The Association between the L3 Skeletal Muscle Index Derived from Computed Tomography and Clinical Outcomes in Patients with Urinary Tract Infection in the Emergency Department
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Data Collection
2.4. Skeletal Muscle Index
2.5. Outcome Variables
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
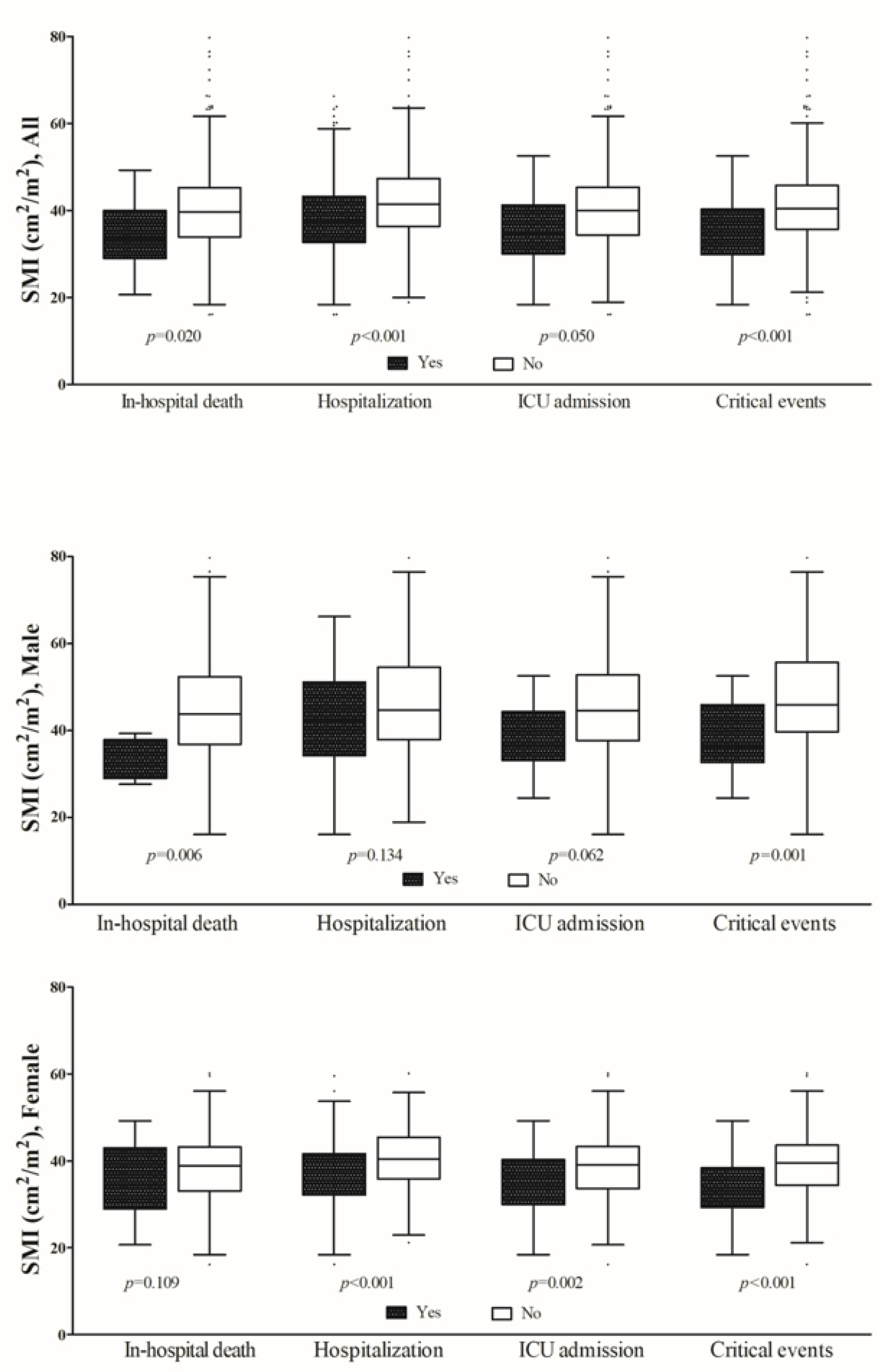
3.2. Clinical Outcomes
3.3. In-Hospital Mortality
3.4. Analysis for Predicting of Critical Events
3.5. Prognostic Value of Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zilberberg, M.D.; Nathanson, B.H.; Sulham, K.; Shorr, A.F. Descriptive epidemiology and outcomes of emergency department visits with complicated urinary tract infections in the United States, 2016–2018. J. Am. Coll. Emerg. Physicians Open 2022, 3, e12694. [Google Scholar] [CrossRef]
- Ohyama, K.; Watanabe, M.; Nosaki, Y.; Hara, T.; Iwai, K.; Mokuno, K. Correlation Between Skeletal Muscle Mass Deficit and Poor Functional Outcome in Patients with Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 104623. [Google Scholar] [CrossRef]
- Han, J.W.; Song, H.; Kim, S.H. The association between L1 skeletal muscle index derived from routine CT and in-hospital mortality in CAP patients in the ED. Am. J. Emerg. Med. 2021, 42, 49–54. [Google Scholar] [CrossRef]
- Xia, W.; Barazanchi, A.W.H.; MacFater, W.S.; Hill, A.G. The impact of computed tomography-assessed sarcopenia on outcomes for trauma patients—A systematic review and meta-analysis. Injury 2019, 50, 1565–1576. [Google Scholar] [CrossRef]
- Gomez-Perez, S.; McKeever, L.; Sheean, P. Tutorial: A Step-by-Step Guide (Version 2.0) for Measuring Abdominal Circumference and Skeletal Muscle from a Single Cross-Sectional Computed-Tomography Image Using the National Institutes of Health ImageJ. JPEN J. Parenter. Enter. Nutr. 2020, 44, 419–424. [Google Scholar] [CrossRef]
- Gomez-Perez, S.L.; Haus, J.M.; Sheean, P.; Patel, B.; Mar, W.; Chaudhry, V.; McKeever, L.; Braunschweig, C. Measuring Abdominal Circumference and Skeletal Muscle from a Single Cross-Sectional Computed Tomography Image: A Step-by-Step Guide for Clinicians Using National Institutes of Health ImageJ. JPEN J. Parenter. Enter. Nutr. 2016, 40, 308–318. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.K.; Lee, S.; Kim, K.W.; Lee, J.E.; Hwang, J.A.; Park, T.; Lee, J. Reference Values for Skeletal Muscle Mass at the Third Lumbar Vertebral Level Measured by Computed Tomography in a Healthy Korean Population. Endocrinol Metab. 2021, 36, 672–677. [Google Scholar] [CrossRef]
- Chu, C.M.; Lowder, J.L. Diagnosis and treatment of urinary tract infections across age groups. Am. J. Obstet. Gynecol. 2018, 219, 40–51. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Petrosillo, N.; Granata, G.; Boyle, B.; Doyle, M.M.; Pinchera, B.; Taglietti, F. Preventing sepsis development in complicated urinary tract infections. Expert Rev. Anti-Infect. Ther. 2020, 18, 47–61. [Google Scholar] [CrossRef]
- Tan, C.W.; Chlebicki, M.P. Urinary tract infections in adults. Singap. Med. J. 2016, 57, 485–490. [Google Scholar] [CrossRef] [Green Version]
- Kranz, J.; Schmidt, S.; Lebert, C.; Schneidewind, L.; Schmiemann, G.; Wagenlehner, F. Uncomplicated Bacterial Community-Acquired Urinary Tract Infection in Adults. Dtsch. Arztebl. Int. 2017, 114, 866–873. [Google Scholar] [CrossRef]
- Gomila, A.; Carratala, J.; Eliakim-Raz, N.; Shaw, E.; Wiegand, I.; Vallejo-Torres, L.; Gorostiza, A.; Vigo, J.M.; Morris, S.; Stoddart, M.; et al. Risk factors and prognosis of complicated urinary tract infections caused by Pseudomonas aeruginosa in hospitalized patients: A retrospective multicenter cohort study. Infect. Drug Resist. 2018, 11, 2571–2581. [Google Scholar] [CrossRef] [Green Version]
- Sabih, A.; Leslie, S.W. Complicated Urinary Tract Infections; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Fuchs, G.; Thevathasan, T.; Chretien, Y.R.; Mario, J.; Piriyapatsom, A.; Schmidt, U.; Eikermann, M.; Fintelmann, F.J. Lumbar skeletal muscle index derived from routine computed tomography exams predict adverse post-extubation outcomes in critically ill patients. J. Crit. Care 2018, 44, 117–123. [Google Scholar] [CrossRef]
- Giani, M.; Rezoagli, E.; Grassi, A.; Porta, M.; Riva, L.; Famularo, S.; Barbaro, A.; Bernasconi, D.; Ippolito, D.; Bellani, G.; et al. Low skeletal muscle index and myosteatosis as predictors of mortality in critically ill surgical patients. Nutrition 2022, 101, 111687. [Google Scholar] [CrossRef]
- Weijs, P.J.; Looijaard, W.G.; Dekker, I.M.; Stapel, S.N.; Girbes, A.R.; Oudemans-van Straaten, H.M.; Beishuizen, A. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit. Care 2014, 18, R12. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Sun, J.S.; Lee, Y.H.; Lee, J.H.; Hong, J.; Lee, J.M. Comparative assessment of skeletal muscle mass using computerized tomography and bioelectrical impedance analysis in critically ill patients. Clin. Nutr. 2019, 38, 2747–2755. [Google Scholar] [CrossRef]
- Tourel, C.; Burnol, L.; Lanoisele, J.; Molliex, S.; Viallon, M.; Croisille, P.; Morel, J. Reliability of standardized ultrasound measurements of quadriceps muscle thickness in neurological critically ill patients: A comparison to computed tomography measures. J. Rehabil. Med. 2020, 52, jrm00032. [Google Scholar] [CrossRef] [Green Version]
- Thibault, R.; Genton, L. Accuracy of bioelectrical impedance analysis to measure skeletal muscle mass. Clin. Nutr. 2014, 33, 1157. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Tabara, Y.; Setoh, K.; Kawaguchi, T.; Matsuda, F. Skeletal muscle mass index is independently associated with all-cause mortality in men: The Nagahama study. Geriatr. Gerontol. Int. 2022, 22, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Bernabe-Ortiz, A.; Carrillo-Larco, R.M.; Gilman, R.H.; Smeeth, L.; Checkley, W.; Miranda, J.J. Skeletal muscle mass and all-cause mortality: Findings from the CRONICAS cohort study. Trop. Med. Int. Health 2023, 28, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.V.; Chen, J.D.; Wu, W.T.; Huang, K.C.; Hsu, C.T.; Han, D.S. Association between Loss of Skeletal Muscle Mass and Mortality and Tumor Recurrence in Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Cancer 2018, 7, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Kruse, N.T.; Buzkova, P.; Barzilay, J.I.; Valderrabano, R.J.; Robbins, J.A.; Fink, H.A.; Jalal, D.I. Association of skeletal muscle mass, kidney disease and mortality in older men and women: The cardiovascular health study. Aging 2020, 12, 21023–21036. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.D.; Nepal, P.; Akinlonu, A.; Nekkalapudi, D.; Kim, K.; Cativo, E.H.; Visco, F.; Mushiyev, S.; Pekler, G. Low Skeletal Muscle Mass Independently Predicts Mortality in Patients with Chronic Heart Failure after an Acute Hospitalization. Cardiology 2019, 142, 28–36. [Google Scholar] [CrossRef]
- Meyer, H.J.; Wienke, A.; Surov, A. Computed tomography-defined low skeletal muscle mass as a prognostic marker for short-term mortality in critically ill patients: A systematic review and meta-analysis. Nutrition 2021, 91–92, 111417. [Google Scholar] [CrossRef]
- Miyake, H.; Kanazawa, I.; Tanaka, K.I.; Sugimoto, T. Low skeletal muscle mass is associated with the risk of all-cause mortality in patients with type 2 diabetes mellitus. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819842971. [Google Scholar] [CrossRef]
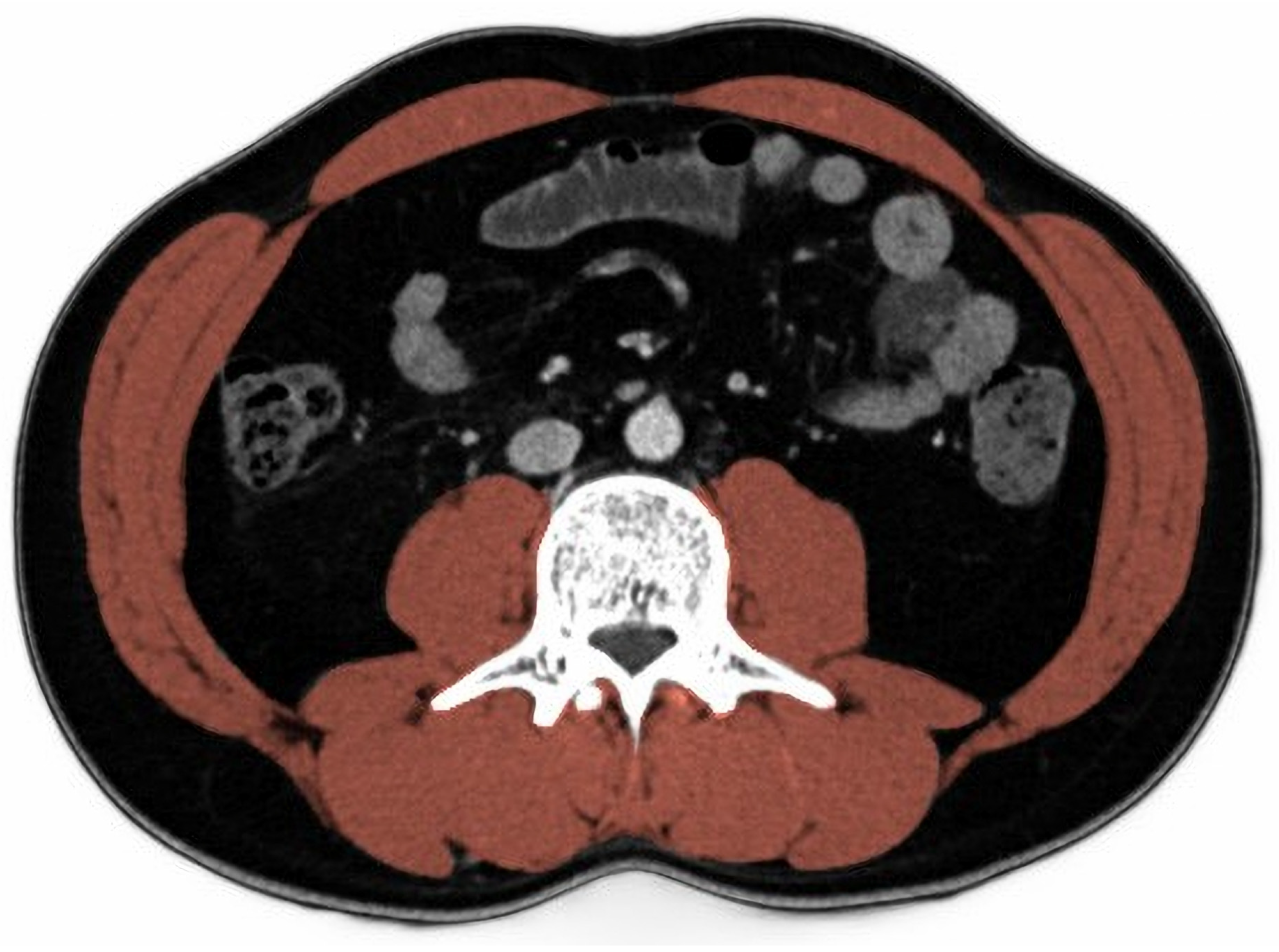
| All | Low SMI | High SMI | p | |
|---|---|---|---|---|
| N = 478 | n = 93 | n = 385 | ||
| Male, n (%) | 120 (25.1) | 47 (50.5) | 73 (19.0) | <0.001 |
| Age (mean ± SD) | 61.6 ± 19.8 | 73.3 ± 17.6 | 58.8 ± 19.3 | <0.001 |
| BMI | 23.4 ± 4.9 | 20.4 ± 4.9 | 24.1 ± 4.6 | <0.001 |
| SMI (cm2/m2) | ||||
| Male | 45.4 ± 13.4 | 33.5 ± 5.5 | 53.0 ± 11.2 | |
| Female | 38.5 ± 7.3 | 26.9 ± 3.2 | 40.2 ± 6.1 | |
| Premorbid disease, n (%) | ||||
| Hypertension | 177 (37.0) | 51 (54.8) | 126 (32.7) | <0.001 |
| Diabetes mellitus | 143 (29.9) | 40 (43.0) | 103 (26.8) | 0.003 |
| Coronary artery disease | 52 (10.9) | 11 (11.8) | 41 (10.6) | 0.887 |
| Cerebrovascular accident | 41 (8.6) | 14 (15.1) | 27 (7.0) | 0.023 |
| Pulmonary disease | 14 (2.9) | 5 (5.4) | 9 (2.3) | 0.161 |
| Chronic kidney disease | 21 (4.4) | 7 (7.5) | 14 (3.6) | 0.152 |
| Clinical findings on admission | ||||
| Altered mentality, n (%) | 43 (9.0) | 13 (14.0) | 30 (7.8) | 0.095 |
| Vital signs (mean ± SD) | ||||
| Mean arterial pressure, mmHg | 93.1 ± 17.2 | 94.6 ± 21.6 | 92.7 ± 15.9 | 0.429 |
| Heart rate/min | 90.6 ± 19.1 | 93.6 ± 21.1 | 89.8 ± 18.5 | 0.083 |
| Respiratory rate/min | 19.0 ± 2.8 | 19.4 ± 3.4 | 18.9 ± 2.6 | 0.211 |
| Temperature, °C | 37.6 ± 1.2 | 37.2 ± 1.1 | 37.7 ± 1.2 | 0.001 |
| qSOFA above 2 points | 12 (2.5) | 7 (7.6) | 5 (1.3) | 0.001 |
| Laboratory findings, median (IQR) | ||||
| White blood cells (×109/L) | 11.0 (8.1–14.8) | 11.7 (7.9–15.5) | 10.9 (8.1–14.6) | 0.548 |
| BUN (mg/dL) | 15.5 (11.8–22.2) | 22.2 (14.7–36.1) | 14.5 (11.4–20.7) | <0.001 |
| Creatinine (mg/dL) | 0.8 (0.7–1.1) | 0.9 (0.7–1.4) | 0.8 (0.6–1.0) | 0.005 |
| C-reactive protein (mg/dL) | 7.5 (1.4–15.2) | 6.6 (1.6–13.4) | 7.6 (1.4–15.4) | 0.756 |
| ESR (mm/h) | 27.0 (10.0–49.0) | 31.0 (9.0–52.0) | 26.0 (10.0–49.0) | 0.535 |
| Lactate, mmol/L | 1.6 (1.1–2.2) | 1.8 (1.2–2.6) | 1.5 (1.1–2.0) | 0.082 |
| pH | 7.43 (7.38–7.47) | 7.42 (7.35–7.47) | 7.43 (7.39–7.47) | 0.367 |
| Bacteriuria | 285 (59.6%) | 50 (53.8%) | 235 (61.0%) | 0.244 |
| All | Low SMI | High SMI | p | |
|---|---|---|---|---|
| N = 478 | n = 93 | n = 385 | ||
| Hospital courses | ||||
| In-hospital mortality | 17 (3.6) | 8 (8.6) | 9 (2.3) | 0.009 |
| Hospitalization | 280 (58.6) | 60 (64.5) | 220 (57.1) | 0.239 |
| ICU admission | 56 (11.7) | 26 (28.0) | 30 (7.8) | <0.001 |
| Length of ICU stay | 4.5 (3.0–10.5) | 4.5 (3.0–14.5) | 4.5 (3.0–7.5) | 0.560 |
| Length of hospital stay | 5.0 (1.0–9.0) | 8.0 (2.0–14.0) | 5.0 (1.0–8.0) | <0.001 |
| Critical events, n (%) | 100 (20.9) | 47 (50.5) | 53 (13.8) | <0.001 |
| Dialysis | 15 (3.1) | 10 (10.8) | 5 (1.3) | <0.001 |
| Use of inotropic | 56 (11.7) | 27 (29.0) | 29 (7.5) | <0.001 |
| Refractory shock | 64 (13.4) | 32 (34.4) | 32 (8.3) | <0.001 |
| Mechanical ventilation | 12 (2.5) | 8 (8.6) | 4 (1.0) | <0.001 |
| Weaning failure, n = 12 | 8 (66.7) | 5 (62.5) | 3 (75.0) | 0.010 |
| Nonsurvivors | Survivors | p | |
|---|---|---|---|
| n = 17 | n = 461 | ||
| Male, n (%) | 4 (23.5) | 116 (25.2) | 0.879 |
| Age (mean ± SD) | 78.8 ± 13.9 | 61.0 ± 19.7 | <0.001 |
| BMI | 20.1 ± 4.7 | 23.5 ± 4.8 | 0.005 |
| SMI (cm2/m2) | 0.020 | ||
| Male | 33.4 ± 4.8 | 45.8 ± 13.4 | |
| Female | 35.3 ± 8.8 | 38.6 ± 7.6 | |
| Premorbid disease, n (%) | |||
| Hypertension | 8 (47.1) | 169 (36.7) | 0.383 |
| Diabetes mellitus | 13 (76.5) | 130 (28.2) | <0.001 |
| Coronary artery disease | 6 (35.3) | 46 (10.0) | 0.001 |
| Cerebrovascular accident | 1 (5.9) | 40 (8.7) | 0.686 |
| Pulmonary disease | 0 (0.0) | 14 (3.0) | 0.466 |
| Chronic kidney disease | 1 (5.9) | 20 (4.3) | 0.760 |
| Clinical findings on admission | |||
| Altered mentality, n (%) | 4 (23.5) | 39 (8.5) | 0.033 |
| Vital signs (mean ± SD) | |||
| Mean arterial pressure, mmHg | 93.2 ± 23.1 | 93.1 ± 16.9 | 0.986 |
| Heart rate/min | 88.7 ± 16.0 | 90.6 ± 19.2 | 0.674 |
| Respiratory rate/min | 19.8 ± 3.5 | 19.0 ± 2.8 | 0.258 |
| Temperature, °C | 36.6 ± 1.2 | 37.6 ± 1.2 | <0.001 |
| qSOFA above 2 points | 2 (11.8) | 10 (2.2) | 0.013 |
| Laboratory finding, median (IQR) | |||
| White blood cells (×109/L) | 12.2 (8.3–17.4) | 11.0 (8.1–14.7) | 0.127 |
| BUN (mg/dl) | 31.7 (24.8–50.9) | 15.2 (11.7–21.8) | 0.010 |
| Creatinine (mg/dl) | 1.2 (0.9–1.8) | 0.8 (0.7–1.1) | <0.001 |
| C-reactive protein (mg/dL) | 5.0 (2.3–18.3) | 7.6 (1.4–15.1) | 0.722 |
| ESR (mm/h) | 24.0 (8.0–60.0) | 27.0 (10.0–49.0) | 0.763 |
| Lactate, mmol/L | 4.7 (1.9–7.0) | 1.5 (1.1–2.0) | <0.001 |
| pH | 7.38 (7.06–7.45) | 7.43 (7.39–7.47) | 0.037 |
| Bacteriuria | 13 (76.5) | 272 (59.0) | 0.149 |
| Hospital course | |||
| ICU admission | 13 (76.5) | 43 (9.3) | <0.001 |
| Length of ICU stay | 8.0 (4.0–14.0) | 4.0 (3.0–9.0) | 0.137 |
| Length of hospital stay | 7.0 (3.0–11.0) | 5.0 (1.0–9.0) | 0.411 |
| Critical events, n (%) | 17 (100.0) | 83 (18.0) | <0.001 |
| Dialysis | 6 (35.3) | 9 (2.0) | <0.001 |
| Use of inotropic | 16 (94.1) | 40 (8.7) | <0.001 |
| Refractory shock | 17 (100.0) | 47 (10.2) | <0.001 |
| Mechanical ventilation | 8 (47.1) | 4 (0.9) | <0.001 |
| aOR (95% CI) | p | |
|---|---|---|
| Model I (adjusted for gender, age, BMI) | ||
| Low SMI | 3.09 (1.72–5.56) | <0.001 |
| Male | 1.22 (0.70–2.13) | 0.473 |
| Age | 1.03 (1.02–1.05) | <0.001 |
| BMI | 0.91 (0.85–0.97) | 0.003 |
| Model II | ||
| Low SMI | 2.87 (1.10–7.53) | 0.031 |
| Diabetes mellitus | 2.81 (1.15–7.25) | 0.025 |
| Altered mentality | 8.15 (1.36–48.9) | 0.022 |
| Lactate, mmol/L | 1.45 (1.05–1.99) | 0.024 |
| Creatinine (mg/dl) | 2.02 (1.03–3.96) | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, J.; Choi, S.P.; Oh, J.H.; Zhu, J.H.; Kim, S.W.; Kim, S.H. The Association between the L3 Skeletal Muscle Index Derived from Computed Tomography and Clinical Outcomes in Patients with Urinary Tract Infection in the Emergency Department. J. Clin. Med. 2023, 12, 5024. https://doi.org/10.3390/jcm12155024
An J, Choi SP, Oh JH, Zhu JH, Kim SW, Kim SH. The Association between the L3 Skeletal Muscle Index Derived from Computed Tomography and Clinical Outcomes in Patients with Urinary Tract Infection in the Emergency Department. Journal of Clinical Medicine. 2023; 12(15):5024. https://doi.org/10.3390/jcm12155024
Chicago/Turabian StyleAn, Jinjoo, Seung Pill Choi, Jae Hun Oh, Jong Ho Zhu, Sung Wook Kim, and Soo Hyun Kim. 2023. "The Association between the L3 Skeletal Muscle Index Derived from Computed Tomography and Clinical Outcomes in Patients with Urinary Tract Infection in the Emergency Department" Journal of Clinical Medicine 12, no. 15: 5024. https://doi.org/10.3390/jcm12155024





