Endocuff Vision-Assisted Resection for Difficult Colonic Lesions—Preliminary Results of a Multicenter, Prospective Randomized Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
3. Definitions
- -
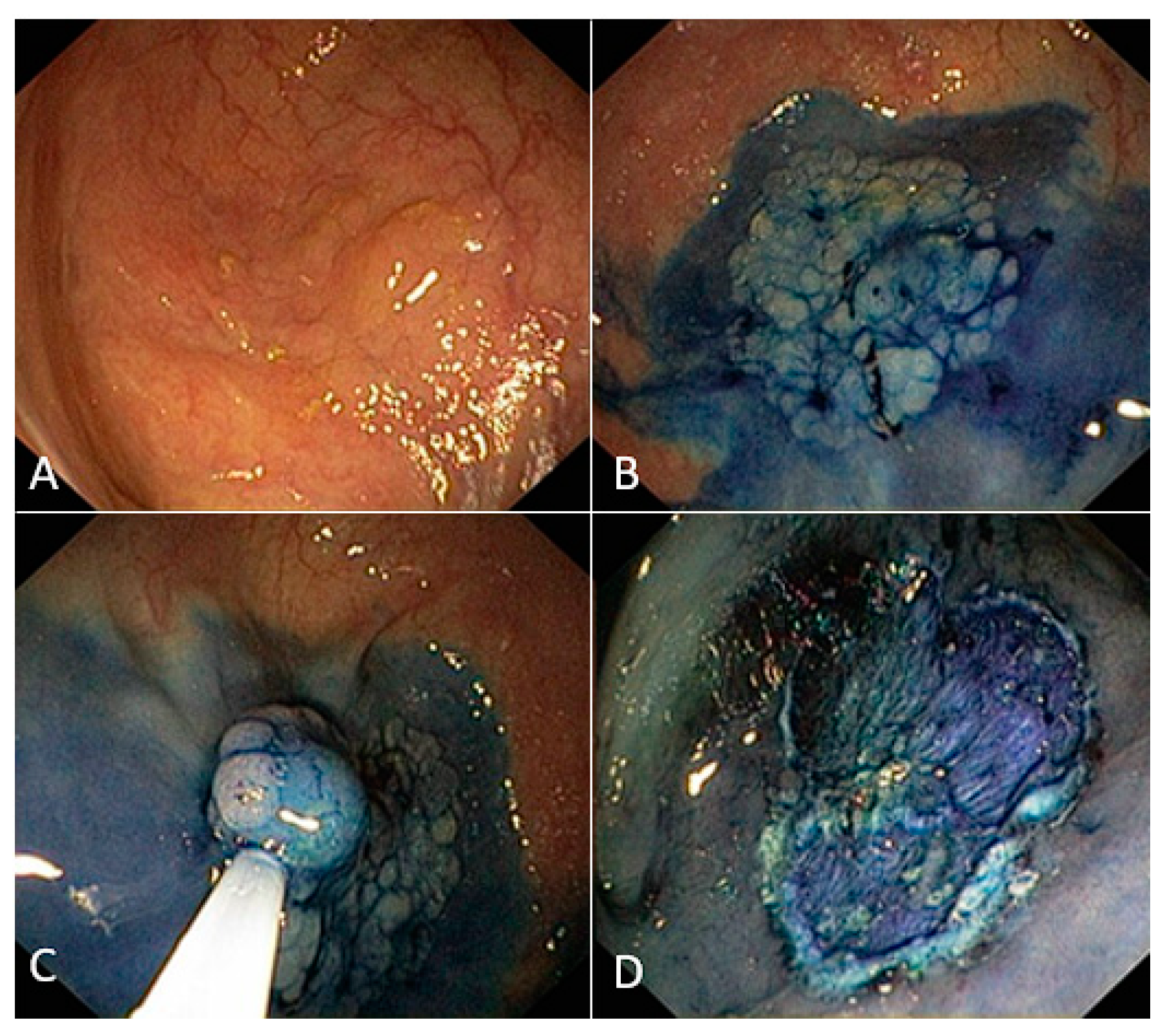
- Difficult polyp: ≥8 score according to the SMSA scoring system.
- -
- Lifting sign: separation of the lesion from the muscularis propria and lifting in response to submucosal injection [15].
- -
- Procedure time: begins with the insertion of the colonoscope, including therapeutic interventions, and ends with the removal of the endoscope.
- -
- Polypectomy time: begins with the submucosal infiltration and ends with retrieval of the polyps.
- -
- Shaking: the number of attempts to maintain the right position of the scope with the subject in the center of the field of view during a polypectomy.
- -
- Withdrawal time: begins with the withdrawal of the colonoscope from the cecal pole, excluding the time spent on interventions, and ends with the removal of the endoscope.
Statistical Analysis
4. Results
- -
- Centre 1 = 8 (EP); 14 (SP);
- -
- Centre 2 = 3 (EP); 5 (SP);
- -
- Centre 3 = 1 (EP); 1 (SP).
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRC | colorectal cancer; |
| ADR | adenoma detection rate; |
| PCCRC | post-colonoscopy colorectal cancer; |
| SMSA | size: morphology, site, access |
| MUPS | Munich Polypectomy Study; |
| ECV | Endocuff Vision: |
| SP | standard polypectomy; |
| EP | ECV-assisted polypectomy; |
| GI | gastro-intestinal: |
| LST | laterally spreading type; |
| GT | granular type: |
References
- Colorectal Cancer. Available online: https://www.cancer.org/cancer/colon-rectal-cancer.html (accessed on 25 January 2021).
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekker, E.; Rex, D.K. Advances in CRC Prevention: Screening and Surveillance. Gastroenterology 2018, 154, 1970–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaminski, M.F.; Wieszczy, P.; Rupinski, M.; Wojciechowska, U.; Didkowska, J.; Kraszewska, E.; Kobiela, J.; Franczyk, R.; Rupinska, M.; Kocot, B.; et al. Increased Rate of Adenoma Detection Associates with Reduced Risk of Colorectal Cancer and Death. Gastroenterology 2017, 153, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand, E.C.; Wallace, M.B. Strategies to Increase Adenoma Detection Rates. Curr. Treat. Options Gastroenterol. 2017, 15, 184–212. [Google Scholar] [CrossRef]
- Ngamruengphong, S.; Pohl, H.; Haito-Chavez, Y.; Khashab, M.A. Update on Difficult Polypectomy Techniques. Curr. Gastroenterol. Rep. 2016, 18, 3. [Google Scholar] [CrossRef]
- Gallegos-Orozco, J.F.; Gurudu, S.R. Complex colon polypectomy. Gastroenterol. Hepatol. 2010, 6, 375–382. [Google Scholar]
- Tholoor, S.; Tsagkournis, O.; Basford, P.; Bhandari, P. Managing difficult polyps: Techniques and pitfalls. Ann. Gastroenterol. 2013, 26, 114–121. [Google Scholar]
- Puli, S.R.; Kakugawa, Y.; Gotoda, T.; Antillon, D.; Saito, Y.; Antillon, M.R. Meta-analysis and systematic review of colorectal endoscopic mucosal resection. World J. Gastroenterol. 2009, 15, 4273–4277. [Google Scholar] [CrossRef]
- Patil, R.; Ona, M.A.; Ofori, E.; Reddy, M. Endocuff-Assisted Colonoscopy-A Novel Accessory in Improving Adenoma Detection Rate: A Review of the Literature. Clin. Endosc. 2016, 49, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Floer, M.; Biecker, E.; Fitzlaff, R.; Röming, H.; Ameis, D.; Heinecke, A.; Kunsch, S.; Ellenrieder, V.; Ströbel, P.; Schepke, M.; et al. Higher adenoma detection rates with endocuff-assisted colonoscopy—A randomized controlled multicenter trial. PLoS ONE 2014, 9, e114267. [Google Scholar] [CrossRef]
- Pioche, M.; Matsumoto, M.; Takamaru, H.; Sakamoto, T.; Nakajima, T.; Matsuda, T.; Abe, S.; Kakugawa, Y.; Otake, Y.; Saito, Y. Endocuff-assisted colonoscopy increases polyp detection rate: A simulated randomized study involving an anatomic colorectal model and 32 international endoscopists. Surg. Endosc. 2016, 30, 288–295. [Google Scholar] [CrossRef]
- Tsiamoulos, Z.P.; Saunders, B.P. A new accessory, endoscopic cuff, improves colonoscopic access for complex polyp resection and scar assessment in the sigmoid colon (with video). Gastrointest. Endosc. 2012, 76, 1242–1245. [Google Scholar] [CrossRef]
- Vleugels, J.L.A.; Hazewinkel, Y.; Dekker, E. Morphological classifications of gastrointestinal lesions. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 359–367. [Google Scholar] [CrossRef]
- Uno, Y.; Munakata, A. The non-lifting sign of invasive colon cancer. Gastrointest. Endosc. 1994, 40, 485–489. [Google Scholar] [CrossRef]
- Lenze, F.; Beyna, T.; Lenz, P.; Heinzow, H.S.; Hengst, K.; Ullerich, H. Endocuff-assisted colonoscopy: A new accessory to improve adenoma detection rate? Technical aspects and first clinical experiences. Endoscopy 2014, 46, 610–614. [Google Scholar] [CrossRef]
- Biecker, E.; Floer, M.; Heinecke, A.; Ströbel, P.; Böhme, R.; Schepke, M.; Meister, T. Novel endocuff-assisted colonoscopy significantly increases the polyp detection rate: A randomized controlled trial. J. Clin. Gastroenterol. 2015, 49, 413–418. [Google Scholar] [CrossRef]
- De Palma, G.D.; Giglio, M.C.; Bruzzese, D.; Gennarelli, N.; Maione, F.; Siciliano, S.; Manzo, B.; Cassese, G.; Luglio, G. Cap cuff-assisted colonoscopy versus standard colonoscopy for adenoma detection: A randomized back-to-back study. Gastrointest. Endosc. 2018, 87, 232–240. [Google Scholar] [CrossRef]
- Ngu, W.S.; Bevan, R.; Tsiamoulos, Z.P.; Bassett, P.; Hoare, Z.; Rutter, M.D.; Clifford, G.; Totton, N.; Lee, T.J.; Ramadas, A.; et al. Improved adenoma detection with Endocuff Vision: The ADENOMA randomised controlled trial. Gut 2019, 68, 280–288. [Google Scholar] [CrossRef]
- Williet, N.; Tournier, Q.; Vernet, C.; Dumas, O.; Rinaldi, L.; Roblin, X.; Phelip, J.-M.; Pioche, M. Effect of Endocuff-assisted colonoscopy on adenoma detection rate: Meta-analysis of randomized controlled trials. Endoscopy 2018, 50, 846–860. [Google Scholar] [CrossRef]
- van Doorn, S.C.; van der Vlugt, M.; Depla, A.; Wientjes, C.A.; Mallant-Hent, R.C.; Siersema, P.D.; Tytgat, K.; Tuynman, H.; Kuiken, S.D.; Houben, G.; et al. Adenoma detection with Endocuff colonoscopy versus conventional colonoscopy: A multicentre randomised controlled trial. Gut 2017, 66, 438–445. [Google Scholar] [CrossRef]
- Walls, M.; Houwen, B.B.S.L.; Rice, S.; Seager, A.; Dekker, E.; Sharp, L.; Rees, C.J. The effect of the endoscopic device Endocuff™/Endocuff vision™ on quality standards in colonoscopy: A systematic review and me-ta-analysis of randomized trials. Color. Dis. 2023, 25, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, M.; Hassan, C.; Battagello, J.; Antonelli, G.; Pantalena, M.; Bulighin, G.; Alicante, S.; Meggiato, T.; Rosa-Rizzotto, E.; Iacopini, F.; et al. Adenoma detection by Endocuff-assisted versus standard colonosco-py in an organized screening program: The “ItaVision” randomized controlled trial. Endoscopy 2022, 54, 138–147. [Google Scholar] [CrossRef]
- Sakamoto, T.; Abe, S.; Yoshida, M.K.; Tanaka, Y.; Saito, Y. Endocuff-assisted underwater snare polypectomy in complex ascending colon neoplasia. Endoscopy 2018, 50, E136–E137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Figura, G.; Hasenöhrl, M.; Haller, B.; Poszler, A.; Ulrich, J.; Brown, H.; Abdelhafez, M.; Schmid, R.M.; von Delius, S.; Klare, P. Endocuff vision-assisted vs. standard polyp resection in the colorectum (the EVASTA study): A prospective randomized study. Endoscopy 2020, 52, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Palma, R.; Panetta, C.; Raniolo, M.; Gallo, G.; Pontone, S. P.05.16 Endocuff vision-assisted resection for “difficult” colonic lesions. efficacy and safety in a pilot randomized study. Dig. Liver Dis. 2018, 50, e172. [Google Scholar] [CrossRef]


| SP | EP | p-Value | |
|---|---|---|---|
| Patients, N | 20 | 12 | |
| Median Age, y | 68.4 ± 10.5 | 70.1 ± 8.1 | ns |
| Males (%) | 11 (55) | 2 (16.7) | 0.033 |
| First colonoscopy (%) | 17 (85) | 6 (50) | ns |
| Diabetes (%) | 5 (25) | 1 (8.3) | ns |
| Hypertension (%) | 10 (50) | 4 (33.3) | ns |
| Acetylsalicylic acid (ASA) (%) | 3 (15) | 2 (16.7) | ns |
| Diverticulosis (%) | 12 (60) | 6 (50) | ns |
| Variable | SP | EP | p-Value |
|---|---|---|---|
| Polyps number | 23 | 14 | |
| Polyp size in mm | 29.8 ± 17.3 | 24.5 ± 17.8 | ns |
| Polyp location | 1R, 3 S, 2 D, 1 T, 7A, 7C | 1 R, 6 S, 1 D, 2 T, 2 A, 2 C | |
| Peridiverticular polyp (%) | 2 (9.5) | 3 (18.7) | ns |
| Variable | SP | EP | p-Value |
|---|---|---|---|
| Polypectomy time * | 25.4 ± 14.3 | 30.8 ± 19.8 | ns |
| Procedure time * | 37.7 ± 14.1 | 52.2 ± 28.7 | <0.05 |
| Withdrawal time in min * | 6.1 ± 5.2 | 9.8 ± 9.5 | ns |
| Bleeding | 4 (20) | 2 (16.7) | ns |
| Perforation | 1 (5%) | 0 | ns |
| Shaking | 3.8 ± 2.9 | 3.1 ± 3.9 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma, R.; Andrisani, G.; Fanello, G.; Lauro, A.; Panetta, C.; Eberspacher, C.; Di Matteo, F.M.; Vaccari, S.; Zorzetti, N.; D’Andrea, V.; et al. Endocuff Vision-Assisted Resection for Difficult Colonic Lesions—Preliminary Results of a Multicenter, Prospective Randomized Pilot Study. J. Clin. Med. 2023, 12, 4980. https://doi.org/10.3390/jcm12154980
Palma R, Andrisani G, Fanello G, Lauro A, Panetta C, Eberspacher C, Di Matteo FM, Vaccari S, Zorzetti N, D’Andrea V, et al. Endocuff Vision-Assisted Resection for Difficult Colonic Lesions—Preliminary Results of a Multicenter, Prospective Randomized Pilot Study. Journal of Clinical Medicine. 2023; 12(15):4980. https://doi.org/10.3390/jcm12154980
Chicago/Turabian StylePalma, Rossella, Gianluca Andrisani, Gianfranco Fanello, Augusto Lauro, Cristina Panetta, Chiara Eberspacher, Francesco Maria Di Matteo, Samuele Vaccari, Noemi Zorzetti, Vito D’Andrea, and et al. 2023. "Endocuff Vision-Assisted Resection for Difficult Colonic Lesions—Preliminary Results of a Multicenter, Prospective Randomized Pilot Study" Journal of Clinical Medicine 12, no. 15: 4980. https://doi.org/10.3390/jcm12154980





