Comparing Left Ventricular Diastolic Function between Peritoneal Dialysis and Non-Dialysis Patients with Stage 5 Chronic Kidney Disease: A Propensity Score-Matched Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. Conventional Echocardiographic Study
2.3. Assessment of the Volume Status
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kuznetsova, T.; Herbots, L.; Lopez, B.; Jin, Y.; Richart, T.; Thijs, L.; Gonzalez, A.; Herregods, M.C.; Fagard, R.H.; Diez, J.; et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ. Heart Fail. 2009, 2, 105–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogelvang, R.; Sogaard, P.; Pedersen, S.A.; Olsen, N.T.; Marott, J.L.; Schnohr, P.; Goetze, J.P.; Jensen, J.S. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation 2009, 119, 2679–2685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuznetsova, T.; Thijs, L.; Knez, J.; Herbots, L.; Zhang, Z.; Staessen, J.A. Prognostic value of left ventricular diastolic dysfunction in a general population. J. Am. Heart Assoc. 2014, 3, e000789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, A.C.; Bienstock, S.W.; Sharma, R.; Skorecki, K.; Beerkens, F.; Samtani, R.; Coyle, A.; Kim, T.; Baber, U.; Camaj, A.; et al. A personalized approach to chronic kidney disease and cardiovascular disease: JACC review topic of the week. J. Am. Coll. Cardiol. 2021, 77, 1470–1479. [Google Scholar] [CrossRef]
- Farshid, A.; Pathak, R.; Shadbolt, B.; Arnolda, L.; Talaulikar, G. Diastolic function is a strong predictor of mortality in patients with chronic kidney disease. BMC Nephrol. 2013, 14, 280. [Google Scholar] [CrossRef] [Green Version]
- Otsuka, T.; Suzuki, M.; Yoshikawa, H.; Sugi, K. Left ventricular diastolic dysfunction in the early stage of chronic kidney disease. J. Cardiol. 2009, 54, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Hsu, C.Y.; Li, Y.M.; Mishra, R.K.; Keane, M.; Rosas, S.E.; Dries, D.; Xie, D.W.; Chen, J.; He, J.; et al. Associations between kidney function and subclinical cardiac abnormalities in CKD. J. Am. Soc. Nephrol. 2012, 23, 1725–1734. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, J.C.; Farand, P.; Do, H.D.; Brochu, M.C.; Bonenfant, F.; Lepage, S. Effect of age and sex on echocardiographic left ventricular diastolic function parameters in patients with preserved ejection fraction and normal valvular function. Cardiol. J. 2013, 20, 513–518. [Google Scholar] [CrossRef] [Green Version]
- Russo, C.; Jin, Z.; Homma, S.; Rundek, T.; Elkind, M.S.; Sacco, R.L.; Di Tullio, M.R. Effect of diabetes and hypertension on left ventricular diastolic function in a high-risk population without evidence of heart disease. Eur. J. Heart Fail. 2010, 12, 454–461. [Google Scholar] [CrossRef] [Green Version]
- Russo, C.; Jin, Z.; Homma, S.; Rundek, T.; Elkind, M.S.; Sacco, R.L.; Di Tullio, M.R. Effect of obesity and overweight on left ventricular diastolic function: A community-based study in an elderly cohort. J. Am. Coll. Cardiol. 2011, 57, 1368–1374. [Google Scholar] [CrossRef] [Green Version]
- Maragiannis, D.; Schutt, R.C.; Gramze, N.L.; Chaikriangkrai, K.; McGregor, K.; Chin, K.; Nabi, F.; Little, S.H.; Nagueh, S.F.; Chang, S.M. Association of left ventricular diastolic dysfunction with subclinical coronary atherosclerotic disease burden using coronary artery calcium scoring. J. Atheroscler. Thromb. 2015, 22, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Law, J.P.; Pickup, L.; Pavlovic, D.; Townend, J.N.; Ferro, C.J. Hypertension and cardiomyopathy associated with chronic kidney disease: Epidemiology, pathogenesis and treatment considerations. J. Hum. Hypertens. 2023, 37, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Escoli, R.; Carvalho, M.J.; Cabrita, A.; Rodrigues, A. Diastolic dysfunction, an underestimated new callenge in dalysis. Ther. Apher. Dial. 2019, 23, 108–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, T.; Nitta, K. Clinical impact of left ventricular diastolic dysfunction in chronic kidney disease. Contrib. Nephrol. 2018, 195, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Han, B.G.; Lee, J.Y.; Kim, M.R.; Shin, H.; Kim, J.S.; Yang, J.W.; Kim, J.Y. Fluid overload is a determinant for cardiac structural and functional impairments in type 2 diabetes mellitus and chronic kidney disease stage 5 not undergoing dialysis. PLoS ONE 2020, 15, e0235640. [Google Scholar] [CrossRef]
- Kim, J.S.; Yang, J.W.; Yoo, J.S.; Choi, S.O.; Han, B.G. Association between E/e´ ratio and fluid overload in patients with predialysis chronic kidney disease. PLoS ONE 2017, 12, e0184764. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Chiu, Y.W.; Tsai, J.C.; Kuo, H.T.; Hung, C.C.; Hwang, S.J.; Chen, T.H.; Kuo, M.C.; Chen, H.C. Association of fluid overload with cardiovascular morbidity and all-cause mortality in stages 4 and 5 CKD. Clin. J. Am. Soc. Nephrol. 2015, 10, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Tuegel, C.; Bansal, N. Heart failure in patients with kidney disease. Heart 2017, 103, 1848–1853. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [Green Version]
- Moissl, U.M.; Wabel, P.; Chamney, P.W.; Bosaeus, I.; Levin, N.W.; Bosy-Westphal, A.; Korth, O.; Muller, M.J.; Ellegard, L.; Malmros, V.; et al. Body fluid volume determination via body composition spectroscopy in health and disease. Physiol. Meas. 2006, 27, 921–933. [Google Scholar] [CrossRef]
- Chamney, P.W.; Wabel, P.; Moissl, U.M.; Muller, M.J.; Bosy-Westphal, A.; Korth, O.; Fuller, N.J. A whole-body model to distinguish excess fluid from the hydration of major body tissues. Am. J. Clin. Nutr. 2007, 85, 80–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wabel, P.; Chamney, P.; Moissl, U.; Jirka, T. Importance of whole-body bioimpedance spectroscopy for the management of fluid balance. Blood Purif. 2009, 27, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Waki, M.; Kral, J.G.; Mazariegos, M.; Wang, J.; Pierson, R.N., Jr.; Heymsfield, S.B. Relative expansion of extracellular fluid in obese vs. nonobese women. Am. J. Physiol. 1991, 261, E199–E203. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011, 10, 150–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, S.; James, M.; Wiebe, N.; Hemmelgarn, B.; Manns, B.; Klarenbach, S.; Tonelli, M. Cause of death in patients with reduced kidney function. J. Am. Soc. Nephrol. 2015, 26, 2504–2511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, S.H.; Oh, T.R.; Choi, H.S.; Kim, C.S.; Bae, E.H.; Oh, K.H.; Choi, K.H.; Oh, Y.K.; Ma, S.K.; Kim, S.W. Association of left ventricular diastolic dysfunction with cardiovascular outcomes in patients with pre-dialysis chronic kidney disease: Findings from KNOW-CKD study. Front. Cardiovasc. Med. 2022, 9, 844312. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Kim, B.; Lee, J.Y.; Kim, J.S.; Han, B.G.; Choi, S.O.; Yang, J.W. Tissue Doppler-derived E/e′ ratio as a parameter for assessing diastolic heart failure and as a predictor of mortality in patients with chronic kidney disease. Korean J. Intern. Med. 2013, 28, 35–44. [Google Scholar] [CrossRef]
- Ogawa, T.; Koeda, M.; Nitta, K. Left ventricular diastolic dysfunction in end-stage kidney disease: Pathogenesis, diagnosis, and treatment. Ther. Apher. Dial. 2015, 19, 427–435. [Google Scholar] [CrossRef]
- Hsu, H.C.; Norton, G.R.; Robinson, C.; Woodiwiss, A.J.; Dessein, P.H. Potential determinants of the E/e’ ratio in non-dialysis compared with dialysis patients. Nephrology 2021, 26, 988–998. [Google Scholar] [CrossRef]
- Miyajima, Y.; Toyama, T.; Mori, M.; Nakade, Y.; Sato, K.; Yamamura, Y.; Ogura, H.; Yoneda-Nakagawa, S.; Oshima, M.; Miyagawa, T.; et al. Relationships between kidney dysfunction and left ventricular diastolic dysfunction: A hospital-based retrospective study. J. Nephrol. 2021, 34, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shapiro, J.I. Evolving concepts in the pathogenesis of uraemic cardiomyopathy. Nat. Rev. Nephrol. 2019, 15, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Takeda, K.; Turuya, K.; Mukai, H.; Muto, Y.; Okuda, H.; Furusho, M.; Ueno, T.; Nakashita, S.; Miura, S.; et al. Left ventricular mass index is an independent determinant of diastolic dysfunction in patients on chronic hemodialysis: A tissue Doppler imaging study. Nephron Clin. Pract. 2011, 117, C67–C73. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.R.; Lee, Y.K.; Park, H.C.; Kim, D.H.; Cho, A.; Kang, M.K.; Choi, S. Clinical significance of central systolic blood pressure in LV diastolic dysfunction and CV mortality. PLoS ONE 2021, 16, e0250653. [Google Scholar] [CrossRef]
- Okura, H. Subclinical diastolic dysfunction in diabetes: How to detect, how to manage? Eur. Heart J. Cardiovasc. Imaging 2020, 21, 885–886. [Google Scholar] [CrossRef]
- Bouthoorn, S.; Valstar, G.B.; Gohar, A.; den Ruijter, H.M.; Reitsma, H.B.; Hoes, A.W.; Rutten, F.H. The prevalence of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction in men and women with type 2 diabetes: A systematic review and meta-analysis. Diab. Vasc. Dis. Res. 2018, 15, 477–493. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Wang, H.; Jessup, J.A.; Lindsey, S.H.; Chappell, M.C.; Groban, L. Role of estrogen in diastolic dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H628–H640. [Google Scholar] [CrossRef] [Green Version]
- van Ommen, A.; Canto, E.D.; Cramer, M.J.; Rutten, F.H.; Onland-Moret, N.C.; Ruijter, H.M.D. Diastolic dysfunction and sex-specific progression to HFpEF: Current gaps in knowledge and future directions. BMC Med. 2022, 20, 496. [Google Scholar] [CrossRef]
- Ronco, C.; Verger, C.; Crepaldi, C.; Pham, J.; De Los Rios, T.; Gauly, A.; Wabel, P.; Van Biesen, W.; Ipod-Pd Study Group. Baseline hydration status in incident peritoneal dialysis patients: The initiative of patient outcomes in dialysis (IPOD-PD study). Nephrol. Dial. Transplant. 2015, 30, 849–858. [Google Scholar] [CrossRef]
- Oh, K.H.; Baek, S.H.; Joo, K.W.; Kim, D.K.; Kim, Y.S.; Kim, S.; Oh, Y.K.; Han, B.G.; Chang, J.H.; Chung, W.; et al. Does routine bioimpedance-guided fluid management provide additional benefit to non-anuric peritoneal dialysis patients? Results from COMPASS clinical trial. Perit. Dial. Int. 2018, 38, 131–138. [Google Scholar] [CrossRef]
- Dekker, M.J.E.; van der Sande, F.M.; van den Berghe, F.; Leunissen, K.M.L.; Kooman, J.P. Fluid overload and inflammation axis. Blood Purif. 2018, 45, 159–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, M.; Tian, N.; Liu, Y.Q.; Li, W.; Lin, H.; Fan, R.; Li, C.L.; Liu, D.H.; Yao, F.J. High serum phosphorus level is associated with left ventricular diastolic dysfunction in peritoneal dialysis patients. PLoS ONE 2016, 11, e0163659. [Google Scholar] [CrossRef]
- Valdivielso More, S.; Vicente Elcano, M.; Garcia Alonso, A.; Pascual Sanchez, S.; Galceran Herrera, I.; Barbosa Puig, F.; Belarte-Tornero, L.C.; Ruiz-Bustillo, S.; Morales Murillo, R.O.; Barrios, C.; et al. Characteristics of patients with heart failure and advanced chronic kidney disease (Stages 4–5) not undergoing renal replacement therapy (ERCA-IC Study). J. Clin. Med. 2023, 12, 2339. [Google Scholar] [CrossRef] [PubMed]
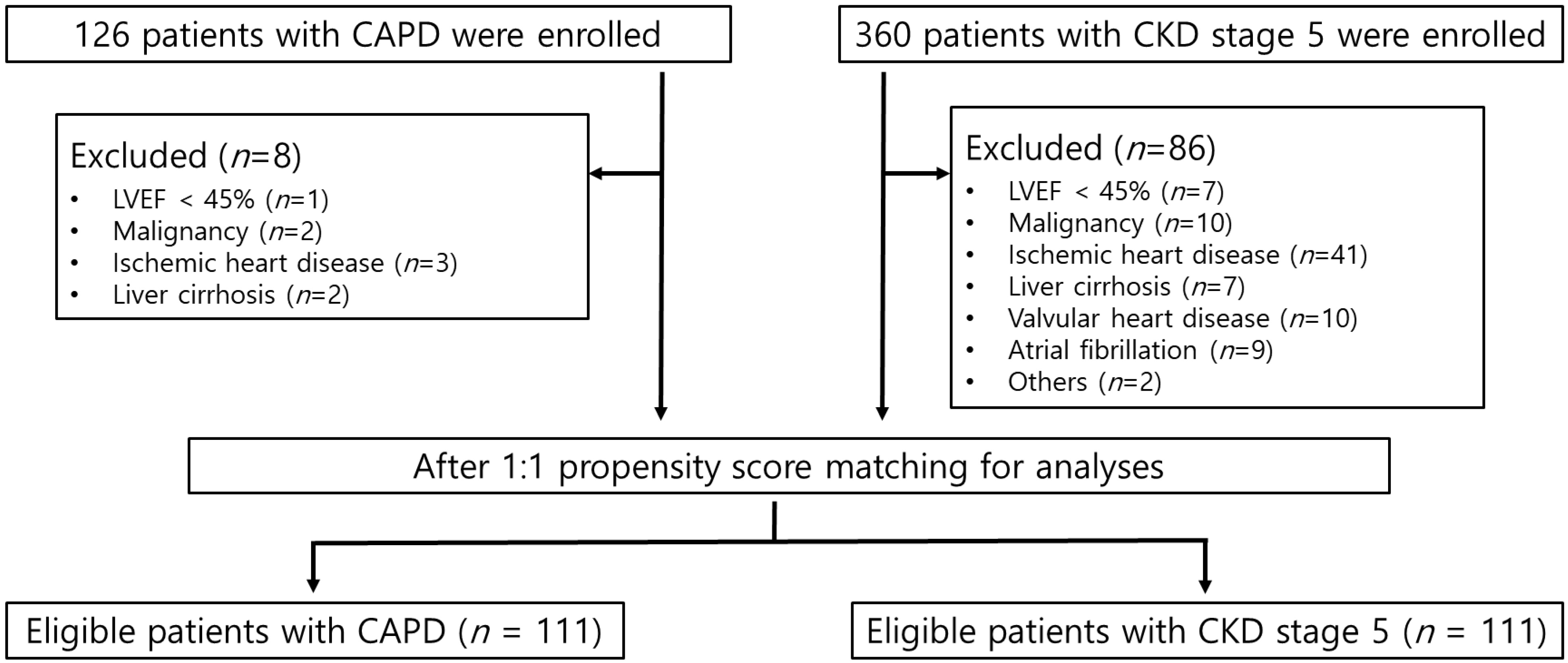
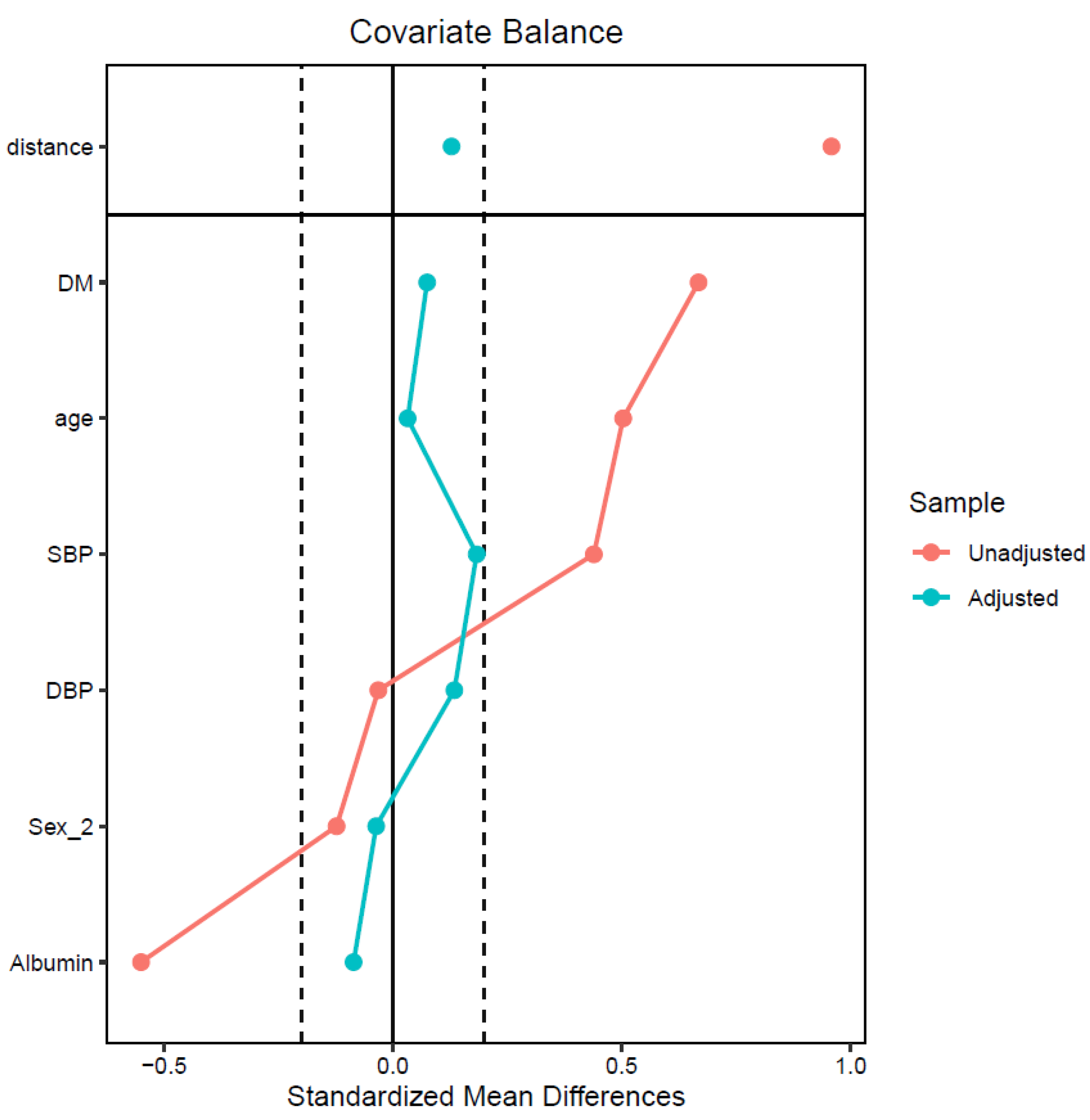
| Before Propensity Score Matching | After Propensity Score Matching | |||||
|---|---|---|---|---|---|---|
| CAPD | CKD5 | SMD | CAPD | CKD5 | SMD | |
| No. of patients | 118 | 274 | 111 | 111 | ||
| Age, years | 52.40 ± 13.06 | 59.28 ± 13.69 | 0.50 | 53.75 ± 12.12 | 54.19 ± 13.60 | 0.03 |
| Sex | 1.49 | 1.43 | −0.12 | 1.50 | 1.48 | −0.04 |
| Diabetes | 0.31 | 0.64 | 0.67 | 0.33 | 0.37 | 0.07 |
| SBP, mmHg | 134.36 | 142.92 | 0.44 | 133.56 | 137.12 | 0.18 |
| DBP, mmHg | 81.02 | 80.65 | −0.03 | 80.42 | 81.96 | 0.13 |
| Albumin, g/dL | 3.80 | 3.50 | −0.55 | 3.79 | 3.74 | −0.09 |
| Variables | Total (n = 222) | CAPD (n = 111) | CKD5 (n = 111) | |||
|---|---|---|---|---|---|---|
| Correlation Coefficient | p-Value | Correlation Coefficient | p-Value | Correlation Coefficient | p-Value | |
| Age, years | 0.093 | 0.168 | 0.127 | 0.183 | 0.062 | 0.520 |
| SBP, mmHg | 0.235 | <0.001 | 0.141 | 0.139 | 0.302 | 0.001 |
| DBP, mmHg | 0.053 | 0.433 | −0.018 | 0.850 | 0.093 | 0.329 |
| BMI, kg/m2 | 0.152 | 0.023 | 0.118 | 0.219 | 0.097 | 0.309 |
| LAD, cm | 0.182 | 0.007 | 0.093 | 0.330 | 0.306 | 0.001 |
| LAVI, mL/m2 | 0.345 | <0.001 | 0.362 | <0.001 | 0.394 | <0.001 |
| LVEDV, mL | 0.252 | <0.001 | 0.159 | 0.105 | 0.231 | 0.015 |
| LVMI, g/m2 | 0.325 | <0.001 | 0.219 | 0.021 | 0.409 | <0.001 |
| LVEF, % | −0.097 | 0.151 | −0.235 | 0.014 | 0.036 | 0.709 |
| hs-CRP, mg/dL | 0.085 | 0.213 | 0.219 | 0.021 | 0.020 | 0.836 |
| iPTH, pg/mL | −0.032 | 0.639 | −0.151 | 0.118 | −0.003 | 0.973 |
| Hemoglobin, g/dL | −0.148 | 0.028 | −0.037 | 0.697 | −0.069 | 0.470 |
| Total protein, g/dL | −0.169 | 0.012 | −0.046 | 0.628 | −0.184 | 0.053 |
| Albumin, g/dL | −0.176 | 0.009 | −0.129 | 0.177 | −0.197 | 0.039 |
| Calcium, mg/dL | −0.162 | 0.016 | 0.051 | 0.597 | −0.178 | 0.061 |
| Phosphorus, mg/dL | 0.139 | 0.093 | 0.060 | 0.534 | 0.114 | 0.233 |
| OH/ECW, % | 0.291 | <0.001 | 0.147 | 0.124 | 0.399 | <0.001 |
| Variables | CAPD (n = 111) | CKD5 (n = 111) | |||||
|---|---|---|---|---|---|---|---|
| E/e′ Ratio ≤ 15 (n = 96) | E/e′ Ratio > 15 (n = 15) | p-Value | E/e′ Ratio ≤ 15 (n = 84) | E/e′ Ratio > 15 (n = 27) | p-Value | ||
| E/e′ ratio | 9.8672 ± 2.65 | 19.38 ± 4.52 | <0.001 | 10.82 ± 2.76 | 19.37 ± 3.84 | <0.001 | |
| Age, years | 53.01 ± 12.48 | 58.47 ± 8.43 | 0.105 | 54.35 ± 13.87 | 53.70 ± 12.99 | 0.832 | |
| Sex | Male | 50 (89.3%) | 6 (10.7%) | 0.384 | 45 (77.6%) | 13 (22.4%) | 0.624 |
| Female | 46 (83.6%) | 9 (16.4%) | 39 (73.6%) | 14 (26.4%) | |||
| Diabetes | Yes | 27 (73.0%) | 10 (27.0%) | 0.003 | 24 (58.5%) | 17 (41.5%) | 0.001 |
| No | 69 (93.2%) | 5 (6.8%) | 60 (85.7%) | 10 (14.3%) | |||
| OH/ECW | <15% | 74 (85.1%) | 13 (14.9%) | 0.517 | 70 (83.3%) | 14 (16.7%) | 0.001 |
| ≥15% | 22 (91.7%) | 2 (8.3%) | 14 (51.9%) | 13 (48.1%) | |||
| SBP, mmHg | 132.61 ± 22.66 | 139.60 ± 17.50 | 0.257 | 135.24 ± 20.78 | 143.00 ± 22.40 | 0.100 | |
| DBP, mmHg | 80.76 ± 12.64 | 78.27 ± 10.41 | 0.469 | 82.04 ± 13.29 | 81.74 ± 11.85 | 0.918 | |
| BMI, kg/m2 | 22.97 ± 3.16 | 24.52 ± 4.06 | 0.094 | 25.03 ± 4.19 | 25.33 ± 3.45 | 0.735 | |
| LAD, cm | 4.48 ± 0.51 | 4.54 ± 0.49 | 0.613 | 4.38 ± 0.46 | 4.58 ± 0.44 | 0.053 | |
| LAVI, mL/m2 | 35.51 ± 14.52 | 43.39 ± 17.80 | 0.078 | 33.91 ± 7.94 | 40.85 ± 13.65 | 0.017 | |
| LVEDV, mL | 98.86 ± 44.02 | 107.26 ± 52.10 | 0.518 | 139.94 ± 25.59 | 150.07 ± 28.36 | 0.084 | |
| LVMI, g/m2 | 101.90 ± 28.23 | 112.60 ± 28.49 | 0.176 | 104.07 ± 24.72 | 120.41 ± 32.33 | 0.021 | |
| LVEF, % | 62.43 ± 5.14 | 60.58 ± 10.67 | 0.520 | 62.66 ± 5.12 | 61.63 ± 5.48 | 0.375 | |
| hs-CRP, mg/dL | 0.19 ± 0.40 | 0.55 ± 1.00 | 0.192 | 0.90 ± 2.35 | 0.54 ± 1.14 | 0.475 | |
| iPTH, pg/mL | 289.28 ± 214.61 | 181.56 ± 119.29 | 0.010 | 347.09 ± 254.10 | 342.21 ± 164.83 | 0.926 | |
| Hemoglobin, g/dL | 10.60 ± 1.46 | 10.49 ± 1.34 | 0.777 | 9.08 ± 1.27 | 8.97 ± 0.80 | 0.602 | |
| Total protein, g/dL | 6.64 ± 0.55 | 6.56 ± 0.68 | 0.631 | 6.36 ± 0.72 | 6.15 ± 0.80 | 0.211 | |
| Albumin, g/dL | 3.80 ± 0.35 | 3.70 ± 0.36 | 0.305 | 3.76 ± 0.49 | 3.66 ± 0.52 | 0.363 | |
| Calcium, mg/dL | 8.84 ± 0.89 | 8.71 ± 0.87 | 0.615 | 7.89 ± 1.21 | 7.75 ± 1.14 | 0.612 | |
| Phosphorus, mg/dL | 5.16 ± 1.14 | 5.00 ± 1.09 | 0.603 | 5.79 ± 1.55 | 5.92 ± 1.13 | 0.692 | |
| OH/ECW, % | 9.22 ± 10.07 | 10.14 ± 5.24 | 0.591 | 6.38 ± 13.03 | 17.46 ± 15.66 | 0.002 | |
| Total (n = 222) | CAPD (n = 111) | CKD5 (n = 111) | ||||
|---|---|---|---|---|---|---|
| B (95% CI) | p-Value | B (95% CI) | p-Value | B (95% CI) | p-Value | |
| Group | 1.616 (0.551, 2.682) | 0.003 | ||||
| OH/ECW, % | 0.044 (−0.006, 0.095) | 0.087 | −0.015 (−0.109, 0.078) | 0.744 | 0.064 (0.001, 0.126) | 0.047 |
| Age, years | 0.031 (−0.011, 0.073) | 0.151 | 0.035 (−0.109, 0.078) | 0.289 | 0.025 (−0.032, 0.083) | 0.387 |
| Sex | 2.145 (1.042, 3.247) | <0.001 | 2.729 (1.087, 4.370) | 0.001 | 1.481 (−0.055, 3.017) | 0.059 |
| Diabetes | 2.583 (1.312, 3.853) | <0.001 | 3.180 (1.303, 5.058) | 0.001 | 2.372 (0.594, 4.151) | 0.009 |
| LVMI, g/m2 | 0.053 (0.032, 0.073) | <0.001 | 0.049 (0.020, 0.078) | 0.001 | 0.060 (0.031, 0.090) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, B.-G.; Seol, J.H.; Choi, S.; Shin, D.; Kim, J.-S.; Kim, Y.H. Comparing Left Ventricular Diastolic Function between Peritoneal Dialysis and Non-Dialysis Patients with Stage 5 Chronic Kidney Disease: A Propensity Score-Matched Analysis. J. Clin. Med. 2023, 12, 5092. https://doi.org/10.3390/jcm12155092
Han B-G, Seol JH, Choi S, Shin D, Kim J-S, Kim YH. Comparing Left Ventricular Diastolic Function between Peritoneal Dialysis and Non-Dialysis Patients with Stage 5 Chronic Kidney Disease: A Propensity Score-Matched Analysis. Journal of Clinical Medicine. 2023; 12(15):5092. https://doi.org/10.3390/jcm12155092
Chicago/Turabian StyleHan, Byoung-Geun, Jae Hee Seol, Sooyeon Choi, Donghui Shin, Jae-Seok Kim, and Yong Hyuk Kim. 2023. "Comparing Left Ventricular Diastolic Function between Peritoneal Dialysis and Non-Dialysis Patients with Stage 5 Chronic Kidney Disease: A Propensity Score-Matched Analysis" Journal of Clinical Medicine 12, no. 15: 5092. https://doi.org/10.3390/jcm12155092





