Sex-Based Differences in Clinical Characteristics and Outcomes among Patients with Peripheral Artery Disease: A Retrospective Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Variables
- Demographic data: age and sex.
- Clinical presentation at the ED admission including dyspnoea, fever, pain (defined as any degree and type of pain in the lower extremities), chest pain and neurological symptoms.
- Peripheral artery disease grade at ED presentation, according to Rutherford classification.
- Coexistent acute infections diagnosed at ED admission, categorized as bloodstream infections, lower limb and other infections not included in the previous categories.
- Laboratory findings including blood creatinine, eGFR (estimated Glomerular Filtration Rate calculated according to the CKD-EPI equation), urea, glucose, glycated haemoglobin (HbA1c), C-reactive protein (CRP), total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides, haemoglobin (Hb) and platelets (PLTs).
- Clinical history and comorbidities: CAD, congestive heart failure (CHF), cerebral vascular disease—previous stroke (CVD), type 2 diabetes mellitus (T2DM), chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD) and dementia. Overall comorbidity severity was assessed by Charlson Comorbidity Index (CCI) [20].
2.3. Study Endpoints
2.4. Statistical Analysis
3. Results
3.1. Study Cohort and Baseline Characteristics
3.2. MALE Composite Outcome
3.3. MACE Composite Outcome
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Criqui, M.H.; Aboyans, V. Epidemiology of Peripheral Artery Disease. Circ. Res. 2015, 116, 1509–1526. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.I.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, Regional, and National Prevalence and Risk Factors for Peripheral Artery Disease in 2015: An Updated Systematic Review and Analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef]
- Hirsch, A.T. Peripheral Arterial Disease Detection, Awareness, and Treatment in Primary Care. JAMA 2001, 286, 1317. [Google Scholar] [CrossRef]
- Fowkes, F.G.R.; Aboyans, V.; Fowkes, F.J.I.; McDermott, M.M.; Sampson, U.K.A.; Criqui, M.H. Peripheral Artery Disease: Epidemiology and Global Perspectives. Nat. Rev. Cardiol. 2017, 14, 156–170. [Google Scholar] [CrossRef]
- Biscetti, F.; Bonadia, N.; Santini, F.; Angelini, F.; Nardella, E.; Pitocco, D.; Santoliquido, A.; Filipponi, M.; Landolfi, R.; Flex, A. Sortilin Levels Are Associated with Peripheral Arterial Disease in Type 2 Diabetic Subjects. Cardiovasc. Diabetol. 2019, 18, 5. [Google Scholar] [CrossRef]
- Biscetti, F.; Ferraro, P.M.; Hiatt, W.R.; Angelini, F.; Nardella, E.; Cecchini, A.L.; Santoliquido, A.; Pitocco, D.; Landolfi, R.; Flex, A. Inflammatory Cytokines Associated with Failure of Lower-Extremity Endovascular Revascularization (LER): A Prospective Study of a Population with Diabetes. Diabetes Care 2019, 42, 1939–1945. [Google Scholar] [CrossRef]
- Vaccarino, V.; Rathore, S.S.; Wenger, N.K.; Frederick, P.D.; Abramson, J.L.; Barron, H.V.; Manhapra, A.; Mallik, S.; Krumholz, H.M. Sex and Racial Differences in the Management of Acute Myocardial Infarction, 1994 through 2002. N. Engl. J. Med. 2005, 353, 671–682. [Google Scholar] [CrossRef]
- Vyas, M.V.; Silver, F.L.; Austin, P.C.; Yu, A.Y.X.; Pequeno, P.; Fang, J.; Laupacis, A.; Kapral, M.K. Stroke Incidence by Sex Across the Lifespan. Stroke 2021, 52, 447–451. [Google Scholar] [CrossRef]
- Rexrode, K.M.; Madsen, T.E.; Yu, A.Y.X.; Carcel, C.; Lichtman, J.H.; Miller, E.C. The Impact of Sex and Gender on Stroke. Circ. Res. 2022, 130, 512–528. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Bairey Merz, C.N.; Berry, C.; Samuel, R.; Saw, J.; Smilowitz, N.R.; de Souza, A.C.D.A.; Sykes, R.; Taqueti, V.R.; Wei, J. Coronary Arterial Function and Disease in Women with No Obstructive Coronary Arteries. Circ. Res. 2022, 130, 529–551. [Google Scholar] [CrossRef] [PubMed]
- Canto, J.G.; Rogers, W.J.; Goldberg, R.J.; Peterson, E.D.; Wenger, N.K.; Vaccarino, V.; Kiefe, C.I.; Frederick, P.D.; Sopko, G.; Zheng, Z.-J.; et al. Association of Age and Sex with Myocardial Infarction Symptom Presentation and In-Hospital Mortality. JAMA 2012, 307, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Lanzi, S.; Pousaz, A.; Calanca, L.; Mazzolai, L. Sex-Based Differences in Supervised Exercise Therapy Outcomes for Symptomatic Peripheral Artery Disease. Vasc. Med. 2023, 28, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Vakhtangadze, T.; Tak, R.S.; Singh, U.; Baig, M.S.; Bezsonov, E. Gender Differences in Atherosclerotic Vascular Disease: From Lipids to Clinical Outcomes. Front. Cardiovasc. Med. 2021, 8, 637. [Google Scholar] [CrossRef] [PubMed]
- Pabon, M.; Cheng, S.; Altin, S.E.; Sethi, S.S.; Nelson, M.D.; Moreau, K.L.; Hamburg, N.; Hess, C.N. Sex Differences in Peripheral Artery Disease. Circ. Res. 2022, 130, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.; Aday, A.W. Sex as a Key Determinant of Peripheral Artery Disease: Epidemiology, Differential Outcomes, and Proposed Biological Mechanisms. Can. J. Cardiol. 2022, 38, 601–611. [Google Scholar] [CrossRef]
- Altin, S.E.; Gitto, M.; Secemsky, E.A.; Rao, S.V.; Hess, C.N. Sex-Based Differences in Periprocedural Complications Following Lower Extremity Peripheral Vascular Intervention. Circ. Cardiovasc. Interv. 2022, 15, e011768. [Google Scholar] [CrossRef]
- Kröger, K.; Suckel, A.; Hirche, H.; Rudofsky, G. Different Prevalence of Asymptomatic Atherosclerotic Lesions in Males and Females. Vasc. Med. 1999, 4, 61–65. [Google Scholar] [CrossRef]
- Santoro, L.; Flex, A.; Nesci, A.; Ferraro, P.M.; De Matteis, G.; Di Giorgio, A.; Giupponi, B.; Saviano, L.; Gambaro, G.; Franceschi, F.; et al. Association between Peripheral Arterial Disease and Cardiovascular Risk Factors: Role of Ultrasonography versus Ankle-Brachial Index. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3160–3165. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Lin, J.; Chen, Y.; Jiang, N.; Li, Z.; Xu, S. Burden of Peripheral Artery Disease and Its Attributable Risk Factors in 204 Countries and Territories From 1990 to 2019. Front. Cardiovasc. Med. 2022, 9, 868370. [Google Scholar] [CrossRef]
- Curry, S.J.; Krist, A.H.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; Kubik, M.; et al. Screening for Peripheral Artery Disease and Cardiovascular Disease Risk Assessment With the Ankle-Brachial Index: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 320, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Ghare, M.I.; Chandrasekhar, J.; Mehran, R.; Ng, V.; Grines, C.; Lansky, A. Sex Disparities in Cardiovascular Device Evaluations. JACC Cardiovasc. Interv. 2019, 12, 301–308. [Google Scholar] [CrossRef]
- Abtan, J.; Bhatt, D.L.; Elbez, Y.; Sorbets, E.; Eagle, K.; Reid, C.M.; Baumgartner, I.; Wu, D.; Hanson, M.E.; Hannachi, H.; et al. Geographic Variation and Risk Factors for Systemic and Limb Ischemic Events in Patients with Symptomatic Peripheral Artery Disease: Insights from the REACH Registry. Clin. Cardiol. 2017, 40, 710–718. [Google Scholar] [CrossRef]
- Détriché, G.; Guédon, A.; Mohamedi, N.; Sellami, O.; Cheng, C.; Galloula, A.; Goudot, G.; Khider, L.; Mortelette, H.; Sitruk, J.; et al. Women Specific Characteristics and 1-Year Outcome Among Patients Hospitalized for Peripheral Artery Disease: A Monocentric Cohort Analysis in a Tertiary Center. Front. Cardiovasc. Med. 2022, 9, 824466. [Google Scholar] [CrossRef] [PubMed]
- Sigvant, B.; Lundin, F.; Wahlberg, E. The Risk of Disease Progression in Peripheral Arterial Disease Is Higher than Expected: A Meta-Analysis of Mortality and Disease Progression in Peripheral Arterial Disease. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 395–403. [Google Scholar] [CrossRef]
- Freisinger, E.; Malyar, N.M.; Reinecke, H.; Unrath, M. Low Rate of Revascularization Procedures and Poor Prognosis Particularly in Male Patients with Peripheral Artery Disease—A Propensity Score Matched Analysis. Int. J. Cardiol. 2018, 255, 188–194. [Google Scholar] [CrossRef]
- Dreyer, R.P.; van Zitteren, M.; Beltrame, J.F.; Fitridge, R.; Denollet, J.; Vriens, P.W.; Spertus, J.A.; Smolderen, K.G. Gender Differences in Health Status and Adverse Outcomes among Patients with Peripheral Arterial Disease. J. Am. Heart Assoc. 2015, 4, e000863. [Google Scholar] [CrossRef]
- Haine, A.; Kavanagh, S.; Berger, J.S.; Hess, C.N.; Norgren, L.; Fowkes, F.G.R.; Katona, B.G.; Mahaffey, K.W.; Blomster, J.I.; Patel, M.R.; et al. Sex-Specific Risks of Major Cardiovascular and Limb Events in Patients with Symptomatic Peripheral Artery Disease. J. Am. Coll. Cardiol. 2020, 75, 608–617. [Google Scholar] [CrossRef]
- Lee, M.S.; Choi, B.G.; Hollowed, J.; Han, S.K.; Baek, M.J.; gi Ryu, Y.; Choi, S.Y.; Byun, J.K.; Mashaly, A.; Park, Y.; et al. Assessment of Sex Differences in 5-Year Clinical Outcomes Following Endovascular Revascularization for Peripheral Artery Disease. Cardiovasc. Revascularization Med. 2020, 21, 110–115. [Google Scholar] [CrossRef]
- Comsa, H.; Gusetu, G.; Cismaru, G.; Caloian, B.; Rosu, R.; Zdrenghea, D.; David, A.; Dutu, B.; Tomoaia, R.; Fringu, F.; et al. Predictors for the Development of Major Adverse Limb Events after Percutaneous Revascularization—Gender-Related Characteristics. Medicina 2023, 59, 480. [Google Scholar] [CrossRef] [PubMed]
- Denegri, A.; Magnani, G.; Kraler, S.; Bruno, F.; Klingenberg, R.; Mach, F.; Gencer, B.; Räber, L.; Rodondi, N.; Rossi, V.A.; et al. History of Peripheral Artery Disease and Cardiovascular Risk of Real-World Patients with Acute Coronary Syndrome: Role of Inflammation and Comorbidities. Int. J. Cardiol. 2023, 382, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Matetzky, S.; Natanzon, S.S.; Shlomo, N.; Atar, S.; Pollak, A.; Yosefy, C.; Zahger, D.; Fefer, P.; Iakobishvili, Z.; Mazin, I.; et al. Peripheral Arterial Disease in Patients With Acute Coronary Syndrome: Results from a Large Real-World Registry. Heart Lung Circ. 2022, 31, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.; Ding, N.; Ballew, S.H.; Salameh, M.J.; Martin, S.S.; Selvin, E.; Heiss, G.; Ballantyne, C.M.; Matsushita, K.; Hoogeveen, R.C. Conventional and Novel Lipid Measures and Risk of Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1229–1238. [Google Scholar] [CrossRef]
- Rowe, V.L.; Weaver, F.A.; Lane, J.S.; Etzioni, D.A. Racial and Ethnic Differences in Patterns of Treatment for Acute Peripheral Arterial Disease in the United States, 1998–2006. J Vasc Surg 2010, 51, S21–S26. [Google Scholar] [CrossRef]
- Biscetti, F.; Nardella, E.; Bonadia, N.; Angelini, F.; Pitocco, D.; Santoliquido, A.; Filipponi, M.; Landolfi, R.; Flex, A. Association between Plasma Omentin-1 Levels in Type 2 Diabetic Patients and Peripheral Artery Disease. Cardiovasc. Diabetol. 2019, 18, 74. [Google Scholar] [CrossRef]
- Biscetti, F.; Nardella, E.; Rando, M.M.; Cecchini, A.L.; Bonadia, N.; Bruno, P.; Angelini, F.; Di Stasi, C.; Contegiacomo, A.; Santoliquido, A.; et al. Sortilin Levels Correlate with Major Cardiovascular Events of Diabetic Patients with Peripheral Artery Disease Following Revascularization: A Prospective Study. Cardiovasc. Diabetol. 2020, 19, 147. [Google Scholar] [CrossRef]
- Giordano, S.; Xing, D.; Chen, Y.-F.; Allon, S.; Chen, C.; Oparil, S.; Hage, F.G. Estrogen and Cardiovascular Disease: Is Timing Everything? Am. J. Med. Sci. 2015, 350, 27–35. [Google Scholar] [CrossRef]
- Wang, G.J.; Shaw, P.A.; Townsend, R.R.; Anderson, A.H.; Xie, D.; Wang, X.; Nessel, L.C.; Mohler, E.R.; Sozio, S.M.; Jaar, B.G.; et al. Sex Differences in the Incidence of Peripheral Artery Disease in the Chronic Renal Insufficiency Cohort. Circ. Cardiovasc. Qual. Outcomes 2016, 9, S86–S93. [Google Scholar] [CrossRef]
- Vavra, A.K.; Kibbe, M.R. Women and Peripheral Arterial Disease. Women’s Health 2009, 5, 669–683. [Google Scholar] [CrossRef]
- Lakka, H.-M. The Metabolic Syndrome and Total and Cardiovascular Disease Mortality in Middle-Aged Men. JAMA 2002, 288, 2709. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.; Higgins, A. Epidemiology of Peripheral Arterial Disease in Women. J. Epidemiol. 2003, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
| All Cases N 1640 | Male N 1103 | Female N 537 | p Value | |
|---|---|---|---|---|
| Age | 72 [64–81] | 71 [63–80] | 75 [69–84] | <0.001 |
| Clinical presentation | ||||
| Fever | 425 (25.9%) | 301 (27.3%) | 124 (23.1%) | 0.038 |
| Pain | 704 (42.9%) | 463 (42%) | 241 (44.9%) | 0.144 |
| Chest pain | 107 (6.5%) | 82 (7.4%) | 25 (23.4%) | 0.019 |
| Dyspnoea | 184 (11.2%) | 136 (12.3%) | 48 (8.9%) | 0.024 |
| Neurological symptoms | 67 (4.1%) | 48 (4.4%) | 19 (3.5%) | 0.261 |
| PAD grade (according to Rutherford classification) | ||||
| Rutherford Grade I-3 | 101 (6.2%) | 73 (6.6%) | 28 (5.2%) | |
| Rutherford Grade II-4 | 171 (10.4%) | 106 (9.6%) | 65 (12.1%) | |
| Rutherford Grade III-5 | 161 (9.8%) | 107 (9.7%) | 54 (10.1%) | 0.158 |
| Rutherford Grade III-6 | 888 (54.1%) | 595 (53.9%) | 293 (54.6%) | |
| Previous amputation | 265 (16.2%) | 197 (17.9%) | 68 (12.7%) | 0.004 |
| Presence of infections | ||||
| Bloodstream infection | 130 (7.9%) | 92 (8.3%) | 38 (7.1%) | 0.215 |
| Lover limb infections | 888 (54.1%) | 595 (53.9%) | 293 (54.6%) | 0.813 |
| Other infections | 1043 (63.6%) | 710 (64.4%) | 333 (62.0%) | 0.352 |
| Laboratory findings | ||||
| Creatinine (mg/dL) | 1.95 [0.83–2.07] | 2.1 [0.86–2.32] | 1.64 [0.74–1.71] | <0.001 |
| eGFR (ml/min) | 54.3 [28.5–84.2] | 50.8 [29.4–76.7] | 55.6 [28.0–88.7] | 0.024 |
| Urea (mg/dL) | 33.2 [18–40] | 33.6 [18–42] | 32.4 [18–39] | 0.186 |
| Glucose (mg/dL) | 173 [107–207] | 172 [108–207] | 174 [104–208] | 0.443 |
| HbA1c (mmol) | 61 [45–72] | 62 [44–74] | 61 [45–71] | 0.848 |
| C-reactive protein (mg/L) | 95 [19–148] | 99 [25–152] | 88 [14–134] | 0.160 |
| Total cholesterol (mg/dL) | 129 [102–150] | 124 [97–147] | 138 [110–161] | <0.001 |
| HDL (mg/dL) | 32 [24–38] | 30 [23–36] | 36 [27–43] | <0.001 |
| LDL (mg/dL) | 75 [53–90] | 72 [48–85] | 74 [53–90] | 0.077 |
| Triglycerides (mg/dL) | 128 [86–154] | 122 [83–145] | 139 [93–173] | <0.001 |
| Hb (gr/dL) | 11.3 [9.8–12.9] | 11.53 [9.8–13.2] | 11 [9.7–12.4] | <0.001 |
| PLTs (×109/L) | 302 [204–369] | 292 [196–357] | 323 [218–400] | 0.001 |
| Comorbidities | ||||
| CCI | 4 [3–6] | 4 [3–6] | 4 [2–5] | 0.020 |
| CAD | 699 (42.6%) | 497 (45.1%) | 202 (37.6%) | 0.002 |
| Congestive heart failure | 447 (27.3%) | 314 (28.5%) | 133 (24.8%) | 0.064 |
| Cerebrovascular disease | 149 (9.1%) | 98 (8.9%) | 51 (9.5%) | 0.374 |
| Dementia | 109 (6.6%) | 67 (6.1%) | 42 (7.8%) | 0.111 |
| COPD | 196 (12%) | 138 (12.5%) | 58 (10.8%) | 0.179 |
| Diabetes | 1280 (78%) | 880 (79.8%) | 400 (74.5%) | 0.009 |
| Chronic kidney disease | 565 (34.5%) | 391 (35.4%) | 174 (32.4%) | 0.122 |
| Outcomes | ||||
| Death (all causes) | 127 (7.7%) | 79 (7.2%) | 48 (8.9%) | 0.123 |
| Length of stay (LOS) | 16 [7–20] | 16 [7–21] | 15 [7–19] | 0.148 |
| Acute limb ischaemia (ALI) | 288 (17.6%) | 187 (17.0%) | 101 (18.8%) | 0.357 |
| Overall revascularizations | 447 (27.3%) | 286 (25.9%) | 161 (30.1%) | 0.048 |
| Angioplasty | 442 (27.0%) | 282 (25.6%) | 160 (29.8%) | 0.040 |
| Stenting | 23 (1.4%) | 14 (1.3%) | 9 (1.7%) | 0.325 |
| Urgent revascularizations | 267 (16.3%) | 168 (15.2%) | 99 (18.4%) | 0.058 |
| Amputations | 302 (18.4%) | 220 (19.9%) | 82 (15.3%) | 0.012 |
| MALE cumulative events | 660 (40.2%) | 454 (41.2%) | 206 (38.4%) | 0.151 |
| Myocardial infarction | 60 (3.7%) | 40 (3.6%) | 20 (3.7%) | 0.510 |
| Stroke | 28 (1.7%) | 18 (1.6%) | 10 (1.9%) | 0.438 |
| MACE cumulative events | 123 (7.5%) | 78 (7.1%) | 45 (8.4%) | 0.199 |
| Controls N 980 | MALE Cumulative Events N 660 | Unadjusted p Value | Adjusted Odds for MALE [95% Confidence Interval] | Multivariate p Value | |
|---|---|---|---|---|---|
| Age | 72 [65–81] | 71 [64–80] | 0.030 | 0.99 [0.98–1.01] | 0.484 |
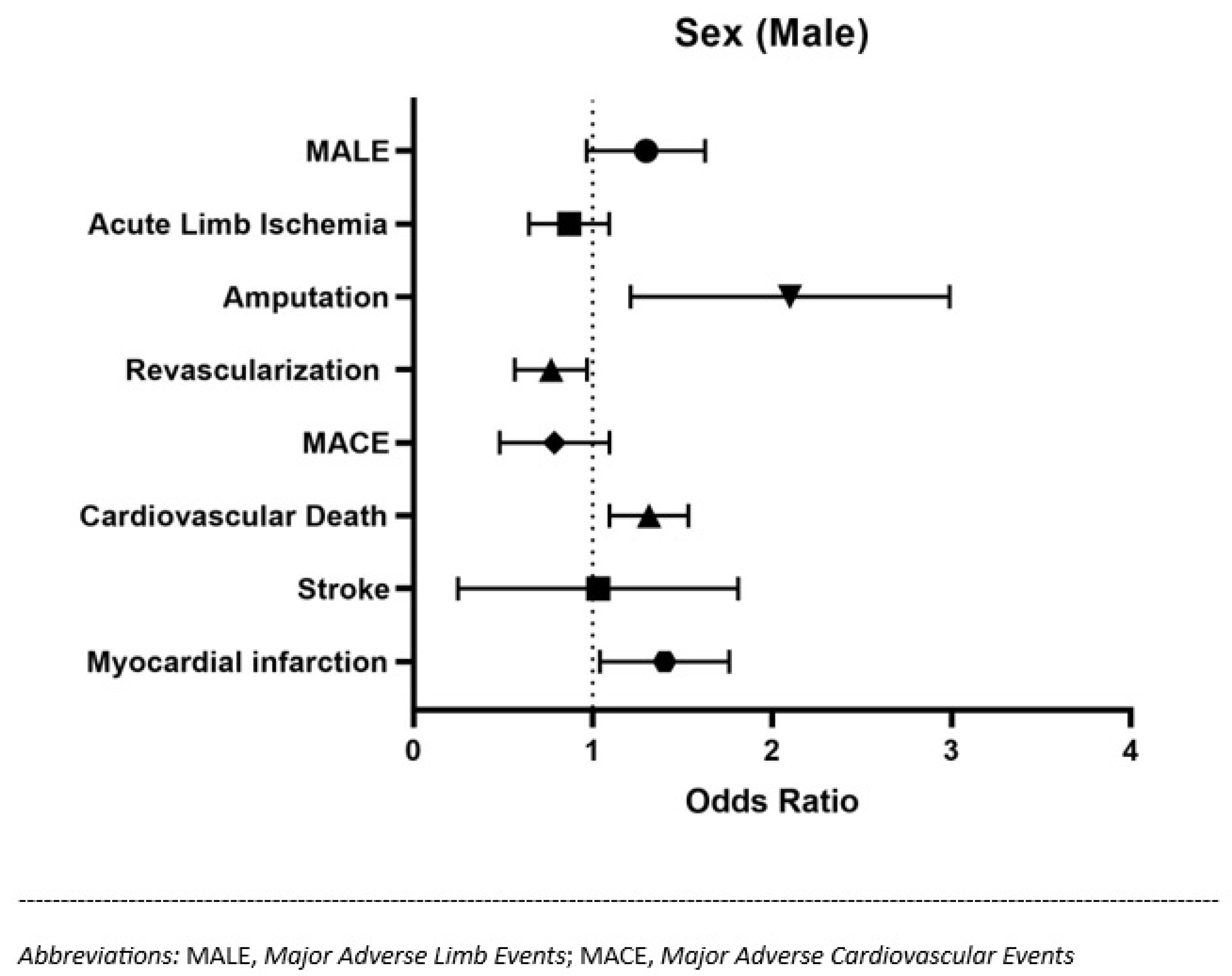
| Sex (male) | 649 (66.2%) | 454 (68.8%) | 0.151 | 1.27 [0.99–1.64] | 0.059 |
| Clinical presentation | |||||
| Fever | 265 (62.4%) | 160 (37.6%) | 0.110 | ||
| Pain (any) | 333 (47.3%) | 371 (52.7%) | <0.001 | 3.65 [2.85–4.70] | <0.001 |
| Chest pain | 91 (9.3%) | 16 (2.4%) | <0.001 | 0.25 [0.13–0.46] | 0.003 |
| Dyspnoea | 166 (16.9%) | 18 (2.7%) | <0.001 | 0.28 [0.16–0.49] | 0.006 |
| Neurological symptoms | 45 (4.6%) | 22 (3.3%) | 0.047 | ||
| Laboratory findings | |||||
| Creatinine (mg/dL) | 1.86 [0.84–2.02] | 2.08 [0.82–2.2] | 0.770 | ||
| eGFR | 54.4 [29.3–82.3] | 53.8 [26.5–88.4] | 0.977 | ||
| Urea (mg/dL) | 33.6 [18–41] | 32 [18–39] | 0.610 | ||
| Glucose (mg/dL) | 174 [107–202] | 171 [107–215] | 0.780 | ||
| HbA1C (mmol) | 57 [44–69] | 66 [51–78] | <0.001 | ||
| C-reactive protein (mg/L) | 87 [16–128] | 107 [27–161] | <0.001 | ||
| Total cholesterol (mg/dL) | 133 [104–157] | 123 [99–145] | <0.001 | ||
| HDL (mg/dL) | 32 [25–39] | 33 [23–37] | 0.140 | ||
| LDL (mg/dL) | 73 [50–88] | 73 [48–85] | 0.399 | ||
| Triglycerides (mg/dL) | 131 [87–158] | 123 [86–151] | 0.315 | ||
| Hb (gr/dL) | 11.4 [9.8–13] | 11.2 [9.7–12.9] | 0.054 | ||
| PLT (×109/L) | 284 [194–344] | 328 [230–404] | <0.001 | ||
| PAD grade (according to Rutherford classification) | |||||
| Rutherford Grade I-3 | 67 (6.8%) | 34 (5.2%) | |||
| Rutherford Grade II-4 | 125 (12.8%) | 46 (7.0%) | |||
| Rutherford Grade III-5 | 123 (12.6%) | 38 (5.8%) | <0.001 | ||
| Rutherford Grade III-6 | 359 (36.6%) | 529 (59.6%) | |||
| Previous amputation | 141 (14.4%) | 124 (18.8%) | 0.011 | 0.91 [0.67–1.25] | 0.574 |
| Presence of infections | |||||
| Bloodstream infection | 62 (6.3%) | 68 (10.3%) | 0.004 | 1.75 [1.11–2.76] | 0.016 |
| Lower limb infections | 359 (36.6%) | 529 (80.2%) | <0.001 | 18.9 [9.72–36.87] | 0.001 |
| Other infections | 503 (51.3%) | 540 (81.8%) | <0.001 | 0.27 [0.13–0.55] | <0.001 |
| Comorbidities | |||||
| CCI | 4 [2–5] | 4 [3–6] | 0.003 | 1.03 [0.98–1.08] | 0.267 |
| CAD | 412 (42%) | 287 (43.5%) | 0.576 | ||
| Congestive heart failure | 282 (28.8%) | 165 (25%) | 0.101 | ||
| Cerebrovascular disease | 99 (10.1%) | 50 (7.6%) | 0.015 | ||
| Dementia | 70 (7.1%) | 39 (5.9%) | 0.360 | ||
| COPD | 124 (12.7%) | 72 (10.9%) | 0.161 | ||
| Diabetes | 749 (76.4%) | 531 (80.5%) | 0.008 | ||
| Chronic kidney disease | 324 (57.3%) | 241 (42.7%) | 0.015 | ||
| Outcomes | |||||
| Length of stay (LOS) | 13.1 [7.1–16.5] | 19.3 [8.5–25.5] | <0.001 | ||
| Death (all causes) | 76 (7.8%) | 51 (7.7%) | 0.075 | ||
| Controls N 1517 | MACE Cumulative Events N 123 | Unadjusted p Value | Adjusted Odds for MACE [95% Confidence Interval] | Multivariate p Value | |
|---|---|---|---|---|---|
| Age | 72 [64–81] | 74 [68–83] | 0.093 | ||
| Sex (male) | 1025 (63.4%) | 78 (67.8%) | 0.020 | 0.75 [0.50–1.11] | 0.153 |
| Clinical presentation | |||||
| Fever | 402 (26.5%) | 23 (18.7%) | 0.034 | ||
| Pain | 658 (43.4%) | 46 (37.4%) | 0.030 | ||
| Chest pain | 83 (5.5%) | 24 (19.5%) | <0.001 | 3.46 [1.82–6.56] | <0.001 |
| Dyspnoea | 162 (10.7%) | 22 (17.9%) | 0.015 | 1.19 [0.69–2.04] | 0.520 |
| Neurological symptoms | 55 (3.6%) | 12 (9.8%) | 0.002 | 2.96 [1.49–5.89] | <0.001 |
| Laboratory findings | |||||
| Creatinine (mg/dL) | 1.94 [0.82–2.02] | 2.09 [0.94–2.63] | 0.018 | ||
| eGFR (ml/min) | 54.7 [29.2–85.2] | 45.2 [22.6–70.6] | 0.012 | ||
| Urea (mg/dL) | 33 [18–40] | 36 [19.7–49] | 0.035 | ||
| Glucose (mg/dL) | 174 [107–210] | 160 [107–184] | 0.160 | ||
| HbA1c (mmol) | 61 [45–73] | 54 [43–62] | <0.001 | ||
| C-reactive protein (mg/L) | 96 [19–148] | 88 [16–149] | 0.660 | ||
| Total cholesterol (mg/dL) | 128 [101–150] | 135 [104–162] | 0.230 | ||
| HDL (mg/dL) | 32 [24–38] | 31 [21–40] | 0.830 | ||
| LDL (mg/dL) | 72 [50–86] | 84 [49–114] | 0.070 | ||
| Triglycerides (mg/dL) | 127 [86–153] | 139 [98–177] | 0.048 | ||
| Hb (gr/dL) | 11.3 [9.5–13] | 11.2 [9.6–13.1] | 0.430 | ||
| PLT (×109/L) | 306 [210–373] | 249 [174–294] | <0.001 | ||
| PAD grade (according to Rutherford classification) | |||||
| Rutherford Grade I-3 | 88 (5.8%) | 13 (10.6%) | Reference | ||
| Rutherford Grade II-4 | 147 (9.7%) | 24 (19.5%) | 0.71 [0.32–1.71] | 0.398 | |
| Rutherford Grade III-5 | 152 (10%) | 9 (7.3%) | <0.001 | 0.44 [0.19–0.97] | 0.041 |
| Rutherford Grade III-6 | 847 (55.8%) | 41 (33.3%) | 0.45 [0.21–0.90] | 0.026 | |
| Previous amputation | 254 (16.7%) | 11 (8.9%) | 0.007 | 0.96 [0.37–2.50] | 0.012 |
| Presence of infections | |||||
| Bloodstream infection | 119 (7.8%) | 11 (8.9%) | 0.12 | ||
| Lower limb infections | 847 (55.8%) | 41 (33.3%) | <0.001 | 0.61 [0.29–1.27] | 0.332 |
| Other infections | 988 (65.1%) | 55 (44.7%) | <0.001 | 0.73 [0.38–1.38] | 0.336 |
| Comorbidities | |||||
| CCI | 4 [2–5] | 4 [3–6] | 0.003 | 1.08 [1.01–1.17] | 0.047 |
| CAD | 626 (41.3%) | 73 (59.3%) | <0.001 | ||
| Congestive heart failure | 405 (26.7%) | 42 (34.1%) | 0.017 | ||
| Cerebrovascular disease | 130 (8.6%) | 19 (15.4%) | 0.021 | ||
| Dementia | 101 (6.7%) | 8 (6.5%) | 0.57 | ||
| COPD | 179 (11.8%) | 17 (13.8%) | 0.29 | ||
| Diabetes | 1193 (78.6%) | 87 (70.7%) | 0.05 | ||
| Chronic kidney disease | 521 (34.3%) | 44 (35.8%) | 0.41 | ||
| Outcomes | |||||
| Length of stay (LOS) | 15.73 [7.4–20.2] | 18.8 [7.5–26] | 0.011 | ||
| Death (all causes) | 81 (5.3%) | 46 (37.4%) | <0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Matteis, G.; Biscetti, F.; Della Polla, D.A.; Serra, A.; Burzo, M.L.; Fuorlo, M.; Nicolazzi, M.A.; Novelli, A.; Santoliquido, A.; Gambassi, G.; et al. Sex-Based Differences in Clinical Characteristics and Outcomes among Patients with Peripheral Artery Disease: A Retrospective Analysis. J. Clin. Med. 2023, 12, 5094. https://doi.org/10.3390/jcm12155094
De Matteis G, Biscetti F, Della Polla DA, Serra A, Burzo ML, Fuorlo M, Nicolazzi MA, Novelli A, Santoliquido A, Gambassi G, et al. Sex-Based Differences in Clinical Characteristics and Outcomes among Patients with Peripheral Artery Disease: A Retrospective Analysis. Journal of Clinical Medicine. 2023; 12(15):5094. https://doi.org/10.3390/jcm12155094
Chicago/Turabian StyleDe Matteis, Giuseppe, Federico Biscetti, Davide Antonio Della Polla, Amato Serra, Maria Livia Burzo, Mariella Fuorlo, Maria Anna Nicolazzi, Angela Novelli, Angelo Santoliquido, Giovanni Gambassi, and et al. 2023. "Sex-Based Differences in Clinical Characteristics and Outcomes among Patients with Peripheral Artery Disease: A Retrospective Analysis" Journal of Clinical Medicine 12, no. 15: 5094. https://doi.org/10.3390/jcm12155094
APA StyleDe Matteis, G., Biscetti, F., Della Polla, D. A., Serra, A., Burzo, M. L., Fuorlo, M., Nicolazzi, M. A., Novelli, A., Santoliquido, A., Gambassi, G., Gasbarrini, A., Flex, A., Franceschi, F., & Covino, M. (2023). Sex-Based Differences in Clinical Characteristics and Outcomes among Patients with Peripheral Artery Disease: A Retrospective Analysis. Journal of Clinical Medicine, 12(15), 5094. https://doi.org/10.3390/jcm12155094










