Swiss Vascular Biobank: Evaluation of Optimal Extraction Method and Admission Solution for Preserving RNA from Human Vascular Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Samples
2.2. RNA Extraction
2.3. Determination of the RNA Quality
2.4. Time-Dependent Comparison of Different Protective Solutions
2.5. Quantitative Real-Time PCR Analysis
- ACTB: Hs_ACTB_2_SG, amplicon length 104 bp
- GAPDH: Hs_GAPDH_vb.1_SG, amplicon length 112 bp (exons 2/3)
- GAPDH: Hs_GAPDH_1_SG, amplicon length 95 bp (exons 6/7)
- ribosomal Gene 18S: Hs_RPS18_1_SG, amplicon length 61 bp
- ribosomal Gene 28S: Hs_RPS28_1_SG, amplicon length 77 bp
- MALAT1: Hs00273907_s1, amplicon length 117 bp
- H19: Hs00399294_g1, amplicon length 62 bp (exons 2/3)
2.6. Statistical Analysis
3. Results
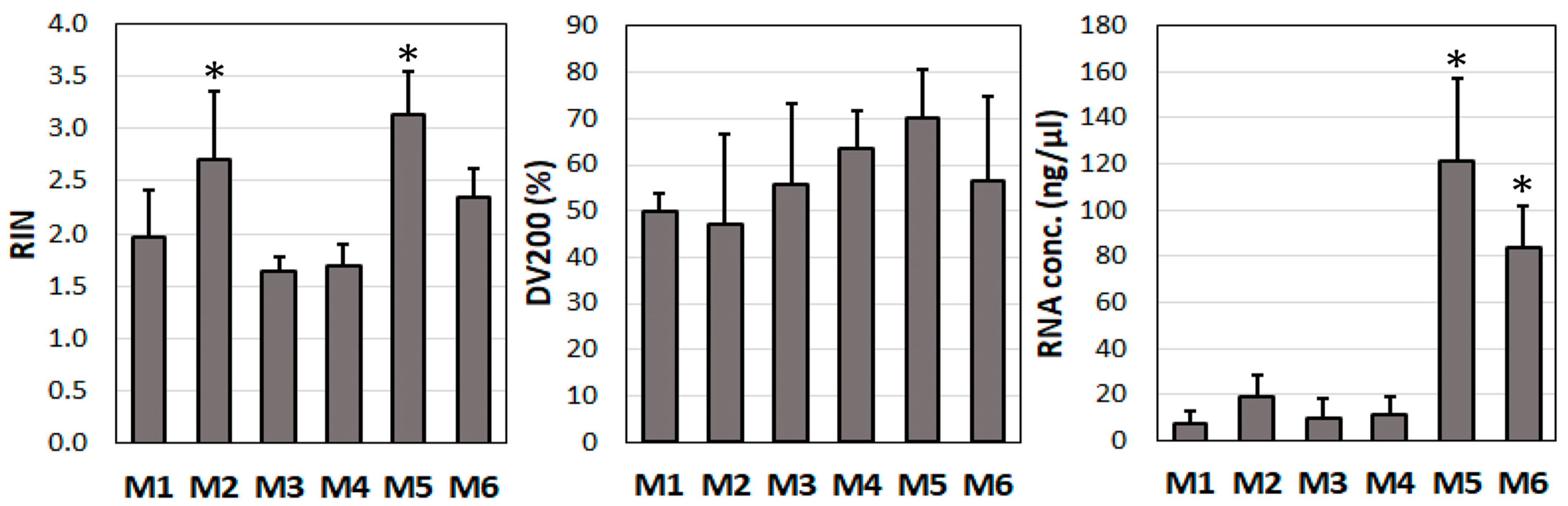
3.1. Evaluation of Optimal Method of Extracting RNA from Vascular Tissue
3.2. Determination of RIN and DV200 Values in the Study Tissue Samples
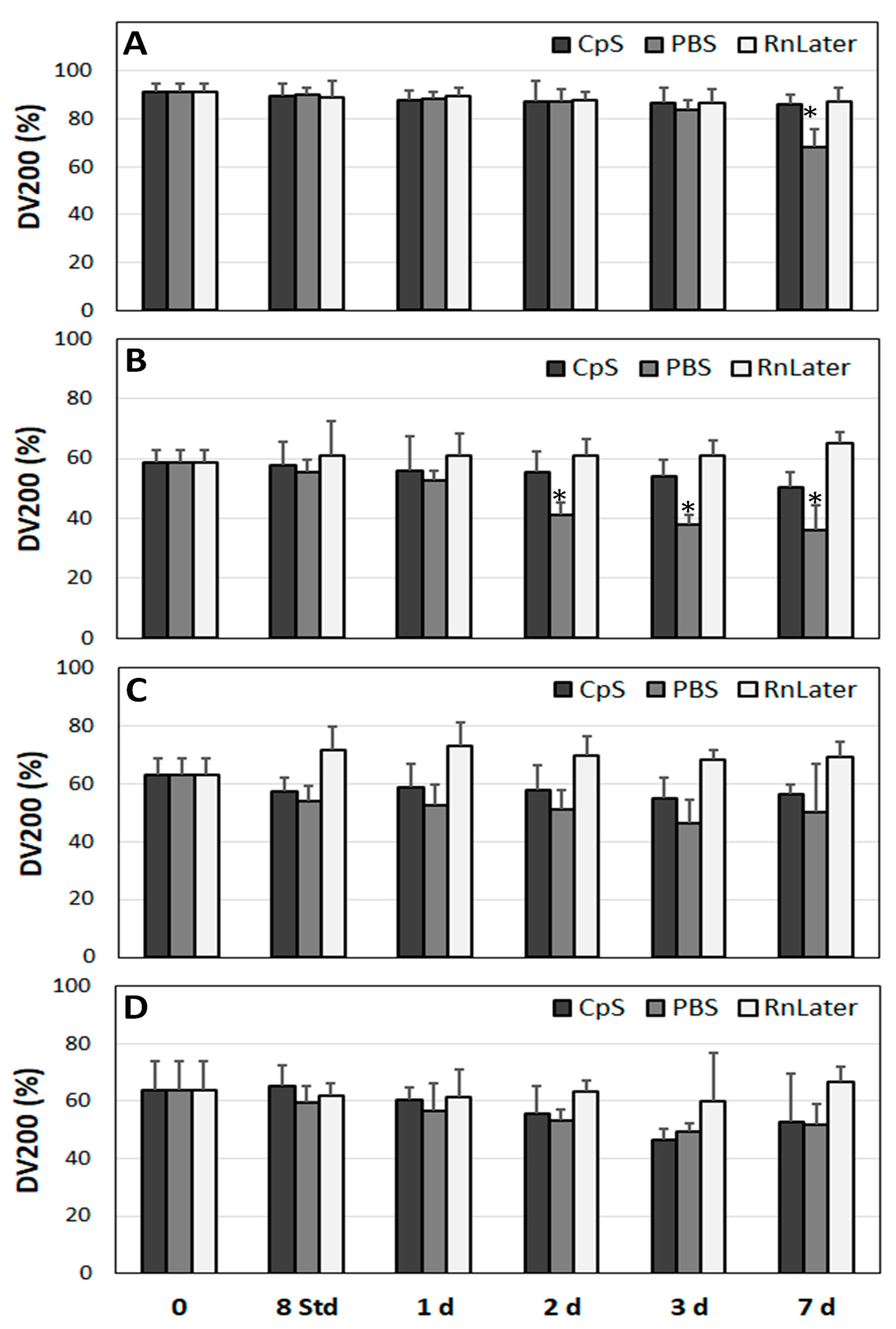
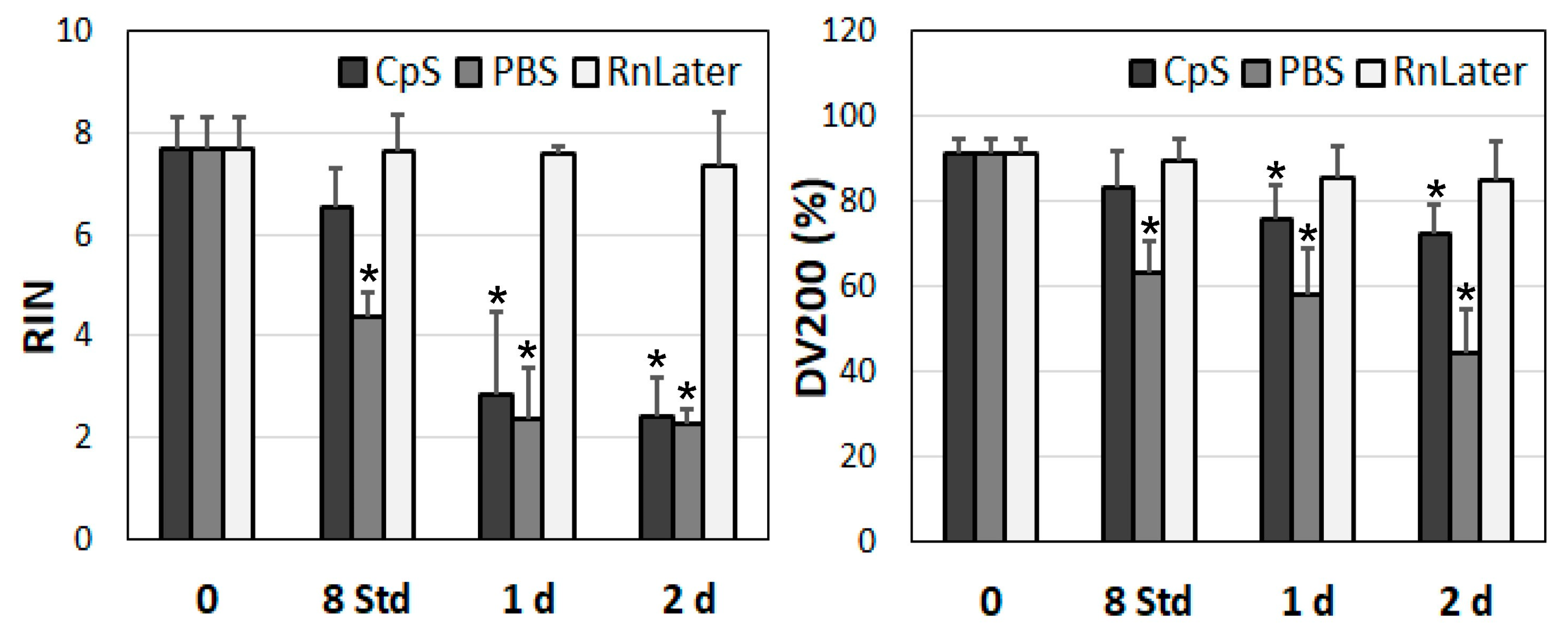
3.3. Stability of RNA over Time
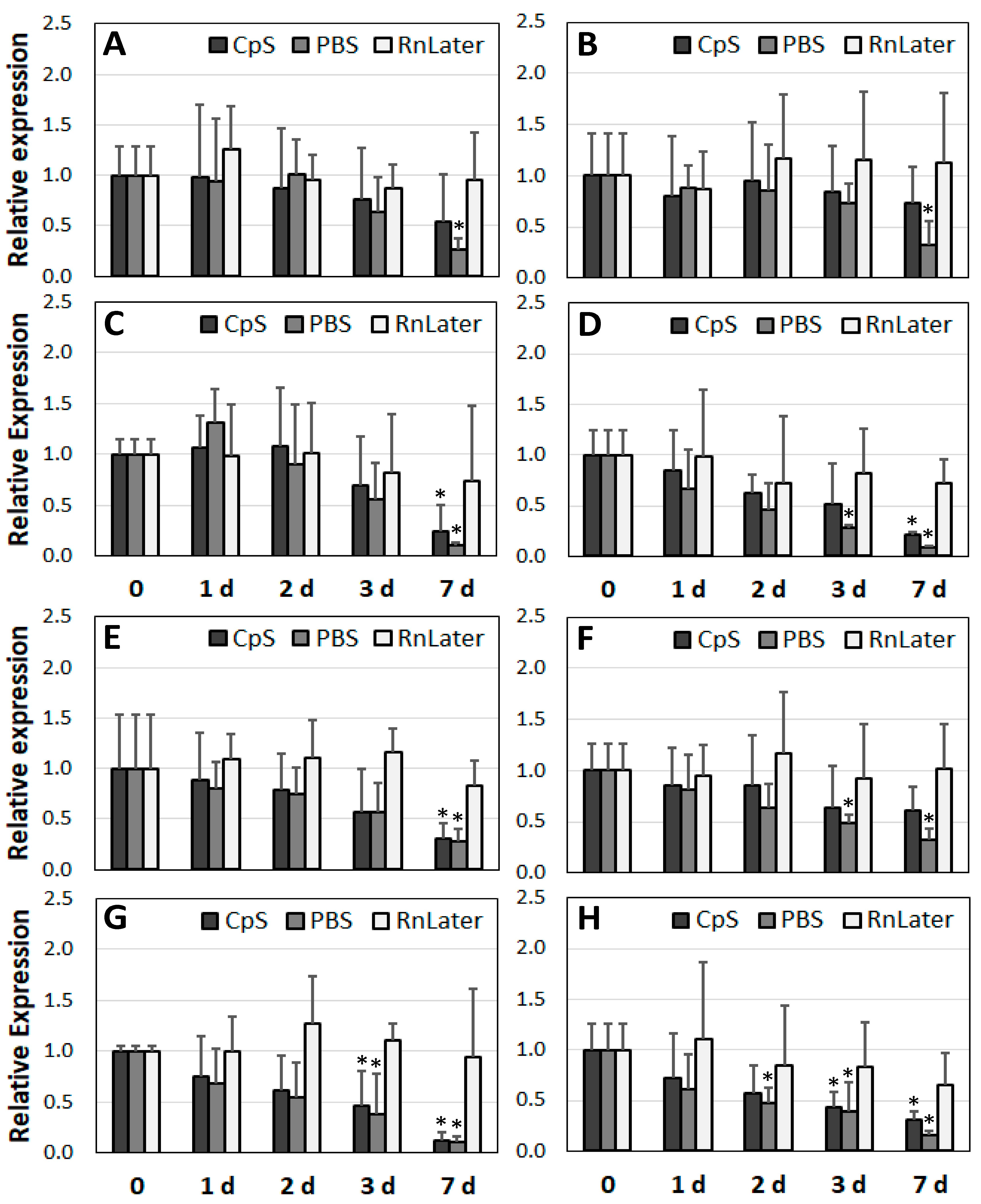
3.4. Changes in Gene Expression over Time
3.5. Correlation between RIN, DV200, and Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hentze, J.L.; Kringelbach, T.M.; Novotny, G.W.; Hamid, B.H.; Ravn, V.; Christensen, I.J.; Hogdall, C.; Hogdall, E. Optimized Biobanking Procedures for Preservation of RNA in Tissue: Comparison of Snap-Freezing and RNAlater-Fixation Methods. Biopreserv. Biobank. 2019, 17, 562–569. [Google Scholar] [CrossRef]
- Pelisek, J.; Hegenloh, R.; Bauer, S.; Metschl, S.; Pauli, J.; Glukha, N.; Busch, A.; Reutersberg, B.; Kallmayer, M.; Trenner, M.; et al. Biobanking: Objectives, Requirements, and Future Challenges-Experiences from the Munich Vascular Biobank. J. Clin. Med. 2019, 8, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Keegan, A.; Hicks, C.W. Surgical Decision-Making and Outcomes in Open Versus Endovascular Repair for Various Vascular Diseases. Anesthesiol. Clin. 2022, 40, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakos, J.; Lal, B.K. Abdominal aortic aneurysms. Prog. Cardiovasc. Dis. 2021, 65, 34–43. [Google Scholar] [CrossRef]
- Klaus, V.; Tanios-Schmies, F.; Reeps, C.; Trenner, M.; Matevossian, E.; Eckstein, H.H.; Pelisek, J. Association of Matrix Metalloproteinase Levels with Collagen Degradation in the Context of Abdominal Aortic Aneurysm. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 549–558. [Google Scholar] [CrossRef] [Green Version]
- Li, D.Y.; Busch, A.; Jin, H.; Chernogubova, E.; Pelisek, J.; Karlsson, J.; Sennblad, B.; Liu, S.; Lao, S.; Hofmann, P.; et al. H19 Induces Abdominal Aortic Aneurysm Development and Progression. Circulation 2018, 138, 1551–1568. [Google Scholar] [CrossRef] [PubMed]
- Pelisek, J.; Eckstein, H.H.; Zernecke, A. Pathophysiological mechanisms of carotid plaque vulnerability: Impact on ischemic stroke. Arch. Immunol. Ther. Exp. 2012, 60, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Reeps, C.; Kehl, S.; Tanios, F.; Biehler, J.; Pelisek, J.; Wall, W.A.; Eckstein, H.H.; Gee, M.W. Biomechanics and gene expression in abdominal aortic aneurysm. J. Vasc. Surg. 2014, 60, 1640–1647.e2. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Shaffer, A.; Geddes, T.; Studzinski, D.; Mitton, K.; Pruetz, B.; Long, G.; Shanley, C. Evaluation of optimal RNA extraction method from human carotid atherosclerotic plaque. Cardiovasc. Pathol. 2015, 24, 187–190. [Google Scholar] [CrossRef]
- Martinet, W.; de Meyer, G.R.; Herman, A.G.; Kockx, M.M. Reactive oxygen species induce RNA damage in human atherosclerosis. Eur. J. Clin. Investig. 2004, 34, 323–327. [Google Scholar] [CrossRef]
- Martinet, W.; De Meyer, G.R.; Herman, A.G.; Kockx, M.M. RNA damage in human atherosclerosis: Pathophysiological significance and implications for gene expression studies. RNA Biol. 2005, 2, 4–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallego Romero, I.; Pai, A.A.; Tung, J.; Gilad, Y. RNA-seq: Impact of RNA degradation on transcript quantification. BMC Biol. 2014, 12, 42. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, T.; Soh, J.; Morita, M.; Uwabo, T.; Tomida, S.; Fujiwara, T.; Kanazawa, S.; Toyooka, S.; Hirasawa, A. DV200 Index for Assessing RNA Integrity in Next-Generation Sequencing. Biomed. Res. Int. 2020, 2020, 9349132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Towler, B.P.; Newbury, S.F. Regulation of cytoplasmic RNA stability: Lessons from Drosophila. Wiley Interdiscip. Rev. RNA 2018, 9, e1499. [Google Scholar] [CrossRef]
- Bakhach, J. The cryopreservation of composite tissues: Principles and recent advancement on cryopreservation of different type of tissues. Organogenesis 2009, 5, 119–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, G.D.; Wang, S.; Fuller, B.J. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 2017, 76, 74–91. [Google Scholar] [CrossRef]
- Muller-Schweinitzer, E. Cryopreservation of vascular tissues. Organogenesis 2009, 5, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.J.; Weegman, B.P.; Baicu, S.C.; Giwa, S.E. New Approaches to Cryopreservation of Cells, Tissues, and Organs. Transfus. Med. Hemother. 2019, 46, 197–215. [Google Scholar] [CrossRef]
- Cao, M.; Luo, H.; Li, D.; Wang, S.; Xuan, L.; Sun, L. Research advances on circulating long noncoding RNAs as biomarkers of cardiovascular diseases. Int. J. Cardiol. 2022, 353, 109–117. [Google Scholar] [CrossRef]
- Josefs, T.; Boon, R.A. The Long Non-coding Road to Atherosclerosis. Curr. Atheroscler. Rep. 2020, 22, 55. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhou, Q.; Chen, Y. Impact of RNA degradation on next-generation sequencing transcriptome data. Genomics 2022, 114, 110429. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.G.; Whetzel, A.M.; Serrano, G.; Sue, L.I.; Lue, L.F.; Beach, T.G. Characterization of RNA isolated from eighteen different human tissues: Results from a rapid human autopsy program. Cell Tissue Bank. 2016, 17, 361–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tutino, V.M.; Fricano, S.; Frauens, K.; Patel, T.R.; Monteiro, A.; Rai, H.H.; Waqas, M.; Chaves, L.; Poppenberg, K.E.; Siddiqui, A.H. Isolation of RNA from Acute Ischemic Stroke Clots Retrieved by Mechanical Thrombectomy. Genes 2021, 12, 1617. [Google Scholar] [CrossRef]
- Habran, M.; De Beule, J.; Jochmans, I. IGL-1 preservation solution in kidney and pancreas transplantation: A systematic review. PLoS ONE 2020, 15, e0231019. [Google Scholar] [CrossRef]
- Kosieradzki, M.; Rowinski, W. Ischemia/reperfusion injury in kidney transplantation: Mechanisms and prevention. Transplant. Proc. 2008, 40, 3279–3288. [Google Scholar] [CrossRef]
- Kim, S.M.; Huh, J.W.; Kim, E.Y.; Shin, M.K.; Park, J.E.; Kim, S.W.; Lee, W.; Choi, B.; Chang, E.J. Endothelial dysfunction induces atherosclerosis: Increased aggrecan expression promotes apoptosis in vascular smooth muscle cells. BMB Rep. 2019, 52, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Solomon, C.U.; McVey, D.G.; Andreadi, C.; Gong, P.; Turner, L.; Stanczyk, P.J.; Khemiri, S.; Chamberlain, J.C.; Yang, W.; Webb, T.R.; et al. Effects of Coronary Artery Disease-Associated Variants on Vascular Smooth Muscle Cells. Circulation 2022, 146, 917–929. [Google Scholar] [CrossRef]
- Bustin, S.; Huggett, J. qPCR primer design revisited. Biomol. Detect. Quantif. 2017, 14, 19–28. [Google Scholar] [CrossRef]
- Bustin, S.; Nolan, T. Talking the talk, but not walking the walk: RT-qPCR as a paradigm for the lack of reproducibility in molecular research. Eur. J. Clin. Investig. 2017, 47, 756–774. [Google Scholar] [CrossRef] [Green Version]
- Julian, G.S.; de Oliveira, R.W.; Perry, J.C.; Tufik, S.; Chagas, J.R. Validation of housekeeping genes in the brains of rats submitted to chronic intermittent hypoxia, a sleep apnea model. PLoS ONE 2014, 9, e109902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, W.; Smith, A.O.; Sun, T.; Zhao, P.; Jiang, Y.; Liu, L.; Zhang, T.; Qi, K.; Qiao, J.; Xu, K.; et al. Validation of Housekeeping Genes as Reference for Reverse-Transcription-qPCR Analysis in Busulfan-Injured Microvascular Endothelial Cells. Biomed. Res. Int. 2018, 2018, 4953806. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yan, J.; Wu, A.; Zhao, W.; Qin, J.; Pogwizd, S.M.; Wu, X.; Yuan, S.; Ai, X. Alterations of housekeeping proteins in human aged and diseased hearts. Pflügers Arch.-Eur. J. Physiol. 2021, 473, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Blomquist, D.; Fernandez, J.R.; Miranda, J.; Bello, C.; Silva, J.A.; Estrada, R.C.; Novoa, L.I.; Palenzuela, D.; Bello, I. Selection of reference genes for use in quantitative reverse transcription PCR assays when using interferons in U87MG. Mol. Biol. Rep. 2012, 39, 11167–11175. [Google Scholar] [CrossRef] [PubMed]
- Busscher, D.; Boon, R.A.; Juni, R.P. The multifaceted actions of the lncRNA H19 in cardiovascular biology and diseases. Clin. Sci. 2022, 136, 1157–1178. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wei, Y.T.; Li, H.; Jiang, T.; Zheng, X.L.; Yin, K.; Zhao, G.J. Long non-coding RNA H19 in atherosclerosis: What role? Mol. Med. 2020, 26, 72. [Google Scholar] [CrossRef]
- Skuratovskaia, D.; Vulf, M.; Komar, A.; Kirienkova, E.; Litvinova, L. Promising Directions in Atherosclerosis Treatment Based on Epigenetic Regulation Using MicroRNAs and Long Noncoding RNAs. Biomolecules 2019, 9, 226. [Google Scholar] [CrossRef] [Green Version]
- Schober, A.; Maleki, S.S.; Nazari-Jahantigh, M. Regulatory Non-coding RNAs in Atherosclerosis. Prevention and Treatment of Atherosclerosis: Improving State-of-the-Art Management and Search for Novel Targets. Handb. Exp. Pharmacol. 2022, 270, 463–492. [Google Scholar] [CrossRef]
- Illumina. Evaluating RNA Quality from FFPE Samples. Available online: https://emea.illumina.com/content/dam/illumina-marketing/documents/products/technotes/evaluating-rna-quality-from-ffpe-samples-technical-note-470-2014-001.pdf (accessed on 26 October 2016).


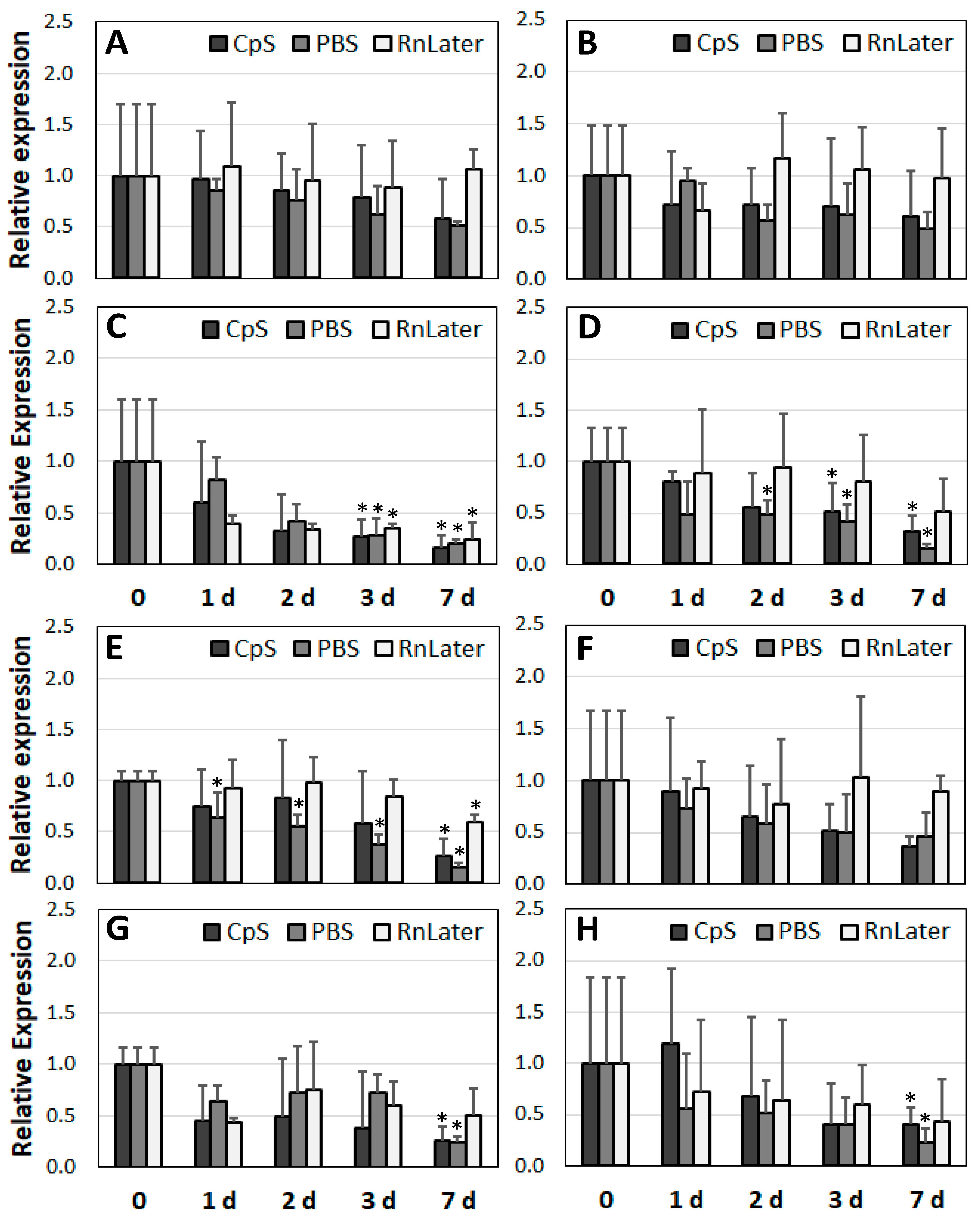
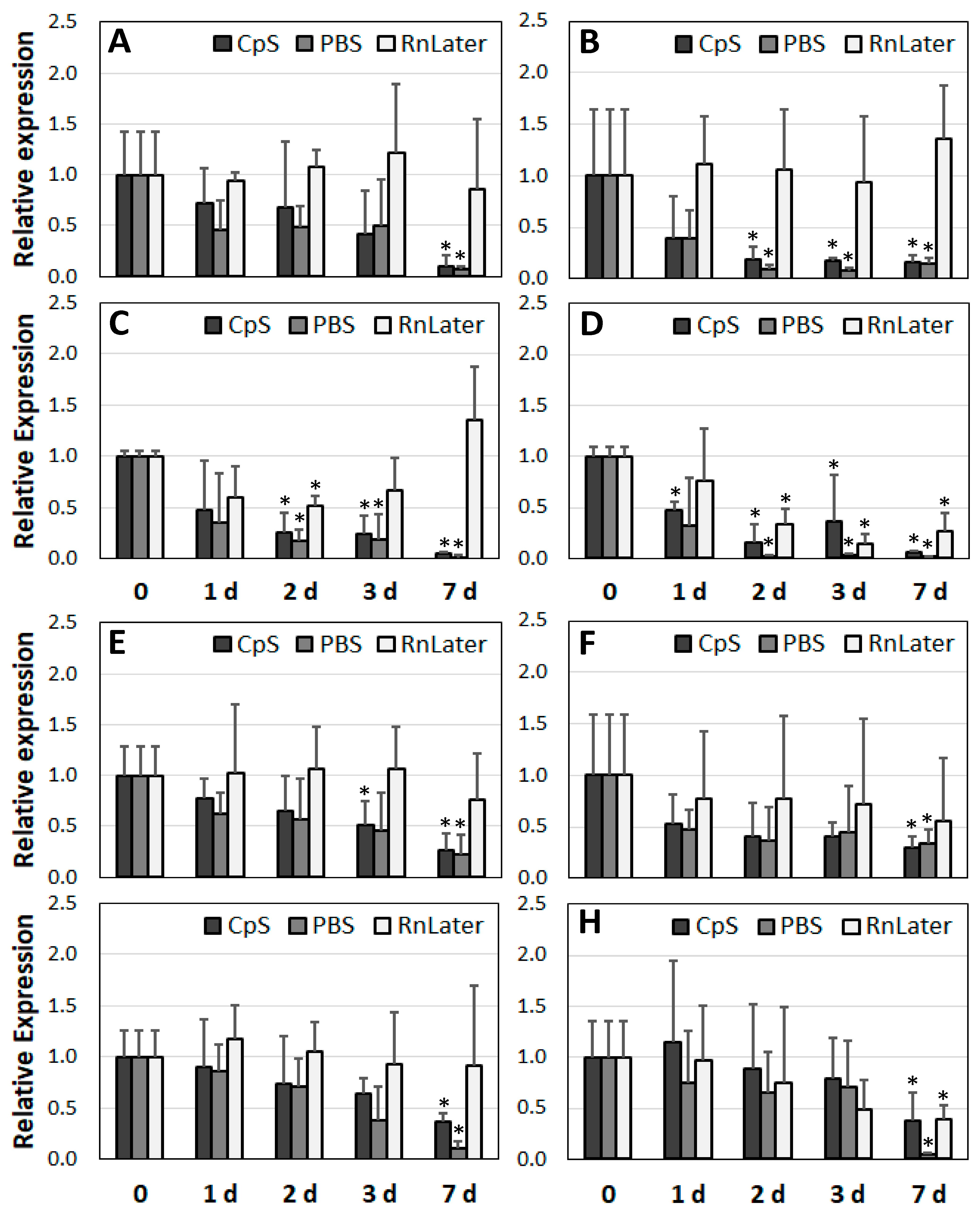
| Muscle | |||||||||
| RIN | DV200 | ACTB | GAPDH | RS18 | RS28 | MALAT1 | H19 | ||
| CpS | Time | −0.706 ** | n.s. | n.s. | −0.564 ** | n.s. | −0.545 ** | −0.632 ** | −0.745 *** |
| RIN | 0.601 * | n.s. | n.s. | 0.715 ** | 0.794 *** | 0.794 *** | 0.683 ** | ||
| DV200 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||
| PBS | Time | −0.922 *** | n.s. | −0.545 * | −0.652 ** | −0.521 * | −0.912 *** | −0.681 ** | −0.692 ** |
| RIN | n.s. | 0.645 ** | 0.583 * | 0.508 * | 0.878 *** | 0.885 *** | 0.686 ** | ||
| DV200 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||
| RNALater | Time | −0.467 * | n.s. | n.s. | n.s. | n.s. | −0.545 * | n.s. | n.s. |
| RIN | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| DV200 | 0.543 * | n.s. | n.s. | n.s. | n.s. | n.s. | |||
| Healthy aorta | |||||||||
| RIN | DV200 | ACTB | GAPDH | RS18 | RS28 | MALAT1 | H19 | ||
| CpS | Time | n.s. | n.s. | n.s. | n.s. | n.s. | −0.520 * | −0.707 *** | −0.647 ** |
| RIN | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| DV200 | n.s. | n.s. | 0.510 * | 0.713 ** | n.s. | n.s. | |||
| PBS | Time | −0.685 ** | −0.873 *** | −0.615 ** | −0.771 *** | −0.623 ** | −0.529 * | −0.737 *** | −0.561 * |
| RIN | 0.743 ** | n.s. | 0.533 * | 0.517 * | n.s. | 0.490 * | n.s. | ||
| DV200 | 0.523 * | 0.785 *** | 0.629 * | 0.553 * | 0.763 *** | 0.577 * | |||
| RNALater | Time | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| RIN | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| DV200 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||
| AAA | |||||||||
| RIN | DV200 | ACTB | GAPDH | RS18 | RS28 | MALAT1 | H19 | ||
| CpS | Time | −0.650 ** | n.s. | −0.540 * | −0.705 ** | −0.720 *** | −0.557 * | −0.764 *** | −0.591 ** |
| RIN | 0.541 * | n.s. | 0.674 ** | 0.621 ** | n.s. | 0.497 * | 0.520 * | ||
| DV200 | n.s. | n.s. | n.s. | 0.676 ** | 0.558 * | n.s. | |||
| PBS | Time | −0.542 * | n.s. | −0.506 * | −0.863 *** | −0.779 *** | −0.644 ** | −0.914 *** | −0.669 ** |
| RIN | n.s. | n.s. | n.s. | 0.503 * | n.s. | 0.569 * | 0.458 * | ||
| DV200 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||
| RNALater | Time | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| RIN | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0.459 * | ||
| DV200 | 0.608 * | n.s. | 0.625 * | n.s. | n.s. | n.s. | |||
| PAD | |||||||||
| RIN | DV200 | ACTB | GAPDH | RS18 | RS28 | MALAT1 | H19 | ||
| CpS | Time | −0.604 ** | −0.505 * | −0.644 ** | −0.739 *** | −0.798 *** | −0.578 ** | −0.771 *** | n.s. |
| RIN | n.s. | 0.518 * | n.s. | n.s. | n.s. | n.s. | 0.584 ** | ||
| DV200 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||
| PBS | Time | −0.550 * | −0.640 * | −0.776 *** | −0.831 *** | −0.836 *** | −0.703 *** | −0.815 *** | −0.466 * |
| RIN | n.s. | 0.465 * | n.s. | n.s. | n.s. | 0.486 * | n.s. | ||
| DV200 | 0.732 ** | n.s. | n.s. | n.s. | 0.764 ** | n.s. | |||
| RNALater | Time | n.s. | n.s. | n.s. | n.s. | n.s. | −0.481 * | −0.751 ** | n.s. |
| RIN | n.s. | n.s. | n.s. | n.s. | n.s. | 0.454 * | n.s. | ||
| DV200 | n.s. | n.s. | n.s. | n.s. | n.s. | 0.536 * | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelisek, J.; Yundung, Y.; Reutersberg, B.; Meuli, L.; Rössler, F.; Rabin, L.; Kopp, R.; Zimmermann, A. Swiss Vascular Biobank: Evaluation of Optimal Extraction Method and Admission Solution for Preserving RNA from Human Vascular Tissue. J. Clin. Med. 2023, 12, 5109. https://doi.org/10.3390/jcm12155109
Pelisek J, Yundung Y, Reutersberg B, Meuli L, Rössler F, Rabin L, Kopp R, Zimmermann A. Swiss Vascular Biobank: Evaluation of Optimal Extraction Method and Admission Solution for Preserving RNA from Human Vascular Tissue. Journal of Clinical Medicine. 2023; 12(15):5109. https://doi.org/10.3390/jcm12155109
Chicago/Turabian StylePelisek, Jaroslav, Yankey Yundung, Benedikt Reutersberg, Lorenz Meuli, Fabian Rössler, Laetitia Rabin, Reinhard Kopp, and Alexander Zimmermann. 2023. "Swiss Vascular Biobank: Evaluation of Optimal Extraction Method and Admission Solution for Preserving RNA from Human Vascular Tissue" Journal of Clinical Medicine 12, no. 15: 5109. https://doi.org/10.3390/jcm12155109
APA StylePelisek, J., Yundung, Y., Reutersberg, B., Meuli, L., Rössler, F., Rabin, L., Kopp, R., & Zimmermann, A. (2023). Swiss Vascular Biobank: Evaluation of Optimal Extraction Method and Admission Solution for Preserving RNA from Human Vascular Tissue. Journal of Clinical Medicine, 12(15), 5109. https://doi.org/10.3390/jcm12155109








