Metabolic Syndrome Rather Than Other Phenotypes in PCOS as a Predictive Indicator for Clinical Outcomes in IVF: Comprehensive Phenotypic Assessment across All PCOS Classifications
Abstract
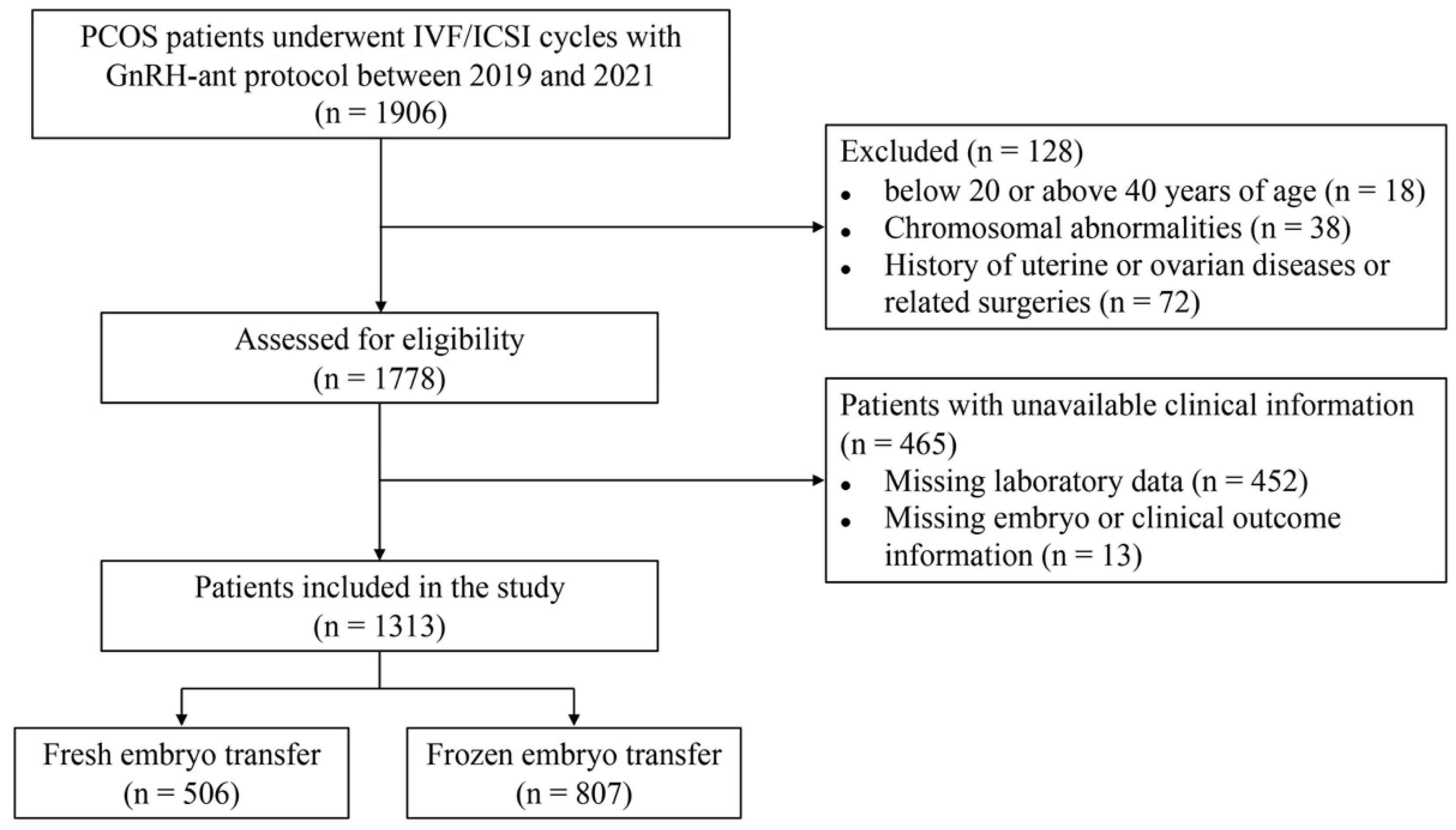
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Laboratory Tests
2.3. The GnRH-Ant Protocol
2.4. Measurement of Outcomes
2.5. Statistical Analysis
3. Results
3.1. The Characteristics of Patients with Different Classic Phenotypes of PCOS
3.2. The Characteristics of Patients with or without MetS
3.3. Pregnancy Outcomes in Women with Different Phenotypes of PCOS
3.4. Factors Associated with Pregnancy Outcomes in Women with PCOS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conway, G.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Franks, S.; Gambineri, A.; Kelestimur, F.; Macut, D.; Micic, D.; Pasquali, R.; et al. The polycystic ovary syndrome: A position statement from the European Society of Endocrinology. Eur. J. Endocrinol. 2014, 171, P1–P29. [Google Scholar] [PubMed] [Green Version]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.; Taylor, A.E.; et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: The complete task force report. Fertil. Steril. 2009, 91, 456–488. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.J.; Taylor, A.E.; et al. Positions statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An Androgen Excess Society guideline. J. Clin. Endocrinol. Metab. 2006, 91, 4237–4245. [Google Scholar] [CrossRef] [Green Version]
- McCartney, C.R.; Marshall, J.C. Clinical practice. Polycystic Ovary Syndrome. N. Engl. J. Med. 2016, 375, 54–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Sun, Q.; Zhu, K.; Hu, C. Elevated IGF-1 induced female rats perpetuates the PCOS phenotype: Pathological mechanism of IGF-1 in polycystic ovary syndrome. Gynecol. Obstet. Investig. 2023, 88, 143–149. [Google Scholar]
- Han, Q.; Wang, J.; Li, W.; Chen, Z.-J.; Du, Y. Androgen-induced gut dysbiosis disrupts glucolipid metabolism and endocrinal functions in polycystic ovary syndrome. Microbiome 2021, 9, 101. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, H.; Li, W.; Chen, D.; Xu, Y.; Xu, A.; Ye, D. Targeting adipokines in polycystic ovary syndrome and related metabolic disorders: From experimental insights to clinical studies. Pharmacol. Ther. 2022, 240, 108284. [Google Scholar] [CrossRef]
- Wekker, V.; Van Dammen, L.; Koning, A.; Heida, K.Y.; Painter, R.C.; Limpens, J.; Laven, J.S.E.; Lennep, J.E.R.V.; Roseboom, T.J.; Hoek, A. Long-term cardiometabolic disease risk in women with PCOS: A systematic review and meta-analysis. Hum. Reprod. Update 2020, 26, 942–960. [Google Scholar] [CrossRef]
- Rotterdam EA-SPcwg. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Joham, A.E.; Norman, R.J.; Stener-Victorin, E.; Legro, R.S.; Franks, S.; Moran, L.J.; Boyle, J.; Teede, H.J. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022, 10, 668–680. [Google Scholar] [CrossRef]
- Orio, F.; Palomba, S. Reproductive endocrinology: New guidelines for the diagnosis and treatment of PCOS. Nat. Rev. Endocrinol. 2014, 10, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Moghetti, P.; Tosi, F.; Bonin, C.; Di Sarra, D.; Fiers, T.; Kaufman, J.-M.; Giagulli, V.A.; Signori, C.; Zambotti, F.; Dall’Alda, M.; et al. Divergences in insulin resistance between the different phenotypes of the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2013, 98, E628–E637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakoly, N.S.; Khomami, M.B.; Joham, A.A.E.; Cooray, S.D.; Misso, M.L.; Norman, R.J.; Harrison, C.L.; Ranasinha, S.; Teede, H.J.; Moran, L.J. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: A systematic review and meta-regression. Hum. Reprod. Update 2018, 24, 455–467. [Google Scholar] [CrossRef]
- Palomba, S.; La Sala, G.B. Pregnancy complications in women with polycystic ovary syndrome: Importance of diagnostic criteria or of phenotypic features? Hum. Reprod. 2016, 31, 223–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palomba, S. Is fertility reduced in ovulatory women with polycystic ovary syndrome? An opinion paper. Hum. Reprod. 2021, 36, 2421–2428. [Google Scholar] [CrossRef]
- Nikolayenkov, I.P.; Kazymova, O.E.; Sudakov, D.S.; Dymarskaya, Y.R. IVF efficiency in different phenotypes of polycystic ovary syndrome. J. Obstet. Womens Dis. 2021, 70, 81–90. [Google Scholar] [CrossRef]
- Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- Norman, R.J.; Dewailly, D.; Legro, R.S.; Hickey, T.E. Polycystic ovary syndrome. Lancet 2007, 370, 685–697. [Google Scholar] [CrossRef] [Green Version]
- Rosner, W.; Auchus, R.J.; Azziz, R.; Sluss, P.M.; Raff, H. Position statement: Utility, limitations, and pitfalls in measuring testosterone: An Endocrine Society position statement. J. Clin. Endocrinol. Metab. 2007, 92, 405–413. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar]
- Singh, S.; Pal, N.; Shubham, S.; Sarma, D.K.; Verma, V.; Marotta, F.; Kumar, M. Polycystic Ovary Syndrome: Etiology, Current Management, and Future Therapeutics. J. Clin. Med. 2023, 12, 1454. [Google Scholar] [CrossRef]
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Zawadri, J. Diagnostic criteria for polycystic ovary syndrome: Towards a rational approach. In Polycystic Ovary Syndrome; Dunaif, A., Givens, J.R., Haseltine, F.P., Merriam, G.R., Eds.; Blackwell Scientific: Boston, MA, USA, 1992; pp. 377–384. [Google Scholar]
- Amisi, C.A. Markers of insulin resistance in Polycystic ovary syndrome women: An update. World J. Diabetes 2022, 13, 129–149. [Google Scholar] [CrossRef]
- Chen, C.; Lu, F.C.; Department of Disease Control Ministry of Health, China. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed. Environ. Sci. 2004, 17, 1–36. [Google Scholar] [PubMed]
- Wang, H.; Zhai, F. Programme and policy options for preventing obesity in China. Obes. Rev. 2013, 14, 134–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Expert Panel on Detection, Evaluation. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Vliet-Ostaptchouk, J.V.; Nuotio, M.-L.; Slagter, S.N.; Doiron, D.; Fischer, K.; Foco, L.; Gaye, A.; Gögele, M.; Heier, M.; Hiekkalinna, T.; et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: A collaborative analysis of ten large cohort studies. BMC Endocr. Disord. 2014, 14, 9. [Google Scholar] [CrossRef] [Green Version]
- Balaban, B.; Brison, D.; Calderon, G.; Catt, J.; Conaghan, J.; Cowan, L.; Ebner, T.; Gardner, D.; Hardarson, T.; Lundin, K.; et al. Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Reprod. Biomed. Online 2011, 22, 632–646. [Google Scholar]
- Gardner, D.K.; Lane, M.; Stevens, J.; Schlenker, T.; Schoolcraft, W.B. Blastocyst score affects implantation and pregnancy outcome: Towards a single blastocyst transfer. Fertil. Steril. 2000, 73, 1155–1158. [Google Scholar] [CrossRef]
- Ozay, A.C.; Ozay, O.E.; Gulekli, B. Comparison of Anti-müllerian Hormone (AMH) and Hormonal Assays for Phenotypic Classification of Polycystic Ovary Syndrome. Ginekol. Polska 2020, 91, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Sova, H.; Unkila-Kallio, L.; Tiitinen, A.; Hippeläinen, M.; Perheentupa, A.; Tinkanen, H.; Puukka, K.; Bloigu, R.; Piltonen, T.; Tapanainen, J.S.; et al. Hormone profiling, including anti-Müllerian hormone (AMH), for the diagnosis of polycystic ovary syndrome (PCOS) and characterization of PCOS phenotypes. Gynecol. Endocrinol. 2019, 35, 595–600. [Google Scholar] [CrossRef] [Green Version]
- Gürsu, T.; Eraslan, A.; Angun, B. Comparison of body mass index, anti-müllerian hormone and insulin resistance parameters among different phenotypes of polycystic ovarian syndrome. Gynecol. Obstet. Clin. Med. 2022, 2, 164–170. [Google Scholar] [CrossRef]
- Soyman, Z. Comparison of serum antimullerian hormone levels among four different phenotypes of polycystic ovary syndrome. Ann. Med. Res. 2021, 28, 1326–1331. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Oberfield, S.E.; Stener-Victorin, E.; Marshall, J.C.; Laven, J.S.; Legro, R.S. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr. Rev. 2015, 36, 487–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Gao, J.; Liu, X.; Qin, H.; Wu, X. Effect of Three Androgen Indexes (FAI, FT, and TT) on Clinical, Biochemical, and Fertility Outcomes in Women with Polycystic Ovary Syndrome. Reprod. Sci. 2021, 28, 775–784. [Google Scholar] [CrossRef]
- Rahmatnezhad, L.; Moghaddam-Banaem, L.; Lak, T.B.; Shiva, A.; Rasuli, J. Free androgen index (FAI)’s relations with oxidative stress and insulin resistance in polycystic ovary syndrome. Sci. Rep. 2023, 13, 5118. [Google Scholar] [CrossRef]
- Krentowska, A.; Łebkowska, A.; Jacewicz-Święcka, M.; Hryniewicka, J.; Leśniewska, M.; Adamska, A.; Kowalska, I. Metabolic syndrome and the risk of cardiovascular complications in young patients with different phenotypes of polycystic ovary syndrome. Endocrine 2021, 72, 400–410. [Google Scholar] [CrossRef]
- VanHise, K.; Wang, E.T.; Norris, K.; Azziz, R.; Pisarska, M.D.; Chan, J.L. Racial and ethnic disparities in polycystic ovary syndrome. Fertil. Steril. 2023, 119, 348–354. [Google Scholar] [CrossRef]
- Goodman, N.F.; Cobin, R.H.; Futterweit, W.; Glueck, J.S.; Legro, R.S.; Carmina, E. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and Pcos Society Disease State Clinical Review: Guide to the Best Practices in the Evaluation and Treatment of Polycystic Ovary Syndrome—Part 2. Endocr. Pract. 2015, 21, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Sendur, S.N.; Yildiz, B.O. Influence of ethnicity on different aspects of polycystic ovary syndrome: A systematic review. Reprod. Biomed. Online 2021, 42, 799–818. [Google Scholar] [CrossRef]
- Chan, J.L.; Kar, S.; Vanky, E.; Morin-Papunen, L.; Piltonen, T.; Puurunen, J.; Tapanainen, J.S.; Maciel, G.A.R.; Hayashida, S.A.Y.; Soares, J.M.; et al. Racial and ethnic differences in the prevalence of metabolic syndrome and its components of metabolic syndrome in women with polycystic ovary syndrome: A regional cross-sectional study. Am. J. Obstet. Gynecol. 2017, 217, 189.e1–189.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glueck, C.J.; Goldenberg, N. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metabolism 2019, 92, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Neubronner, S.A.; Indran, I.R.; Chan, Y.H.; Thu, A.W.P.; Yong, E.-L. Effect of body mass index (BMI) on phenotypic features of polycystic ovary syndrome (PCOS) in Singapore women: A prospective cross-sectional study. BMC Women’s Health 2021, 21, 135. [Google Scholar] [CrossRef]
- Bahadur, A.; Verma, N.; Mundhra, R.; Chawla, L.; Ajmani, M.; Sri, M.S.; Arora, S. Correlation of Homeostatic Model Assessment-Insulin Resistance, Anti-Mullerian Hormone, and BMI in the Characterization of Polycystic Ovary Syndrome. Cureus 2021, 13, e16047. [Google Scholar] [CrossRef] [PubMed]
- Ramezanali, F.; Ashrafi, M.; Hemat, M.; Arabipoor, A.; Jalali, S.; Moini, A. Assisted reproductive outcomes in women with different polycystic ovary syndrome phenotypes: The predictive value of anti-Müllerian hormone. Reprod. Biomed. Online 2016, 32, 503–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Cao, Y.; Ma, Y.; Zhai, J. Association between hyperandrogenism and adverse pregnancy outcomes in patients with different polycystic ovary syndrome phenotypes undergoing in vitro fertilization/intracytoplasmic sperm injection: A systematic review and meta-analysis. Gynecol. Endocrinol. 2021, 37, 694–701. [Google Scholar] [CrossRef]
- De Vos, M.; Pareyn, S.; Drakopoulos, P.; Raimundo, J.M.; Anckaert, E.; Santos-Ribeiro, S.; Polyzos, N.P.; Tournaye, H.; Blockeel, C. Cumulative live birth rates after IVF in patients with polycystic ovaries: Phenotype matters. Reprod. Biomed. Online 2018, 37, 163–171. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Li, P.; Li, X.; Wang, Z.; Yan, L.; Shi, Y. Association of Polycystic Ovary Syndrome Phenotypes With Adverse Pregnancy Outcomes After In-Vitro Fertilization/Intracytoplasmic Sperm Injection. Front. Endocrinol. 2022, 13, 889029. [Google Scholar] [CrossRef]
- Li, H.W.R.; Lee, V.C.Y.; Lau, E.Y.L.; Yeung, W.S.B.; Ho, P.C.; Ng, E.H.Y. Cumulative live-birth rate in women with polycystic ovary syndrome or isolated polycystic ovaries undergoing in-vitro fertilisation treatment. J. Assist. Reprod. Genet. 2014, 31, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Palomba, S.; Daolio, J.; La Sala, G.B. Oocyte Competence in Women with Polycystic Ovary Syndrome. Trends Endocrinol. Metab. 2017, 28, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Rees, A.; Jenkins-Jones, S.; Morgan, C.L. Contemporary Reproductive Outcomes for Patients with Polycystic Ovary Syndrome: A Retrospective Observational Study. J. Clin. Endocrinol. Metab. 2016, 101, 1664–1672. [Google Scholar] [CrossRef] [Green Version]
- Namita, J.; Sonia, M.; Ved, P. Impact of various PCOS phenotypes on oocyte competence in an ART cycle. Clin. J. Obstet. Gynecol. 2022, 5, 067–071. [Google Scholar] [CrossRef]
- Sigala, J.; Sifer, C.; Dewailly, D.; Robin, G.; Bruyneel, A.; Ramdane, N.; Lefebvre-Khalil, V.; Mitchell, V.; Decanter, C. Is polycystic ovarian morphology related to a poor oocyte quality after controlled ovarian hyperstimulation for intracytoplasmic sperm injection? Results from a prospective, comparative study. Fertil. Steril. 2015, 103, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; De Wilde, M.A.; Falbo, A.; Koster, M.P.; LA Sala, G.B.; Fauser, B.C. Pregnancy complications in women with polycystic ovary syndrome. Hum. Reprod. Update 2015, 21, 575–592. [Google Scholar] [CrossRef] [Green Version]
- Mirza, F.G.; Tahlak, M.A.; Rjeili, R.B.; Hazari, K.; Ennab, F.; Hodgman, C.; Khamis, A.H.; Atiomo, W. Polycystic Ovarian Syndrome (PCOS): Does the Challenge End at Conception? Int. J. Environ. Res. Public. Health 2022, 19, 14914. [Google Scholar] [CrossRef]
| Characteristics | Phenotype A (n = 596) | Phenotype B (n = 53) | Phenotype C (n = 135) | Phenotype D (n = 529) | p Value |
|---|---|---|---|---|---|
| Age (years) | 30.14 ± 3.49 | 30.53 ± 2.99 | 30.19 ± 3.45 | 30.59 ± 3.43 | 0.163 |
| BMI (kg/m2) | 24.97 ± 3.81 | 24.99 ± 4.32 | 25.80 ± 4.40 | 24.86 ± 4.03 | 0.109 |
| TT (nmol/L) | 1.31 ± 0.64 | 1.18 ± 0.53 | 1.41 ± 0.78 | 1.06 ± 3.63 | 0.232 |
| AND (nmol/L) | 14.93 ± 4.68 | 13.66 ± 6.31 | 14.96 ± 5.97 | 6.66 ± 2.94 | <0.001 |
| DHEAS (μmol/L) | 6.79 ± 2.91 | 8.11 ± 3.57 | 7.33 ± 3.37 | 5.33 ± 1.86 | <0.001 |
| SHBG (nmol/L) | 32.28 ± 29.32 | 41.44 ± 35.64 | 36.52 ± 39.93 | 44.83 ± 46.01 | 0.018 |
| FAI | 5.89 ± 4.48 | 4.47 ± 4.14 | 7.25 ± 7.45 | 3.29 ± 2.25 | <0.001 |
| LH/FSH | 1.05 ± 0.61 | 1.13 ± 0.91 | 1.09 ± 0.58 | 0.99 ± 0.72 | 0.153 |
| AMH (ng/mL) | 9.25 ± 4.98 | 6.88 ± 3.88 | 8.96 ± 4.55 | 7.16 ± 3.64 | <0.001 |
| IGF-1 (ng/mL) | 222.66 ± 62.18 | 222.52 ± 66.20 | 224.55 ± 65.12 | 217.41 ± 67.74 | 0.536 |
| HOMA-IR | 2.94 ± 2.06 | 2.92 ± 1.90 | 3.26 ± 2.69 | 2.64 ± 2.78 | 0.039 |
| MetS | |||||
| No | 349 (58.6%) | 32 (60.4%) | 74 (54.8%) | 341 (64.5%) | 0.101 * |
| Yes | 247 (41.4%) | 21 (39.6%) | 61 (45.2%) | 188 (35.5%) | |
| Number of retrieved oocytes | 18.52 ± 10.46 | 16.40 ± 7.19 | 18.59 ± 11.17 | 16.37 ± 9.06 | 0.001 |
| Maturation rate (within ICSI, %) | 78.99 ± 17.22 | 72.40 ± 16.12 | 81.27 ± 17.56 | 81.49 ± 17.64 | 0.282 |
| Insemination method | |||||
| Conventional IVF | 432 (72.5%) | 42 (79.2%) | 94 (69.6%) | 366 (69.2%) | 0.608 |
| ICSI | 145 (24.3%) | 11 (20.8%) | 37 (27.4%) | 144 (27.2%) | |
| Half-ICSI | 19 (3.2%) | 0 (0%) | 4 (3.0%) | 19 (3.6%) | |
| Fertilization rate (%) | |||||
| IVF | 79.3 ± 19.1 | 79.6 ± 17.0 | 80.5 ± 17.4 | 80.3 ± 18.0 | 0.849 |
| ICSI | 78.7 ± 15.8 | 78.7 ± 14.8 | 72.6 ± 18.2 | 75.7 ± 19.8 | 0.218 |
| 2PN rate (%) | |||||
| IVF | 64.3 ± 19.7 | 63.5 ± 19.0 | 62.1 ± 19.0 | 64.0 ± 20.8 | 0.807 |
| ICSI | 71.3 ± 18.2 | 68.9 ± 16.9 | 67.3 ± 17.1 | 68.2 ± 21.9 | 0.520 |
| Rate of good quality embryos (%) | 74.1 ± 24.8 | 71.5 ± 22.0 | 74.3 ± 25.1 | 73.2 ± 25.7 | 0.826 |
| Endometrial thickness on the trigger day | 9.88 ± 1.76 | 9.93 ± 1.82 | 10.01 ± 1.59 | 10.25 ± 1.68 | 0.023 |
| Transfer strategy | |||||
| Fresh ET | 202 (33.9%) | 19 (35.8%) | 50 (37.0%) | 235 (44.4%) | 0.004 |
| Frozen ET | 394 (66.1%) | 34 (64.2%) | 85 (63.0%) | 294 (55.6%) | |
| Days of ET in fresh cycles | |||||
| D3 | 198 (98.0%) | 19 (100%) | 50 (100%) | 226 (96.2%) | 0.309 |
| D5/6 | 4 (2.0%) | 0 (0%) | 0 (0%) | 9 (3.8%) | |
| Number of embryos transferred in fresh cycles | |||||
| 1 | 19 (9.4%) | 1 (5.3%) | 3 (6.0%) | 34 (14.5%) | 0.162 |
| 2 | 183 (90.6%) | 18 (94.7%) | 47 (94.0%) | 201 (85.5%) |
| Characteristics | Normal Androgen (n = 529) | HA (n = 784) | p Value | Normal Weight <24 kg/m2 (n = 580) | Overweight ≥24 kg/m2 (n = 733) | p Value |
|---|---|---|---|---|---|---|
| Age (years) | 30.59 ± 3.43 | 30.18 ± 3.45 | 0.034 | 30.30 ± 3.41 | 30.38 ± 3.48 | 0.671 |
| BMI (kg/m2) | 24.86 ± 4.03 | 25.11 ± 3.96 | 0.267 | 21.47 ±1.75 | 27.82 ± 2.88 | <0.001 |
| TT (nmol/L) | 1.06 ± 3.63 | 1.32 ± 0.66 | 0.048 | 1.09 ± 0.55 | 1.31 ± 3.09 | 0.055 |
| AND (nmol/L) | 6.66 ± 2.94 | 14.85 ± 5.05 | <0.001 | 11.77 ± 6.07 | 11.73 ± 5.78 | 0.911 |
| DHEAS (μmol/L) | 5.33 ± 1.86 | 6.97 ± 3.05 | <0.001 | 6.20 ± 2.49 | 6.50 ± 3.00 | 0.130 |
| SHBG (nmol/L) | 44.83 ± 46.01 | 33.61 ± 31.73 | 0.008 | 51.52 ± 45.91 | 28.76 ± 28.31 | <0.001 |
| FAI | 3.29 ± 2.25 | 6.02 ± 5.10 | <0.001 | 3.56 ± 3.69 | 6.02 ± 4.74 | <0.001 |
| LH/FSH | 0.98 ± 0.72 | 1.06 ± 0.63 | 0.043 | 1.13 ± 0.80 | 0.95 ± 0.54 | <0.001 |
| AMH (ng/mL) | 7.16 ± 3.64 | 9.04 ± 4.88 | <0.001 | 9.48 ± 4.88 | 7.33 ± 3.95 | <0.001 |
| IGF-1 (ng/mL) | 217.41 ± 67.74 | 222.97 ± 62.85 | 0.148 | 232.62 ± 66.11 | 211.49 ± 62.43 | <0.001 |
| HOMA-IR | 2.64 ± 2.78 | 2.99 ± 2.17 | 0.011 | 1.90 ± 1.11 | 3.61 ± 2.90 | <0.001 |
| MetS | ||||||
| No | 341 (64.5%) | 455 (58.0%) | 0.019 | 455 (78.4%) | 341 (46.5%) | <0.001 |
| Yes | 188 (35.5%) | 329 (42.0%) | 125 (21.6%) | 392 (53.5%) | ||
| Number of retrieved oocytes | 16.37 ± 9.06 | 18.39 ± 10.40 | <0.001 | 19.32 ± 9.97 | 16.20 ± 9.68 | <0.001 |
| Maturation rate (within ICSI, %) | 81.49 ± 17.64 | 79.05 ± 17.24 | 0.204 | 80.73 ± 16.14 | 79.59 ± 18.41 | 0.552 |
| Insemination method | ||||||
| Conventional IVF | 366 (69.2%) | 568 (72.4%) | 0.420 | 409 (70.5%) | 525 (71.6%) | 0.548 |
| ICSI | 144 (27.2%) | 193 (24.6%) | 149 (25.7%) | 188 (25.6%) | ||
| Half-ICSI | 19 (3.6%) | 23 (2.9%) | 22 (3.8%) | 20 (2.7%) | ||
| Fertilization rate (%) | ||||||
| IVF | 80.3 ± 18.0 | 79.5 ± 18.7 | 0.503 | 80.1 ± 18.7 | 79.6 ± 18.2 | 0.672 |
| ICSI | 75.7 ± 19.8 | 77.5 ± 16.3 | 0.341 | 76.9 ± 17.5 | 76.6 ± 18.3 | 0.879 |
| 2PN rate (%) | ||||||
| IVF | 64.0 ± 20.8 | 63.9 ± 19.5 | 0.907 | 65.0 ± 19.3 | 63.1 ± 20.5 | 0.147 |
| ICSI | 68.2 ± 21.9 | 70.4 ± 17.9 | 0.312 | 69.1 ± 19.3 | 69.7 ± 20.1 | 0.783 |
| Rate of good quality embryos (%) | 73.2 ± 25.7 | 74.0 ± 24.6 | 0.567 | 72.6 ± 24.3 | 74.5 ± 25.7 | 0.166 |
| Endometrial thickness on the trigger day | 10.25 ± 1.68 | 9.90 ± 1.74 | 0.002 | 9.96 ± 1.71 | 10.12 ± 1.73 | 0.156 |
| Transfer strategy | ||||||
| Fresh ET | 235 (44.4%) | 271 (34.6%) | <0.001 | 170 (29.3%) | 336 (45.8%) | <0.001 |
| Frozen ET | 294 (55.6%) | 513 (65.4%) | 410 (70.7%) | 397 (54.2%) | ||
| Days of ET in fresh cycles | ||||||
| D3 | 226 (96.2%) | 267 (98.5%) | 0.095 | 167 (98.2%) | 326 (97.0%) | 0.416 |
| D5/6 | 9 (3.8%) | 4 (1.5%) | 3 (1.8%) | 10 (3.0%) | ||
| Number of embryos transferred in fresh cycles | ||||||
| 1 | 34 (14.5%) | 23 (8.5%) | 0.034 | 16 (9.4%) | 41 (12.2%) | 0.348 |
| 2 | 201 (85.5%) | 248 (91.5%) | 154 (90.6%) | 295 (87.8%) |
| Characteristics | No MetS (n = 796) | MetS (n = 517) | p Value |
|---|---|---|---|
| Age (years) | 30.22 ± 3.39 | 30.52 ± 3.54 | 0.123 |
| BMI (kg/m2) | 23.85 ± 3.74 | 26.79 ± 3.69 | <0.001 |
| TT (nmol/L) | 1.26 ± 2.97 | 1.15 ± 0.62 | 0.413 |
| AND (nmol/L) | 11.57 ± 5.88 | 12.01 ± 5.94 | 0.196 |
| DHEAS (μmol/L) | 6.47 ± 2.69 | 6.22 ± 2.94 | 0.243 |
| SHBG (nmol/L) | 45.45 ± 43.28 | 25.16 ± 21.85 | <0.001 |
| FAI | 4.18 ± 3.78 | 6.56 ± 5.22 | <0.001 |
| LH/FSH | 1.06 ± 0.63 | 0.99 ± 0.72 | 0.086 |
| AMH (ng/mL) | 8.60 ± 4.48 | 7.79 ± 4.53 | 0.001 |
| IGF-1 (ng/mL) | 224.27 ± 64.05 | 215.21 ± 65.88 | 0.019 |
| HOMA-IR | 2.25 ± 1.42 | 3.79 ± 3.25 | <0.001 |
| PCOS phenotypes | |||
| RC-PCOS | |||
| Phenotype A | 349 (43.8%) | 247 (47.8%) | 0.101 |
| Phenotype B | 32 (4.0%) | 21 (4.1%) | |
| Phenotype C | 74 (9.3%) | 61 (11.8%) | |
| Phenotype D | 341 (42.8%) | 188 (36.4%) | |
| HA-based PCOS | |||
| Normal androgen | 341 (42.8%) | 188 (36.4%) | 0.019 |
| HA | 455 (57.2%) | 329 (63.6%) | |
| BMI-based PCOS | |||
| Normal weight | 455 (57.2%) | 125 (24.2%) | <0.001 |
| Overweight | 341 (42.8%) | 392 (75.8%) | |
| Number of retrieved oocytes | 18.33 ± 9.62 | 16.41 ± 10.29 | 0.001 |
| Maturation rate (within ICSI, %) | 80.81 ± 15.74 | 78.84 ± 20.07 | 0.321 |
| Insemination method | |||
| Conventional IVF | 549 (69.0%) | 385 (74.5%) | 0.030 |
| ICSI | 215 (27.0%) | 122 (23.6%) | |
| Half-ICSI | 32 (4.0%) | 10 (1.9%) | |
| Fertilization rate (%) | |||
| IVF | 79.9 ± 18.1 | 79.8 ± 18.8 | 0.931 |
| ICSI | 76.7 ± 17.7 | 76.7 ± 18.4 | 0.987 |
| 2PN rate (%) | |||
| IVF | 64.4 ± 19.8 | 63.3 ± 20.3 | 0.432 |
| ICSI | 68.5 ± 19.7 | 71.2 ± 19.6 | 0.225 |
| Rate of good quality embryos (%) | 73.2 ± 24.8 | 74.3 ± 25.4 | 0.411 |
| Endometrial thickness on the trigger day | 10.03 ± 1.74 | 10.09 ± 1.68 | 0.600 |
| Transfer strategy | |||
| Fresh ET | 274 (34.4%) | 232 (44.9%) | <0.001 |
| Frozen ET | 522 (65.6%) | 285 (55.1%) | |
| Days of ET in fresh cycles | |||
| D3 | 266 (97.1%) | 227 (97.8%) | 0.588 |
| D5/6 | 8 (2.9%) | 5 (2.2%) | |
| Number of embryos transferred in fresh cycles | |||
| 1 | 34 (12.4%) | 23 (9.9%) | 0.376 |
| 2 | 240 (87.6%) | 209 (90.1%) |
| PCOS Phenotypes | Clinical Pregnancy | Live Birth | Preterm Birth | Miscarriage | Twin Pregnancy | GDM | PIH | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | p Value | No | Yes | p Value | No | Yes | p Value | No | Yes | p Value | No | Yes | p Value | No | Yes | p Value | No | Yes | p Value | |
| RC-PCOS | |||||||||||||||||||||
| Phenotype A | 194 | 402 | 0.686 | 323 | 273 | 0.908 | 221 | 52 | 0.014 | 273 | 129 | 0.674 | 220 | 53 | 0.247 | 258 | 15 | 0.142 | 265 | 8 | 0.274 |
| Phenotype B | 19 | 34 | 29 | 24 | 20 | 4 | 24 | 10 | 21 | 3 | 24 | 0 | 23 | 1 | |||||||
| Phenotype C | 51 | 84 | 75 | 60 | 40 | 20 | 60 | 24 | 43 | 17 | 59 | 1 | 60 | 0 | |||||||
| Phenotype D | 180 | 349 | 278 | 251 | 213 | 38 | 251 | 98 | 206 | 45 | 231 | 20 | 248 | 3 | |||||||
| HA-based PCOS | |||||||||||||||||||||
| Normal androgen | 180 | 349 | 0.894 | 278 | 251 | 0.495 | 213 | 38 | 0.056 | 251 | 98 | 0.303 | 206 | 45 | 0.439 | 231 | 20 | 0.073 | 248 | 3 | 0.247 |
| HA | 264 | 520 | 427 | 357 | 281 | 76 | 357 | 163 | 284 | 73 | 341 | 16 | 348 | 9 | |||||||
| BMI-based PCOS | |||||||||||||||||||||
| Normal weight | 161 | 419 | <0.001 | 271 | 309 | <0.001 | 257 | 52 | 0.217 | 309 | 110 | 0.019 | 248 | 61 | 0.833 | 293 | 16 | 0.430 | 299 | 10 | 0.047 |
| Overweight | 283 | 450 | 434 | 299 | 237 | 62 | 299 | 151 | 242 | 57 | 279 | 20 | 297 | 2 | |||||||
| MetS-based PCOS | |||||||||||||||||||||
| No MetS | 238 | 558 | <0.001 | 394 | 402 | <0.001 | 338 | 64 | 0.013 | 402 | 156 | 0.074 | 327 | 75 | 0.513 | 388 | 14 | <0.001 | 395 | 7 | 0.565 |
| MetS | 206 | 311 | 311 | 206 | 156 | 50 | 206 | 105 | 163 | 43 | 184 | 22 | 201 | 5 | |||||||
| Characteristics | Clinical Pregnancy | Live Birth | Preterm Birth | GDM | PIH | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||||||||
| OR (95% CIs) | p Value | OR (95% CIs) | p Value | OR (95% CIs) | p Value | Adjusted OR (95% CIs) | p Value | OR (95% CIs) | p Value | Adjusted OR (95% CIs) | p Value | OR (95% CIs) | p Value | Adjusted OR (95% CIs) | p Value | OR (95% CIs) | p Value | Adjusted OR (95% CIs) | p Value | |
| Age (years) | 1.005 (0.972, 1.039) | 0.763 | / | 0.317 | 0.975 (0.945, 1.007) | 0.120 | / | 0.265 | 0.959 (0.902, 1.020) | 0.183 | / | 0.170 | 1.072 (0.970, 1.185) | 0.175 | / | 0.352 | 1.102 (0.930, 1.305) | 0.264 | / | 0.272 |
| Type of infertility | ||||||||||||||||||||
| Primary | Reference | Reference | Reference | Reference | Reference | |||||||||||||||
| Secondary | 0.836 (0.651, 1.074) | 0.162 | / | 0.136 | 0.912 (0.717, 1.160) | 0.454 | / | 0.399 | 1.203 (0.771, 1.876) | 0.416 | / | 0.285 | 0.736 (0.328, 1.648) | 0.456 | / | 0.580 | 1.897 (0.594, 6.063) | 0.280 | / | 0.394 |
| Infertility duration (years) | 0.966 (0.920, 1.014) | 0.167 | / | 0.467 | 0.969 (0.924, 1.016) | 0.189 | / | 0.916 | 1.016 (0.934, 1.105) | 0.715 | / | 0.429 | 1.105 (0.980, 1.246) | 0.104 | / | 0.114 | 1.209 (1.020, 1.433) | 0.029 | 1.200 (1.005, 1.432) | 0.044 |
| BMI (kg/m2) | 0.973 (0.963, 0.983) | <0.001 | 0.930 (0.901, 0.960) | <0.001 | 0.920 (0.895, 0.947) | <0.001 | 0.918 (0.888, 0.949) | <0.001 | 1.042 (0.988, 1.098) | 0.127 | / | 0.849 | 1.076 (0.989, 1.170) | 0.089 | / | 0.828 | 0.895 (0.757, 1.060) | 0.198 | / | 0.076 |
| LH/FSH | 1.190 (0.986, 1.436) | 0.069 | / | 0.544 | 1.247 (1.049, 1.481) | 0.012 | / | 0.115 | 0.943 (0.701, 1.269) | 0.700 | / | 0.803 | 1.358 (0.998, 1.847) | 0.052 | / | 0.085 | 0.405 (0.111, 1.480) | 0.172 | / | 0.229 |
| AMH (ng/mL) | 1.040 (1.013, 1.068) | < 0.001 | / | 0.182 | 1.032 (1.007, 1.057) | 0.011 | / | 0.450 | 0.977 (0.934, 1.022) | 0.313 | / | 0.540 | 0.949 (0.875, 1.029) | 0.207 | / | 0.103 | 0.974 (0.855, 1.109) | 0.686 | / | 0.645 |
| HOMA-IR | 0.971 (0.927, 1.017) | 0.217 | / | 0.124 | 0.972 (0.926, 1.021) | 0.257 | / | 0.074 | 1.046 (0.984, 1.110) | 0.148 | / | 0.415 | 1.111 (1.021, 1.209) | 0.015 | / | 0.071 | 1.022 (0.888, 1.177) | 0.762 | / | 0.354 |
| MetS | ||||||||||||||||||||
| No | Reference | Reference | Reference | Reference | Reference | |||||||||||||||
| Yes | 0.644 (0.510, 0.812) | <0.001 | / | 0.091 | 0.649 (0.519, 0.812) | <0.001 | 0.748 (0.581, 0.963) | 0.024 | 1.693 (1.117, 2.565) | 0.013 | 1.655 (1.079, 2.537) | 0.021 | 3.314 (1.658, 6.624) | 0.001 | 2.411 (1.151, 5.048) | 0.020 | 1.404 (0.440, 4.478) | 0.567 | / | 0.697 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, M.; Xu, W.; Qi, X.; Jiang, H.; Zhao, Y.; Li, R.; Long, X.; Qiao, J. Metabolic Syndrome Rather Than Other Phenotypes in PCOS as a Predictive Indicator for Clinical Outcomes in IVF: Comprehensive Phenotypic Assessment across All PCOS Classifications. J. Clin. Med. 2023, 12, 5073. https://doi.org/10.3390/jcm12155073
Si M, Xu W, Qi X, Jiang H, Zhao Y, Li R, Long X, Qiao J. Metabolic Syndrome Rather Than Other Phenotypes in PCOS as a Predictive Indicator for Clinical Outcomes in IVF: Comprehensive Phenotypic Assessment across All PCOS Classifications. Journal of Clinical Medicine. 2023; 12(15):5073. https://doi.org/10.3390/jcm12155073
Chicago/Turabian StyleSi, Manfei, Wanxue Xu, Xinyu Qi, Huahua Jiang, Yue Zhao, Rong Li, Xiaoyu Long, and Jie Qiao. 2023. "Metabolic Syndrome Rather Than Other Phenotypes in PCOS as a Predictive Indicator for Clinical Outcomes in IVF: Comprehensive Phenotypic Assessment across All PCOS Classifications" Journal of Clinical Medicine 12, no. 15: 5073. https://doi.org/10.3390/jcm12155073








