Clinical Applications of Nasal Nitric Oxide in Allergic Rhinitis: A Review of the Literature
Abstract
:1. Introduction
2. Nasal Nitric Oxide
2.1. Sources and Biological Mechanisms
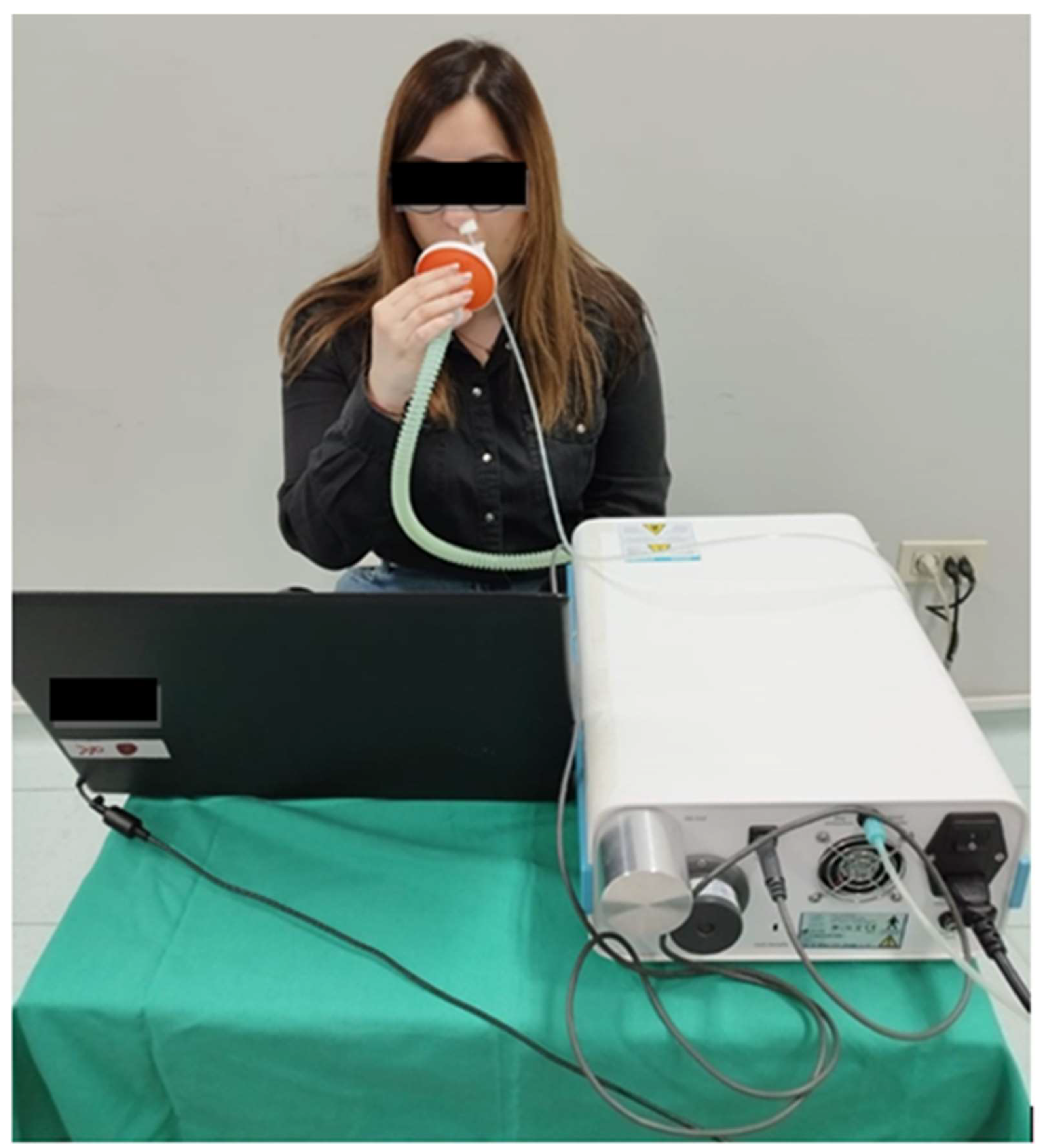
2.2. Sampling and Measurements Methods
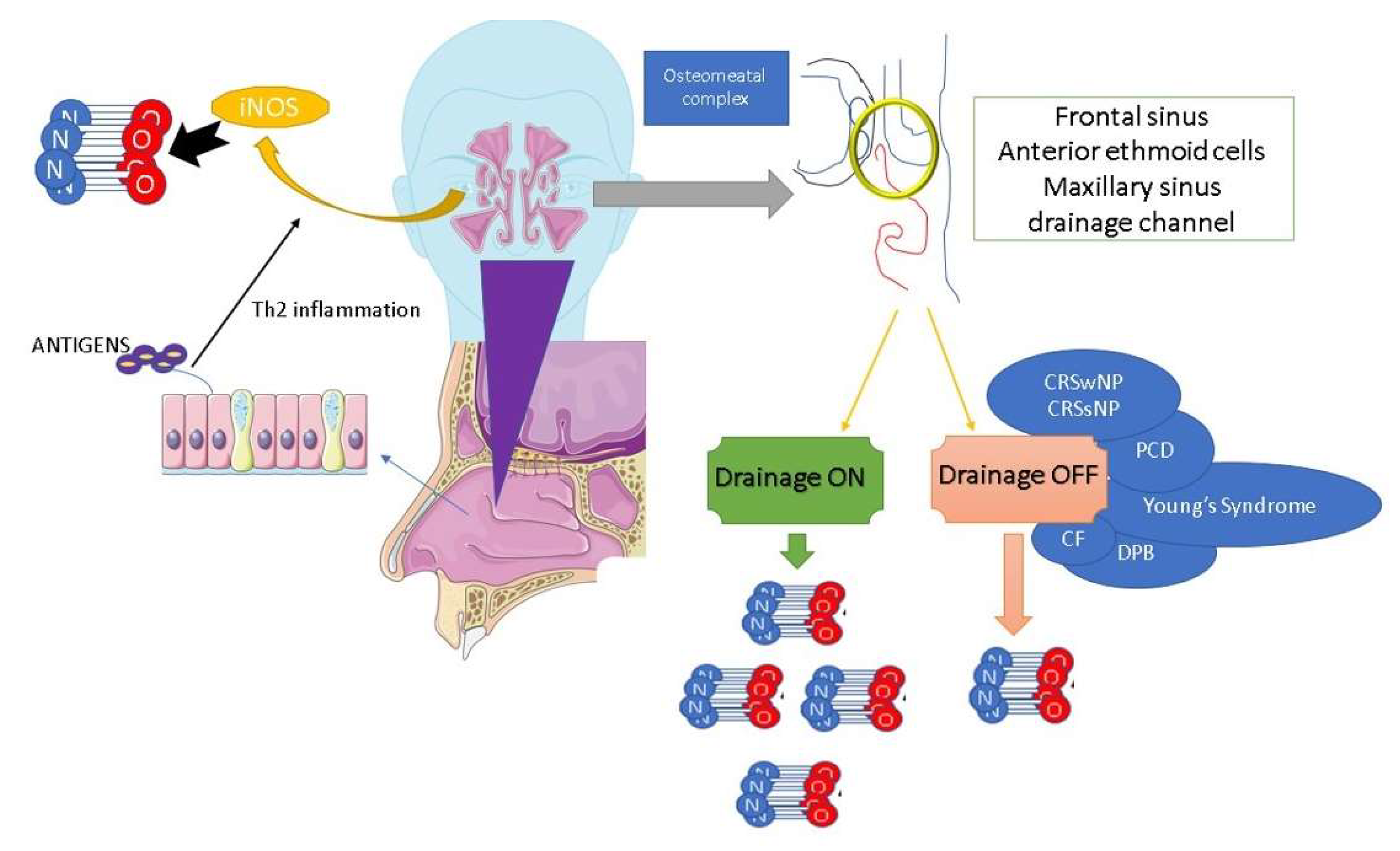
3. Nitric Oxide and Allergic Rhinitis: Clinical and Functional Mechanisms
4. Drug-Induced nNO Levels in Allergic Rhinitis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, B.; Wu, Z.; Wang, F.; Yin, Z.; Shi, L.; Liu, Y. Nasal nitric oxide testing for allergic rhinitis patients: Systematic review and meta-analysis. Immun. Inflamm. Dis. 2021, 9, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Baena-Cagnani, C.E.; Canonica, G.W.; van Weel, C.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008, 63 (Suppl. S86), 8–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nur Husna, S.M.; Tan, H.T.; Md Shukri, N.; Mohd Ashari, N.S.; Wong, K.K. Allergic Rhinitis: A Clinical and Pathophysiological Overview. Front. Med. 2022, 9, 874114. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.J.; Tung, T.H.; Tang, C.S.; Zhao, Z.H. Allergens, air pollutants, and childhood allergic diseases. Int. J. Hyg. Environ. Health 2016, 219, 66–71. [Google Scholar] [CrossRef]
- Hill, D.A.; Grundmeier, R.W.; Ram, G.; Spergel, J.M. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: A retrospective cohort study. BMC Pediatr. 2016, 16, 133. [Google Scholar] [CrossRef] [Green Version]
- Testa, D.; Bari, M.D.; Nunziata, M.; Cristofaro, G.; Massaro, G.; Marcuccio, G.; Motta, G. Allergic rhinitis and asthma assessment of risk factors in pediatric patients: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2020, 129, 109759. [Google Scholar] [CrossRef]
- Ogulur, I.; Pat, Y.; Ardicli, O.; Barletta, E.; Cevhertas, L.; Fernandez-Santamaria, R.; Huang, M.; Bel Imam, M.; Koch, J.; Ma, S.; et al. Advances and highlights in biomarkers of allergic diseases. Allergy 2021, 76, 3659–3686. [Google Scholar] [CrossRef]
- Agache, I.; Akdis, C.A. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J. Clin. Investig. 2019, 129, 1493–1503. [Google Scholar] [CrossRef] [Green Version]
- Wise, S.K.; Damask, C.; Roland, L.T.; Ebert, C.; Levy, J.M.; Lin, S.; Luong, A.; Rodriguez, K.; Sedaghat, A.R.; Toskala, E.; et al. International consensus statement on allergy and rhinology: Allergic rhinitis—2023. Int. Forum. Allergy Rhinol. 2023, 13, 293–859. [Google Scholar] [CrossRef]
- Meltzer, E.O. Allergic Rhinitis: Burden of Illness, Quality of Life, Comorbidities, and Control. Immunol. Allergy Clin. N. Am. 2016, 36, 235–248. [Google Scholar] [CrossRef]
- Pawankar, R. Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organ. J. 2014, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Heffler, E.; Carpagnano, G.E.; Favero, E.; Guida, G.; Maniscalco, M.; Motta, A.; Paoletti, G.; Rolla, G.; Baraldi, E.; Pezzella, V.; et al. Fractional Exhaled Nitric Oxide (FENO) in the management of asthma: A position paper of the Italian Respiratory Society (SIP/IRS) and Italian Society of Allergy, Asthma and Clinical Immunology (SIAAIC). Multidiscip. Respir. Med. 2020, 15, 36. [Google Scholar] [CrossRef] [Green Version]
- Maniscalco, M.; Sofia, M.; Pelaia, G. Nitric oxide in upper airways inflammatory diseases. Inflamm. Res 2007, 56, 58–69. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, H.; Wang, J.; Han, Y.; Shi, Y.; Zhang, Q.; Shen, L.; Jiang, H.; Jia, C.; Yu, Y.; et al. Nasal nitric oxide in healthy Chinese children aged 6-18 years. Front. Pediatr. 2023, 11, 990510. [Google Scholar] [CrossRef]
- Maniscalco, M.; Sofia, M.; Weitzberg, E.; De Laurentiis, G.; Stanziola, A.; Rossillo, V.; Lundberg, J.O. Humming-induced release of nasal nitric oxide for assessment of sinus obstruction in allergic rhinitis: Pilot study. Eur. J. Clin. Investig. 2004, 34, 555–560. [Google Scholar] [CrossRef]
- Zhao, C.; Qin, M.; Jin, L.; Lai, J.; Wang, Y.; Liu, S.; Yu, S. Significance of Exhaled Nitric Oxide and Carbon Monoxide in Auxiliary Diagnosis and Evaluation of Allergic Rhinitis. Mediat. Inflamm. 2022, 2022, 2083057. [Google Scholar] [CrossRef]
- Abdullah Alwi, A.H.; Zahedi, F.D.; Husain, S.; Wan Hamizan, A.K.; Abdullah, B. Diagnostic Value and Clinical Application of Nasal Fractional Exhaled Nitric Oxide in Subjects with Allergic Rhinitis. Am. J. Rhinol. Allergy 2023, 37, 307–312. [Google Scholar] [CrossRef]
- Spector, B.M.; Shusterman, D.J.; Zhao, K. Nasal nitric oxide flux from the paranasal sinuses. Curr. Opin. Allergy Clin. Immunol. 2023, 23, 22–28. [Google Scholar] [CrossRef]
- Thomassen, M.J.; Kavuru, M.S. Human alveolar macrophages and monocytes as a source and target for nitric oxide. Int. Immunopharmacol. 2001, 1, 1479–1490. [Google Scholar] [CrossRef]
- Joly, G.A.; Ayres, M.; Chelly, F.; Kilbourn, R.G. Effects of NG-methyl-L-arginine, NG-nitro-L-arginine, and aminoguanidine on constitutive and inducible nitric oxide synthase in rat aorta. Biochem. Biophys. Res. Commun. 1994, 199, 147–154. [Google Scholar] [CrossRef]
- Soskic, S.S.; Dobutovic, B.D.; Sudar, E.M.; Obradovic, M.M.; Nikolic, D.M.; Djordjevic, J.D.; Radak, D.J.; Mikhailidis, D.P.; Isenovic, E.R. Regulation of Inducible Nitric Oxide Synthase (iNOS) and its Potential Role in Insulin Resistance, Diabetes and Heart Failure. Open Cardiovasc. Med. J. 2011, 5, 153–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.Y.; Chou, P.C.; Wang, T.Y.; Lo, Y.L.; Joa, W.C.; Chen, L.F.; Sheng, T.F.; Chung, K.F.; Wang, C.H.; Kuo, H.P. Exercise-Induced Changes in Exhaled NO Differentiates Asthma With or Without Fixed Airway Obstruction From COPD With Dynamic Hyperinflation. Medicine 2016, 95, e3400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spector, B.M.; Shusterman, D.J.; Goldberg, A.N.; Weaver, E.M.; Farag, A.A.; Otto, B.A.; Zhao, K. Computational modeling of nasal nitric oxide flux from the paranasal sinuses: Validation against human experiment. Comput. Biol. Med. 2021, 136, 104723. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Reske-Kunz, A.B. The role of NO in contact hypersensitivity. Int. Immunopharmacol. 2001, 1, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Kroncke, K.D.; Fehsel, K.; Suschek, C.; Kolb-Bachofen, V. Inducible nitric oxide synthase-derived nitric oxide in gene regulation, cell death and cell survival. Int. Immunopharmacol. 2001, 1, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C.; Rollinghoff, M.; Diefenbach, A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 2000, 12, 64–76. [Google Scholar] [CrossRef]
- Armstrong, R. The physiological role and pharmacological potential of nitric oxide in neutrophil activation. Int. Immunopharmacol. 2001, 1, 1501–1512. [Google Scholar] [CrossRef]
- Flashner, B.M.; Rifas-Shiman, S.L.; Oken, E.; Camargo, C.A., Jr.; Platts-Mills, T.A.E.; Workman, L.; Litonjua, A.A.; Gold, D.R.; Rice, M.B. Contributions of asthma, rhinitis and IgE to exhaled nitric oxide in adolescents. ERJ Open Res. 2021, 7, 00945-2020. [Google Scholar] [CrossRef]
- Bauer, H.; Jung, T.; Tsikas, D.; Stichtenoth, D.O.; Frolich, J.C.; Neumann, C. Nitric oxide inhibits the secretion of T-helper 1- and T-helper 2-associated cytokines in activated human T cells. Immunology 1997, 90, 205–211. [Google Scholar] [CrossRef]
- van der Veen, R.C. Nitric oxide and T helper cell immunity. Int. Immunopharmacol. 2001, 1, 1491–1500. [Google Scholar] [CrossRef]
- Antosova, M.; Mokra, D.; Pepucha, L.; Plevkova, J.; Buday, T.; Sterusky, M.; Bencova, A. Physiology of nitric oxide in the respiratory system. Physiol. Res. 2017, 66, S159–S172. [Google Scholar] [CrossRef]
- Maniscalco, M.; Pelaia, G.; Sofia, M. Exhaled nasal nitric oxide during humming: Potential clinical tool in sinonasal disease? Biomark. Med. 2013, 7, 261–266. [Google Scholar] [CrossRef]
- Berghi, O.N.; Vrinceanu, D.; Cergan, R.; Dumitru, M.; Costache, A. Solanum melongena allergy (A comprehensive review). Exp. Ther. Med. 2021, 22, 1061. [Google Scholar] [CrossRef]
- American Thoracic, S.; European Respiratory, S. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am. J. Respir. Crit. Care Med. 2005, 171, 912–930. [Google Scholar] [CrossRef] [Green Version]
- Maniscalco, M.; Vitale, C.; Vatrella, A.; Molino, A.; Bianco, A.; Mazzarella, G. Fractional exhaled nitric oxide-measuring devices: Technology update. Med. Devices 2016, 9, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Maniscalco, M.; Faraone, S.; Sofia, M.; Molino, A.; Vatrella, A.; Zedda, A. Extended analysis of exhaled and nasal nitric oxide for the evaluation of chronic cough. Respir. Med. 2015, 109, 970–974. [Google Scholar] [CrossRef] [Green Version]
- de Laurentiis, G.; Maniscalco, M.; Cianciulli, F.; Stanziola, A.; Marsico, S.; Lundberg, J.O.; Weitzberg, E.; Sofia, M. Exhaled nitric oxide monitoring in COPD using a portable analyzer. Pulm. Pharmacol. Ther. 2008, 21, 689–693. [Google Scholar] [CrossRef] [Green Version]
- Colantonio, D.; Brouillette, L.; Parikh, A.; Scadding, G.K. Paradoxical low nasal nitric oxide in nasal polyposis. Clin. Exp. Allergy 2002, 32, 698–701. [Google Scholar] [CrossRef]
- Weitzberg, E.; Lundberg, J.O. Humming greatly increases nasal nitric oxide. Am. J. Respir. Crit. Care Med. 2002, 166, 144–145. [Google Scholar] [CrossRef]
- Maniscalco, M.; Weitzberg, E.; Sundberg, J.; Sofia, M.; Lundberg, J.O. Assessment of nasal and sinus nitric oxide output using single-breath humming exhalations. Eur. Respir. J. 2003, 22, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Benedict, J.J.; Lelegren, M.; Han, J.K.; Lam, K. Nasal Nitric Oxide as a Biomarker in the Diagnosis and Treatment of Sinonasal Inflammatory Diseases: A Review of the Literature. Ann. Otol. Rhinol. Laryngol. 2023, 132, 460–469. [Google Scholar] [CrossRef]
- Beydon, N.; Kouis, P.; Marthin, J.K.; Latzin, P.; Colas, M.; Davis, S.D.; Haarman, E.; Harris, A.L.; Hogg, C.; Kilbride, E.; et al. Nasal nitric oxide measurement in children for the diagnosis of primary ciliary dyskinesia: European Respiratory Society technical standard. Eur. Respir. J. 2023, 61, 2202031. [Google Scholar] [CrossRef] [PubMed]
- Suojalehto, H.; Vehmas, T.; Lindstrom, I.; Kennedy, D.W.; Kilpelainen, M.; Plosila, T.; Savukoski, S.; Sipila, J.; Varpula, M.; Wolff, H.; et al. Nasal nitric oxide is dependent on sinus obstruction in allergic rhinitis. Laryngoscope 2014, 124, E213–E218. [Google Scholar] [CrossRef] [PubMed]
- Boot, J.D.; de Kam, M.L.; Mascelli, M.A.; Miller, B.; van Wijk, R.G.; de Groot, H.; Cohen, A.F.; Diamant, Z. Nasal nitric oxide: Longitudinal reproducibility and the effects of a nasal allergen challenge in patients with allergic rhinitis. Allergy 2007, 62, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Nesic, V.S.; Djordjevic, V.Z.; Tomic-Spiric, V.; Dudvarski, Z.R.; Soldatovic, I.A.; Arsovic, N.A. Measuring nasal nitric oxide in allergic rhinitis patients. J. Laryngol. Otol. 2016, 130, 1064–1071. [Google Scholar] [CrossRef]
- Wen, Y.S.; Lin, C.Y.; Yang, K.D.; Hung, C.H.; Chang, Y.J.; Tsai, Y.G. Nasal nitric oxide is a useful biomarker for acute unilateral maxillary sinusitis in pediatric allergic rhinitis: A prospective observational cohort study. World Allergy Organ. J. 2019, 12, 100027. [Google Scholar] [CrossRef] [Green Version]
- Wen, S.; Cheng, S.; Xie, S.; Zhang, H.; Zhang, J.; Wang, F.; Xie, S.; Xie, Z.; Jiang, W. Predictive Value of Nasal Nitric Oxide and Serum NOS2 Levels in the Efficacy of Subcutaneous Immunotherapy in Pediatric Patients with Allergic Rhinitis. Mediat. Inflamm. 2022, 2022, 1679536. [Google Scholar] [CrossRef]
- Ambrosino, P.; Parrella, P.; Formisano, R.; Papa, A.; Spedicato, G.A.; Di Minno, M.N.D.; Motta, A.; Maniscalco, M. Clinical application of nasal nitric oxide measurement in allergic rhinitis: A systematic review and meta-analysis. Ann. Allergy Asthma Immunol. 2020, 125, 447–459.e5. [Google Scholar] [CrossRef]
- Yuksel, H.; Kirmaz, C.; Yilmaz, O.; Pinar, E.; Vatansever, S.; Degirmenci, P.B.; Ozbilgin, K. Nasal mucosal expression of nitric oxide synthases in patients with allergic rhinitis and its relation to asthma. Ann. Allergy Asthma Immunol. 2008, 100, 12–16. [Google Scholar] [CrossRef]
- Kang, B.H.; Chen, S.S.; Jou, L.S.; Weng, P.K.; Wang, H.W. Immunolocalization of inducible nitric oxide synthase and 3-nitrotyrosine in the nasal mucosa of patients with rhinitis. Eur. Arch. Otorhinolaryngol. 2000, 257, 242–246. [Google Scholar] [CrossRef]
- Olthoff, A.; Rohrbach, S.; Faber, M.; Gotz, W.; Laskawi, R. Neuronal nitric oxide synthase immunoreactivity in the nasal mucosa of patients with idiopathic and allergic rhinitis. ORL J. Otorhinolaryngol. Relat. Spec. 2002, 64, 180–185. [Google Scholar] [CrossRef]
- Takeno, S.; Osada, R.; Furukido, K.; Chen, J.H.; Yajin, K. Increased nitric oxide production in nasal epithelial cells from allergic patients--RT-PCR analysis and direct imaging by a fluorescence indicator: DAF-2 DA. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2001, 31, 881–888. [Google Scholar] [CrossRef]
- Giannessi, F.; Fattori, B.; Ursino, F.; Giambelluca, M.A.; Soldani, P.; Scavuzzo, M.C.; Ruffoli, R. Ultrastructural and ultracytochemical study of the human nasal respiratory epithelium in vasomotor rhinitis. Acta Otolaryngol. 2003, 123, 943–949. [Google Scholar] [CrossRef]
- Sadek, A.A.; Abdelwahab, S.; Eid, S.Y.; Almaimani, R.A.; Althubiti, M.A.; El-Readi, M.Z. Overexpression of Inducible Nitric Oxide Synthase in Allergic and Nonallergic Nasal Polyp. Oxid. Med. Cell Longev. 2019, 2019, 7506103. [Google Scholar] [CrossRef]
- Cardell, L.O.; Agusti, C.; Nadel, J.A. Nitric oxide-dependent neutrophil recruitment: Role in nasal secretion. Clin. Exp. Allergy 2000, 30, 1799–1803. [Google Scholar] [CrossRef]
- Maniscalco, M.; Bianco, A.; Mazzarella, G.; Motta, A. Recent Advances on Nitric Oxide in the Upper Airways. Curr. Med. Chem. 2016, 23, 2736–2745. [Google Scholar] [CrossRef]
- Lee, K.J.; Cho, S.H.; Lee, S.H.; Tae, K.; Yoon, H.J.; Kim, S.H.; Jeong, J.H. Nasal and exhaled nitric oxide in allergic rhinitis. Clin. Exp. Otorhinolaryngol. 2012, 5, 228–233. [Google Scholar] [CrossRef]
- Moody, A.; Fergusson, W.; Wells, A.; Bartley, J.; Kolbe, J. Nasal levels of nitric oxide as an outcome variable in allergic upper respiratory tract disease: Influence of atopy and hayfever on nNO. Am. J. Rhinol. 2006, 20, 425–429. [Google Scholar] [CrossRef]
- Henriksen, A.H.; Sue-Chu, M.; Holmen, T.L.; Langhammer, A.; Bjermer, L. Exhaled and nasal NO levels in allergic rhinitis: Relation to sensitization, pollen season and bronchial hyperresponsiveness. Eur. Respir. J. 1999, 13, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Williamson, P.A.; Vaidyanathan, S.; Clearie, K.; Stewart, M.; Lipworth, B.J. Relationship between fractional exhaled nitric oxide and nasal nitric oxide in airways disease. Ann. Allergy Asthma Immunol. 2010, 105, 162–167. [Google Scholar] [CrossRef]
- Hou, J.; Lou, H.; Wang, Y.; He, F.; Cao, F.; Wang, C.; Zhang, L. Nasal ventilation is an important factor in evaluating the diagnostic value of nasal nitric oxide in allergic rhinitis. Int. Forum. Allergy Rhinol. 2018, 8, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, P.; Molino, A.; Spedicato, G.A.; Parrella, P.; Formisano, R.; Motta, A.; Di Minno, M.N.D.; Maniscalco, M. Nasal Nitric Oxide in Chronic Rhinosinusitis with or without Nasal Polyps: A Systematic Review with Meta-Analysis. J. Clin. Med. 2020, 9, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundberg, J.O.; Maniscalco, M.; Sofia, M.; Lundblad, L.; Weitzberg, E. Humming, nitric oxide, and paranasal sinus obstruction. JAMA 2003, 289, 302–303. [Google Scholar] [CrossRef] [PubMed]
- Kiss, H.; Orlos, Z.; Gellert, A.; Megyesfalvi, Z.; Mikaczo, A.; Sarkozi, A.; Vasko, A.; Miklos, Z.; Horvath, I. Exhaled Biomarkers for Point-of-Care Diagnosis: Recent Advances and New Challenges in Breathomics. Micromachines 2023, 14, 391. [Google Scholar] [CrossRef] [PubMed]
- Takahara, D.; Kono, T.; Takeno, S.; Ishino, T.; Hamamoto, T.; Kubota, K.; Ueda, T. Nasal nitric oxide in the inferior turbinate surface decreases with intranasal steroids in allergic rhinitis: A prospective study. Auris Nasus Larynx 2019, 46, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Struben, V.M.; Wieringa, M.H.; Feenstra, L.; de Jongste, J.C. Nasal nitric oxide and nasal allergy. Allergy 2006, 61, 665–670. [Google Scholar] [CrossRef]
- Vo-Thi-Kim, A.; Van-Quang, T.; Nguyen-Thanh, B.; Dao-Van, D.; Duong-Quy, S. The effect of medical treatment on nasal exhaled nitric oxide (NO) in patients with persistent allergic rhinitis: A randomized control study. Adv. Med. Sci. 2020, 65, 182–188. [Google Scholar] [CrossRef]
- Wald, E.R.; Applegate, K.E.; Bordley, C.; Darrow, D.H.; Glode, M.P.; Marcy, S.M.; Nelson, C.E.; Rosenfeld, R.M.; Shaikh, N.; Smith, M.J.; et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics 2013, 132, e262–e280. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.P.; Wang, G.X.; Ge, W.T.; Tang, L.X.; Zhang, J.; Ni, X. Nasal nitric oxide in allergic rhinitis in children and its relationship to severity and treatment. Allergy Asthma Clin. Immunol. 2017, 13, 20. [Google Scholar] [CrossRef] [Green Version]
- Costache, A.; Berghi, O.N.; Cergan, R.; Dumitru, M.; Neagos, A.; Popa, L.G.; Giurcaneanu, C.; Vrinceanu, D. Respiratory allergies: Salicaceae sensitization (Review). Exp. Ther. Med. 2021, 21, 609. [Google Scholar] [CrossRef]
- Duong-Quy, S. Clinical Utility of the Exhaled Nitric Oxide (NO) Measurement with Portable Devices in the Management of Allergic Airway Inflammation and Asthma. J. Asthma Allergy 2019, 12, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Huss-Marp, J.; Kramer, U.; Eberlein, B.; Pfab, F.; Ring, J.; Behrendt, H.; Gulyas, A.F. Reduced exhaled nitric oxide values in children with asthma after inpatient rehabilitation at high altitude. J. Allergy Clin. Immunol. 2007, 120, 471–472. [Google Scholar] [CrossRef]
- Clini, E.; Bianchi, L.; Foglio, K.; Porta, R.; Vitacca, M.; Ambrosino, N. Effect of pulmonary rehabilitation on exhaled nitric oxide in patients with chronic obstructive pulmonary disease. Thorax 2001, 56, 519–523. [Google Scholar] [CrossRef]
- Leigh, M.W.; Hazucha, M.J.; Chawla, K.K.; Baker, B.R.; Shapiro, A.J.; Brown, D.E.; Lavange, L.M.; Horton, B.J.; Qaqish, B.; Carson, J.L.; et al. Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia. Ann. Am. Thorac. Soc. 2013, 10, 574–581. [Google Scholar] [CrossRef] [Green Version]
- Sanders, S.P.; Proud, D.; Permutt, S.; Siekierski, E.S.; Yachechko, R.; Liu, M.C. Role of nasal nitric oxide in the resolution of experimental rhinovirus infection. J. Allergy Clin. Immunol. 2004, 113, 697–702. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcuccio, G.; Ambrosino, P.; Merola, C.; Manzo, F.; Motta, A.; Rea, G.; Cantone, E.; Maniscalco, M. Clinical Applications of Nasal Nitric Oxide in Allergic Rhinitis: A Review of the Literature. J. Clin. Med. 2023, 12, 5081. https://doi.org/10.3390/jcm12155081
Marcuccio G, Ambrosino P, Merola C, Manzo F, Motta A, Rea G, Cantone E, Maniscalco M. Clinical Applications of Nasal Nitric Oxide in Allergic Rhinitis: A Review of the Literature. Journal of Clinical Medicine. 2023; 12(15):5081. https://doi.org/10.3390/jcm12155081
Chicago/Turabian StyleMarcuccio, Giuseppina, Pasquale Ambrosino, Claudia Merola, Fabio Manzo, Andrea Motta, Gaetano Rea, Elena Cantone, and Mauro Maniscalco. 2023. "Clinical Applications of Nasal Nitric Oxide in Allergic Rhinitis: A Review of the Literature" Journal of Clinical Medicine 12, no. 15: 5081. https://doi.org/10.3390/jcm12155081
APA StyleMarcuccio, G., Ambrosino, P., Merola, C., Manzo, F., Motta, A., Rea, G., Cantone, E., & Maniscalco, M. (2023). Clinical Applications of Nasal Nitric Oxide in Allergic Rhinitis: A Review of the Literature. Journal of Clinical Medicine, 12(15), 5081. https://doi.org/10.3390/jcm12155081









