.-Y. Maintenance anaesthetics during remifentanil-based anaesthesia might affect postoperative pain control after breast cancer surgery. Br. J. Anaesth. 2010, 105, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Song, Y.; Lee, J.; Ha, S. The effects of intraoperative adenosine infusion on acute opioid tolerance and opioid induced hyperalgesia induced by remifentanil in adult patients undergoing tonsillectomy. Korean J. Pain. 2011, 24, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, Y.; Xiao, L. Postoperative Pain Management in Total Knee Arthroplasty. Orthop. Surg. 2019, 11, 755–761. [Google Scholar] [CrossRef]
- Soffin, E.M.; Memtsoudis, S.G. Anesthesia and analgesia for total knee arthroplasty. Minerva Anestesiol. 2018, 84, 1406–1412. [Google Scholar] [CrossRef]
- D’Souza, R.S.; Langford, B.J.; Olsen, D.A.; Johnson, R.L. Ultrasound-guided local anesthetic infiltration between the popliteal artery and the capsule of the posterior knee (Ipack) block for primary total knee arthroplasty: A systematic review of ran-domized controlled trials. Local Reg. Anesth. 2021, 14, 85–98. [Google Scholar] [CrossRef]
- Kim, D.; Beathe, J.; Lin, Y.; YaDeau, J.T.; Maalouf, D.B.; Goytizolo, E.; Garnett, C.; Ranawat, A.S.; Su, E.P.; Mayman, D.J.; et al. Addition of infiltration between the popliteal artery and the capsule of the posterior knee and adductor canal block to periarticular injection enhances postoperative pain control in total knee arthroplasty: A randomized controlled trial. Anesth. Analg. 2019, 129, 526–535. [Google Scholar] [CrossRef]
- Zheng, F.-Y.; Liu, Y.-B.; Huang, H.; Xu, S.; Ma, X.-J.; Liu, Y.-Z.; Chu, H.-C. The impact of IPACK combined with adductor canal block under ultrasound guidance on early motor function after total knee arthroplasty. Braz. J. Anesthesiol. 2022, 72, 110–114. [Google Scholar] [CrossRef]
- Rogobete, A.F.; Bedreag, O.H.; Papurica, M.; Popovici, S.E.; Bratu, L.M.; Rata, A.; Barsac, C.R.; Maghiar, A.; Garofil, D.N.; Negrea, M.; et al. Multiparametric monitoring of hyp-nosis and nociception-antinociception balance during general anesthesia—A new era in patient safety standards and healthcare management. Medicina 2021, 57, 132. [Google Scholar] [CrossRef]
- Jensen, E.W.; Valencia, J.F.; López, A.; Anglada, T.; Agustí, M.; Ramos, Y.; Serra, R.; Jospin, M.; Pineda, P.; Gambus, P. Monitoring hypnotic effect and nociception with two EEG-derived indices, qCON and qNOX, during general anaesthesia. Acta Anaesthesiol. Scand. 2014, 58, 933–941. [Google Scholar] [CrossRef]
- Joly, V.; Richebe, P.; Guignard, B.; Fletcher, D.; Maurette, P.; Sessler, D.I.; Chauvin, M. Remifentanil-induced postoperative hyper-algesia and its prevention with small-dose ketamine. Anesthesiology 2005, 103, 147–155. [Google Scholar] [CrossRef]
- Lavand’homme, P. Rebound pain after regional anesthesia in the ambulatory patient. Curr. Opin. Anaesthesiol. 2018, 31, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Uquillas, C.A.; Capogna, B.M.; Rossy, W.H.; Mahure, S.A.; Rokito, A.S. Postoperative pain control after arthroscopic rotator cuff repair. J. Shoulder Elb. Surg. 2016, 25, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.A.; Bottegal, M.T.; Kentor, M.L.; Irrgang, J.J.; Williams, J.P. Rebound Pain Scores as a Function of Femoral Nerve Block Duration after Anterior Cruciate Ligament Reconstruction: Retrospective Analysis of a Prospective, Randomized Clinical Trial. Reg. Anesthesia Pain Med. 2007, 32, 186–192. [Google Scholar] [CrossRef]
- Kim, Y.; Bae, H.; Yoo, S.; Park, S.-K.; Lim, Y.-J.; Sakura, S.; Kim, J.-T. Effect of remifentanil on post-operative analgesic consumption in patients undergoing shoulder arthroplasty after interscalene brachial plexus block: A randomized controlled trial. J. Anesthesia 2022, 36, 506–513. [Google Scholar] [CrossRef]
- Fletcher, D.; Martinez, V. Opioid-induced hyperalgesia in patients after surgery: A systemic review and a meta-analysis. Br. J. Anaesth. 2014, 13, 587–603. [Google Scholar] [CrossRef]
- Ilkjaer, S.; Bach, L.F.; Nielsen, P.A.; Wernberg, M.; Dahl, J.B. Effect of preoperative oral dextromethorphan on immediate and late postoperative pain and hyperalgesia after total abdominal hysterectomy. Pain 2000, 86, 19–24. [Google Scholar] [CrossRef]
- Melia, U.; Gabarron, E.; Agustí, M.; Souto, N.; Pineda, P.; Fontanet, J.; Vallverdu, M.; Jensen, E.W.; Gambus, P. Comparison of the qCON and qNOX indices for the assessment of unconsciousness level and noxious stimulation response during surgery. J. Clin. Monit. Comput. 2017, 31, 1273–1281. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Ou, P.; Lu, X.-H.; Chen, Y.-P.; Xu, J.-M.; Dai, R.-P. Effect of Intraoperative High-Dose Remifentanil on Postoperative Pain: A Prospective, Double Blind, Randomized Clinical Trial. PLoS ONE 2014, 9, e91454. [Google Scholar] [CrossRef]
- Choi, E.; Lee, H.; Park, H.S.; Lee, G.Y.; Kim, Y.J.; Baik, H.-J. Effect of intraoperative infusion of ketamine on remifentanil-induced hyperalgesia. Korean J. Anesthesiol. 2015, 68, 476–480. [Google Scholar] [CrossRef]
- Galos, D.K.; Taormina, D.P.; Crespo, A.; Ding, D.Y.; Sapienza, A.; Jain, S.; Tejwani, N.C. Does brachial plexus blockade result in im-proved pain scores after distal radius fracture fixation? A randomized trial. Clin. Orthop. Relat. Res. 2016, 474, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Dada, O.; Gonzalez, Z.A.; Ongaigui, C.; Echeverria, V.M.; Kushelev, M.; Bergese, S.D.; Moran, K. Does rebound pain after periph-eral nerve block for orthopedic surgery impact postoperative analgesia and opioid consumption? A narrative review. Int. J. Environ. Res. Public Health 2019, 16, 3257. [Google Scholar] [CrossRef]
- Nobre, L.V.; Cunha, G.P.; de Sousa, P.C.C.B.; Takeda, A.; Ferraro, L.H.C. Peripheral nerve block and rebound pain: Literature review. Braz. J. Anesthesiol. 2019, 69, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, F.W.; Halpern, S.H.; Aoyama, K.; Brull, R. Will the real benefits of single-shot interscalene block please stand up? A systematic review and meta-analysis. Anesth. Analg. 2015, 120, 1114–1129. [Google Scholar] [CrossRef] [PubMed]


| Group | ||||
|---|---|---|---|---|
| Variable | Overall (n = 66) | Remifentanil (n = 33) | Control (n = 33) | p |
| History | ||||
| Osteoarthritis | 55 (83.3) | 27 (81.8) | 28 (84.8) | 0.741 * |
| Rheumatoid arthritis | 11 (16.7) | 6 (18.2) | 5 (15.2) | |
| Age (yr) | 71.3 ± 7.2 | 71.7 ± 6.7 | 70.8 ± 7.7 | 0.525 † |
| Gender | ||||
| Male | 13 (19.7) | 6 (18.2) | 7 (21.2) | 0.757 * |
| Female | 53 (80.3) | 27 (81.8) | 26 (78.8) | |
| Height (cm) | 154.6 ± 6.8 | 154.0 ± 6.9 | 155.1 ± 6.7 | 0.525 † |
| Weight (kg) | 63.0 ± 8.7 | 62.6 ± 8.2 | 63.3 ± 9.3 | 0.737 ‡ |
| BMI (kg/m2) | 26.2 ± 2.9 | 26.2 ± 2.6 | 26.3 ± 3.2 | 0.937 ‡ |
| ASA class | ||||
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.786 * |
| 2 | 47 (71.2) | 24 (72.7) | 23 (69.7) | |
| 3 | 19 (28.8) | 9 (27.3) | 10 (30.3) | |
| Surgical site | ||||
| Right | 35 (53.0) | 19 (57.6) | 16 (48.5) | 0.459 * |
| Left | 31 (47.0) | 14 (42.4) | 17 (51.5) | |
| Surgery time (min) | 118.3 ± 11.3 | 116.8 ± 12.3 | 119.9 ± 10.1 | 0.264 ‡ |
| Anesthesia time (min) | 175.3 ± 14.5 | 174.9 ± 17.0 | 175.7 ± 11.7 | 0.814 ‡ |
| Blood loss (ml) | 325 ± 130 | 329 ± 131 | 321 ± 130 | 0.814 ‡ |
| Preoperative pain score (NRS) | 2.6 ± 1.1 | 2.6 ± 1.2 | 2.6 ± 1.1 | 0.989 † |
| Remifentanil total use (µg) | 1161.8 ± 206.7 | 1161.8 ± 206.7 | - | |
| Group | Analysis for Repeated Measures | ||||
|---|---|---|---|---|---|
| Variable | Remifentanil (n = 33) | Control (n = 33) | p * | Source | p † |
| Postoperative IV-PCA consumption (µg) | |||||
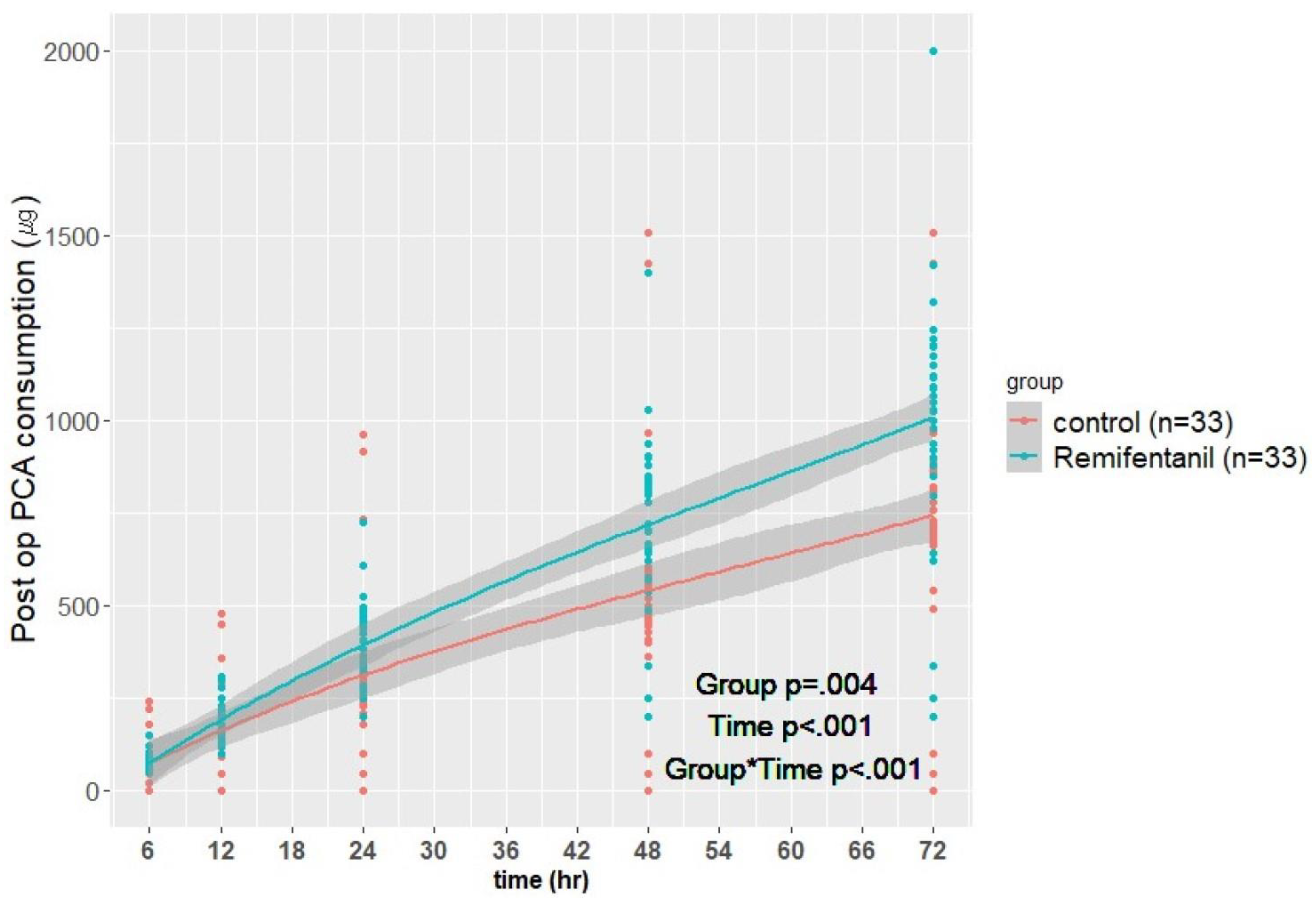
| 6 h | 79.3 ± 18.4 | 77.6 ± 47.9 | 0.019 | Group | 0.004 |
| 12 h | 183.9 ± 55.7 | 155.8 ± 99.5 | <0.001 | Time | <0.001 |
| 24 h | 398.6 ± 108.5 | 316.5 ± 199.3 | <0.001 | Group × Time | <0.001 |
| 48 h | 717.5 ± 224.0 | 541.1 ± 294.5 | <0.001 | ||
| 72 h | 1008.1 ± 339.0 | 744.1 ± 298.0 | <0.001 | ||
| Pain score at rest (NRS) | |||||
| 6 h | 3.2 ± 1.7 | 3.9 ± 1.8 | 0.121 | Group | 0.026 |
| 12 h | 3.9 ± 1.8 | 4.3 ± 1.9 | 0.440 | Time | 0.077 |
| 24 h | 4.2 ± 1.6 | 4.1 ± 1.6 | 0.528 | Group × Time | 0.013 |
| 48 h | 4.2 ± 1.1 | 4.1 ± 1.7 | 0.343 | ||
| 72 h | 4.5 ± 1.5 | 3.8 ± 1.7 | 0.033 | ||
| Pain score at activity (NRS) | |||||
| 24 h | 4.9 ± 1.4 | 4.8 ± 1.7 | 0.434 | Group | 0.031 |
| 48 h | 4.9 ± 1.0 | 4.7 ± 1.8 | 0.257 | Time | 0.382 |
| 72 h | 5.1 ± 1.4 | 4.3 ± 1.8 | 0.017 | Group × Time | 0.012 |
| Group | |||
|---|---|---|---|
| Variable | Remifentanil (n = 33) | Control (n = 33) | p |
| Rescue tramadol use | |||
| Yes | 14 (42.4) | 7 (21.2) | 0.064 * |
| No | 19 (57.6) | 26 (78.8) | |
| Perioperative ephedrine (mg) | 13.0 ± 11.3 | 5.2 ± 7.1 | 0.003 † |
| Nausea or vomiting | |||
| Yes | 11 (33.3) | 11 (33.3) | 1.000 ‡ |
| No | 22 (66.7) | 22 (66.7) | |
| Quality of sleep at 24 h | |||
| Poor | 1 (3.0) | 1 (3.0) | 0.708 ‡ |
| Fair | 30 (90.9) | 27 (81.8) | |
| Good | 2 (6.1) | 5 (15.2) | |
| Satisfaction score (0–100) at 72 h | 76.2 ± 7.5 | 77.6 ± 10.0 | 0.280 † |
| Would undergo the block again (n) at 72 h | |||
| Yes | 32 (97.0) | 32 (97.0) | 1.000 ‡ |
| No | - | - | |
| Ambivalent | 1 (3.0) | 1 (3.0) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, C.; Choi, J.; Lee, T.; Park, S. The Effects of Intraoperative Remifentanil Infusion on Postoperative Opioid Consumption in Patients Who Underwent Total Knee Arthroplasty with Femoral Nerve Block. J. Clin. Med. 2023, 12, 4975. https://doi.org/10.3390/jcm12154975
Chung C, Choi J, Lee T, Park S. The Effects of Intraoperative Remifentanil Infusion on Postoperative Opioid Consumption in Patients Who Underwent Total Knee Arthroplasty with Femoral Nerve Block. Journal of Clinical Medicine. 2023; 12(15):4975. https://doi.org/10.3390/jcm12154975
Chicago/Turabian StyleChung, Chanjong, Jinyoung Choi, Taeyoung Lee, and Sangyoong Park. 2023. "The Effects of Intraoperative Remifentanil Infusion on Postoperative Opioid Consumption in Patients Who Underwent Total Knee Arthroplasty with Femoral Nerve Block" Journal of Clinical Medicine 12, no. 15: 4975. https://doi.org/10.3390/jcm12154975
APA StyleChung, C., Choi, J., Lee, T., & Park, S. (2023). The Effects of Intraoperative Remifentanil Infusion on Postoperative Opioid Consumption in Patients Who Underwent Total Knee Arthroplasty with Femoral Nerve Block. Journal of Clinical Medicine, 12(15), 4975. https://doi.org/10.3390/jcm12154975








