Evaluation of Clinical Manifestations of Hemorrhoidal Disease, Carried Out Surgeries and Prolapsed Anorectal Tissues: Associations with ABO Blood Groups of Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Characteristics
2.2. Histopathological and Histochemical Investigation of Anorectal Tissues
2.3. Immunohistochemical Investigation of Anorectal Tissues
2.4. Statistical Data Analysis
3. Results
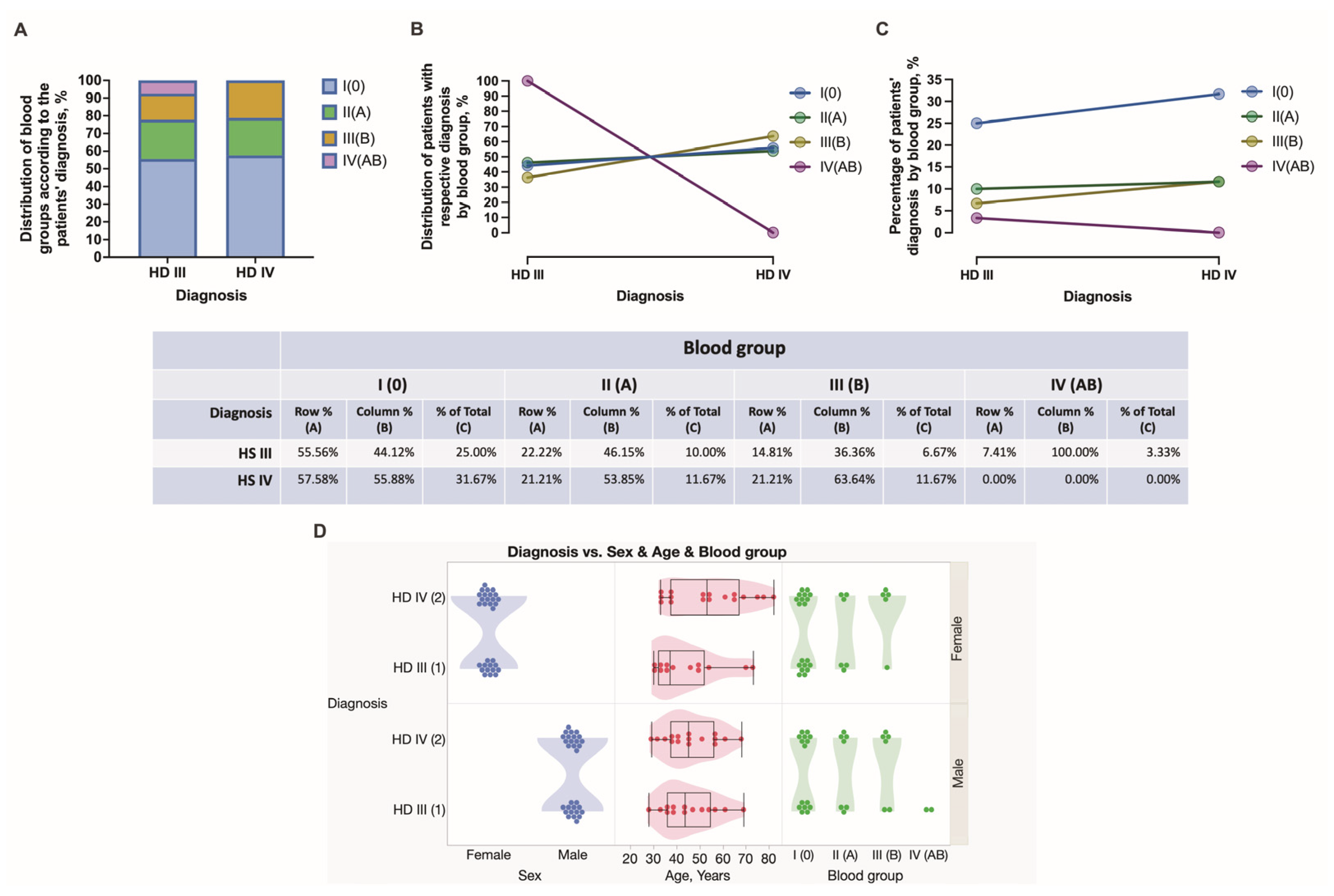
3.1. General Clinical Information
3.2. Comorbidities, HD Patients’ Complaints, and Types of Surgery Used
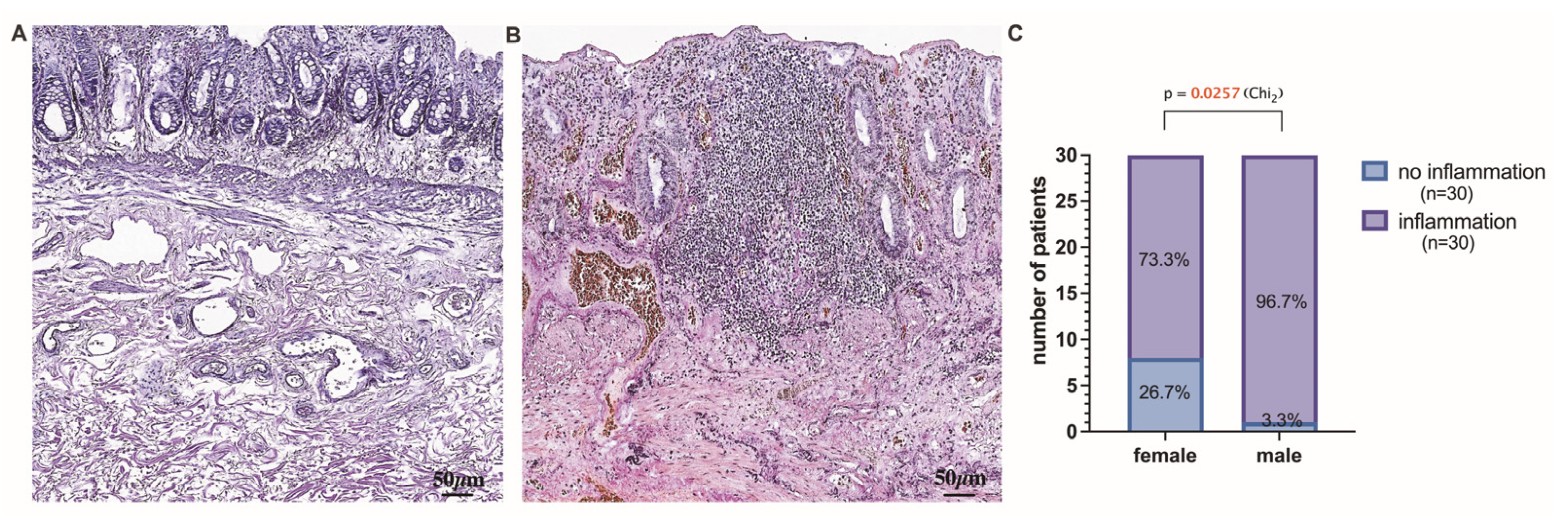
3.3. Histopathological Stratification and Assessment of Tissue Samples Localized above and below the Dentate Line
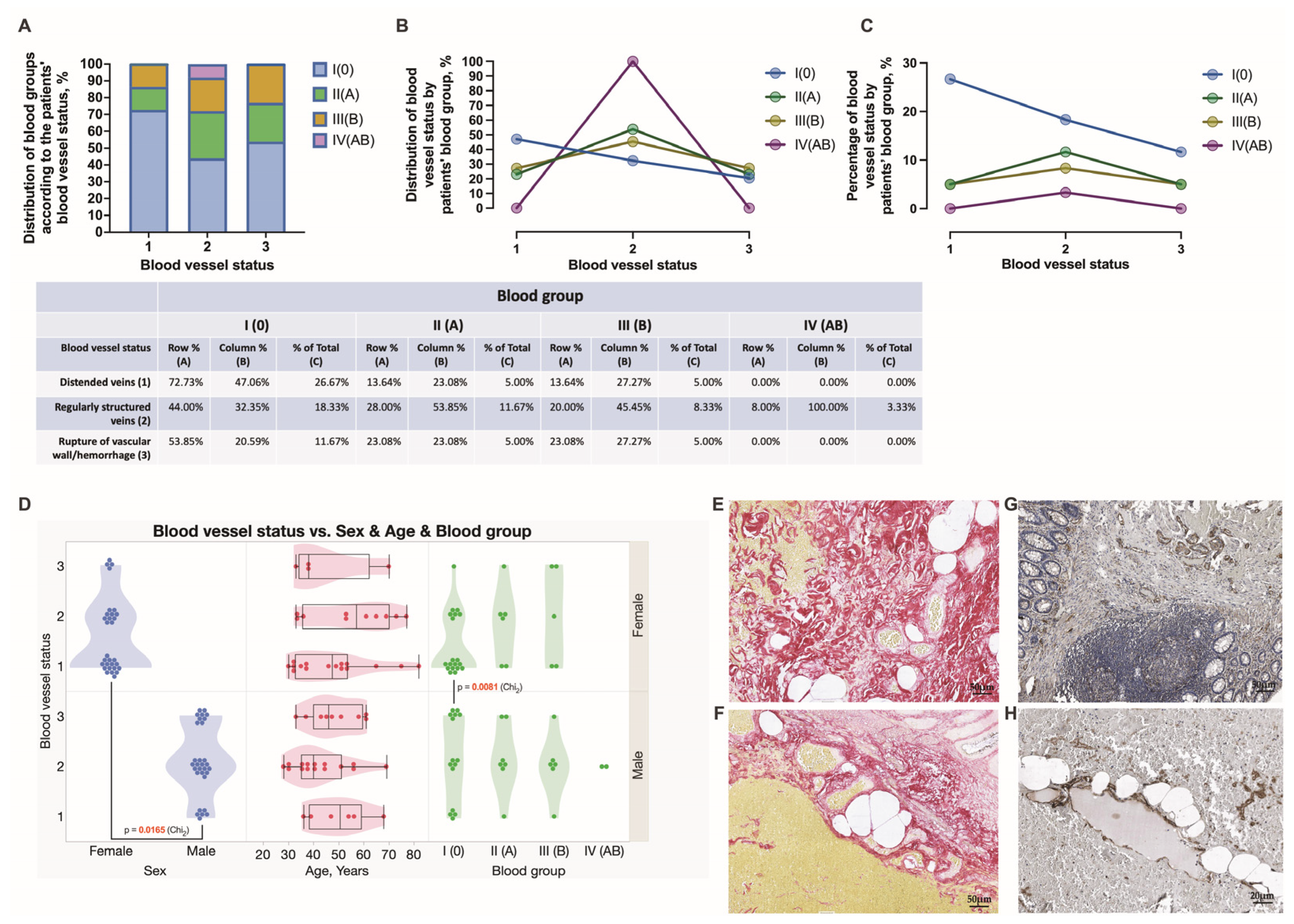
3.4. Assessment of Chronic Anorectal Tissue Injury in Hemorrhoidal Disease Patients
3.5. Hierarchical Clustering Used for the Exploration of Data Similarities and Visualization Using Alluvial Plotting
3.6. Multivariate Analysis of Hemorrhoidal Disease Contributing Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Migaly, J.; Sun, Z. Review of Hemorrhoid Disease: Presentation and Management. Clin. Colon Rectal Surg. 2016, 29, 22–29. [Google Scholar] [CrossRef]
- Lohsiriwat, V. Treatment of hemorrhoids: A coloproctologist’s view. World J. Gastroenterol. 2015, 21, 9245–9252. [Google Scholar] [CrossRef]
- Kibret, A.A.; Oumer, M.; Moges, A.M. Prevalence and associated factors of hemorrhoids among adult patients visiting the surgical outpatient department in the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. PLoS ONE 2021, 16, e0249736. [Google Scholar] [CrossRef] [PubMed]
- Mott, T.; Latimer, K.; Edwards, C. Hemorrhoids: Diagnosis and Treatment Options. Am. Fam. Physician 2018, 97, 172–179. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29431977 (accessed on 3 January 2021). [PubMed]
- Rubbini, M.; Ascanelli, S. Classification and guidelines of hemorrhoidal disease: Present and future. World J. Gastrointest. Surg. 2019, 11, 117–121. [Google Scholar] [CrossRef]
- Gallo, G.; Martellucci, J.; Sturiale, A.; Clerico, G.; Milito, G.; Marino, F.; Cocorullo, G.; Giordano, P.; Mistrangelo, M.; Trompetto, M. Consensus statement of the Italian society of colorectal surgery (SICCR): Management and treatment of hemorrhoidal disease. Tech. Coloproctol. 2020, 24, 145–164. [Google Scholar] [CrossRef] [PubMed]
- De Marco, S.; Tiso, D. Lifestyle and Risk Factors in Hemorrhoidal Disease. Front. Surg. 2021, 8, 729166. [Google Scholar] [CrossRef]
- Pata, F.; Gallo, G.; Pellino, G.; Vigorita, V.; Podda, M.; Di Saverio, S.; D’Ambrosio, G.; Sammarco, G. Evolution of Surgical Management of Hemorrhoidal Disease: An Historical Overview. Front. Surg. 2021, 8, 727059. [Google Scholar] [CrossRef]
- Jayaswal, M.K.; Maurya, O.K. Retrospective Assessment of the Outcome of the Stapled Haemor-rhoidopexy for Haemorrhoids against Conventional Open Technique. Int. J. Pharm. Clin. Res. Orig. Res. Artic. 2022, 14, 705–711. Available online: www.ijpcr.com (accessed on 3 January 2021).
- Hong, Y.S.; Jung, K.U.; Rampal, S.; Zhao, D.; Guallar, E.; Ryu, S.; Chang, Y.; Kim, H.O.; Kim, H.; Chun, H.-K.; et al. Risk factors for hemorrhoidal disease among healthy young and middle-aged Korean adults. Sci. Rep. 2022, 12, 129. [Google Scholar] [CrossRef]
- Mir Mohammad, P.; Sadeghi, P.M.M.; Rabiee, M.; Darestani, N.G.; Alesaheb, F.; Zeinalkhani, F. Short term results of stapled versus conventional hemorrhoidectomy within 1 year follow-up. Int. J. Burn. Trauma 2021, 11, 69–74. [Google Scholar]
- Sahin, M.; Emektar, E.; Kılıç, N.A.; Ozturk, D.; Bulus, H. The role of platelet parameters in thrombosed hemorrhoids. J. Coloproctol. 2020, 40, 362–367. [Google Scholar] [CrossRef]
- Percalli, L.; Passalia, L.; Pricolo, R.; Riccò, M. Pre-operative assessment of internal mucosal rectal prolapse in internal hemorrhoids: Technical details and results from a single institution. Acta Bio Medica Atenei Parm. 2019, 90, 308–315. [Google Scholar] [CrossRef]
- Salgueiro, P.; Caetano, A.C.; Oliveira, A.M.; Rosa, B.; Mascarenhas-Saraiva, M.; Ministro, P.; Amaro, P.; Godinho, R.; Coelho, R.; Gaio, R.; et al. Portuguese Society of Gastroenterology Consensus on the Diagnosis and Management of Hemorrhoidal Disease. GE-Port. J. Gastroenterol. 2020, 27, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Aigner, F.; Gruber, H.; Conrad, F.; Eder, J.; Wedel, T.; Zelger, B.; Engelhardt, V.; Lametschwandtner, A.; Wienert, V.; Böhler, U.; et al. Revised morphology and hemodynamics of the anorectal vascular plexus: Impact on the course of hemorrhoidal disease. Int. J. Color. Dis. 2009, 24, 105–113. [Google Scholar] [CrossRef]
- Watson, A.J.; Cook, J.; Hudson, J.; Kilonzo, M.; Wood, J.; Bruhn, H.; Brown, S.; Buckley, B.; Curran, F.; Jayne, D.; et al. A pragmatic multicentre randomised controlled trial comparing stapled haemorrhoidopexy with traditional excisional surgery for haemorrhoidal disease: The eTHoS study. Health Technol. Assess. 2017, 21, 1–223. [Google Scholar] [CrossRef]
- Roervik, H.D.; Styr, K.; Ilum, L.; McKinstry, G.L.; Dragesund, T.; Campos, A.H.; Brandstrup, B.; Olaison, G. Hemorrhoidal Disease Symptom Score and Short Health ScaleHD: New Tools to Evaluate Symptoms and Health-Related Quality of Life in Hemorrhoidal Disease. Dis. Colon Rectum 2019, 62, 333–342. [Google Scholar] [CrossRef]
- Davis, B.R.; Lee-Kong, S.A.; Migaly, J.; Feingold, D.L.; Steele, S.R. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Hemorrhoids. Dis. Colon Rectum 2018, 61, 284–292. [Google Scholar] [CrossRef]
- Maternini, M.; Guttadauro, A.; Chiarelli, M.; Bianco, G.L.; Pecora, N.; Gabrielli, F. Pervenuto in Redazione Dicembre 2018. Ann. Ital. Chir. 2018, 89, 101–106, pii:S0003469X1802818X. [Google Scholar]
- Alonso-Burgos, A. Interventional Radiology Should be Competitive—If Haemorrhoids are Arteriovenous Connections, It is Now the Time for Liquids? Commentary on “Superior Rectal Artery Embolisation for Haemorrhoids: What do We Know so Far?”. Cardiovasc. Interv. Radiol. 2021, 44, 686–688. [Google Scholar] [CrossRef]
- Serra, R.; Gallelli, L.; Grande, R.; Amato, B.; De Caridi, G.; Sammarco, G.; Ferrari, F.; Butrico, L.; Gallo, G.; Rizzuto, A.; et al. Hemorrhoids and matrix metalloproteinases: A multicenter study on the predictive role of biomarkers. Surgery 2015, 159, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Labidi, A.; Maamouri, F.; Letaief-Ksontini, F.; Maghrebi, H.; Serghini, M.; Boubaker, J. Dietary habits asso-ciated with internal hemorrhoidal disease: A case-control study. Tunis Med. 2019, 97, 572–578. [Google Scholar] [PubMed]
- Zheng, T.; Ellinghaus, D.; Juzenas, S.; Cossais, F.; Burmeister, G.; Mayr, G.; Jørgensen, I.F.; Teder-Laving, M.; Skogholt, A.H.; Chen, S.; et al. Genome-wide analysis of 944 133 individuals provides insights into the etiology of haemorrhoidal disease. Gut 2021, 70, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Said, O.; Khamaysi, I.; Kmail, A.; Sadiq, O.; Saied, B.; Fulder, S.; Abofarekh, B.; Masalha, M.; Amin, R.; Saad, B. Anti-Inflammatory, Antimicrobial, and Vasoconstriction Activities of an Anti-Hemorrhoidal Mixture of Alchemilla vulgaris, Conyza bonariensis, and Nigella sativa: In Vitro and Clinical Evaluations. Immuno 2022, 2, 132–150. [Google Scholar] [CrossRef]
- Lee, K.-C.; Liu, C.-C.; Hu, W.-H.; Lu, C.-C.; Lin, S.-E.; Chen, H.-H. Risk of delayed bleeding after hemorrhoidectomy. Int. J. Color. Dis. 2019, 34, 247–253. [Google Scholar] [CrossRef]
- Ng, K.-S.; Holzgang, M.; Young, C. Still a Case of “No Pain, No Gain”? An Updated and Critical Review of the Pathogenesis, Diagnosis, and Management Options for Hemorrhoids in 2020. Ann. Coloproctol. 2020, 36, 133–147. [Google Scholar] [CrossRef]
- Willis, S.; Junge, K.; Ebrahimi, R.; Prescher, A.; Schumpelick, V. Haemorrhoids—A collagen disease? Color. Dis. 2010, 12, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, V.D.; Tutino, R.; Messina, M.; Santarelli, M.; Nigro, C.; Lo Secco, G.; Piceni, C.; Montanari, E.; Barletta, G.; Venturelli, P.; et al. Altered Gut Microbic Flora and Haemorrhoids: Could They Have a Possible Relationship? J. Clin. Med. 2023, 12, 2198. [Google Scholar] [CrossRef]
- Mehic, D.; Hofer, S.; Jungbauer, C.; Kaider, A.; Haslacher, H.; Eigenbauer, E.; Rejtő, J.; Schwartz, D.; Jilma, B.; Ay, C.; et al. Association of ABO blood group with bleeding severity in patients with bleeding of unknown cause. Blood Adv. 2020, 4, 5157–5164. [Google Scholar] [CrossRef]
- Bayan, K.; Tüzün, Y.; Yılmaz, Ş.; Dursun, M.; Canoruc, F. Clarifying the Relationship Between ABO/Rhesus Blood Group Antigens and Upper Gastrointestinal Bleeding. Dig. Dis. Sci. 2009, 54, 1029–1034. [Google Scholar] [CrossRef]
- Lin, W.-Y.; Hong, M.-Y.; Lin, C.-H.; Chang, P.-P.; Chu, S.-C.; Kao, C.-L. Association of ABO Blood Type with Bleeding Severity in Patients with Acute Gastroesophageal Variceal Bleeding. Medicina 2021, 57, 1323. [Google Scholar] [CrossRef]
- Ewald, D.R.; Sumner, S.C. Blood type biochemistry and human disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 517–535. [Google Scholar] [CrossRef]
- Langenbach, M.R.; Lisovets, R.; Varga-Szabo, D.; Bonicke, L. Decreased collagen ratio type I/III in association with hemorrhoidal disease. J. Transl. Sci. 2018, 5, 1–4. [Google Scholar] [CrossRef][Green Version]
- Sturiale, A.; Dowais, R.; Fabiani, B.; Menconi, C.; Porzio, F.C.; Coli, V.; Naldini, G. Long-term Outcomes of High-Volume Stapled Hemorroidopexy to Treat Symptomatic Hemorrhoidal Disease. Ann. Coloproctol. 2021, 39, 11–16. [Google Scholar] [CrossRef]
- Kim, J.S. Partial Stapled Hemorrhoidopexy Versus Circular Stapled Hemorrhoidopexy. Ann. Coloproctol. 2017, 33, 7–8. [Google Scholar] [CrossRef]
- Maemoto, R.; Tsujinaka, S.; Miyakura, Y.; Machida, E.; Fukui, T.; Kakizawa, N.; Tamaki, S.; Ishikawa, H.; Rikiyama, T. Effect of Antithrombotic Therapy on Secondary Bleeding After Proctological Surgery. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Kraemer, M.; Paulus, W.; Kara, D.; Mankewitz, S.; Rozsnoki, S. Rectal prolapse traumatizes rectal neuromuscular microstructure explaining persistent rectal dysfunction. Int. J. Color. Dis. 2016, 31, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Porrett, L.J.; Porrett, J.K.; Ho, Y.-H. Documented Complications of Staple Hemorrhoidopexy: A Systematic Review. Int. Surg. 2015, 100, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Plocek, M.D.; Kondylis, L.A.; Duhan-Floyd, N.; Reilly, J.C.; Geisler, D.P.; Kondylis, P.D. Hemorrhoidopexy Staple Line Height Predicts Return to Work. Dis. Colon Rectum 2006, 49, 1905–1909. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.K.; Choi, Y.J.; Kang, J.G. Correlation of Histopathology with Anorectal Manometry Following Stapled Hemorrhoidopexy. Ann. Coloproctol. 2013, 29, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Eberspacher, C.; Magliocca, F.M.; Pontone, S.; Mascagni, P.; Fralleone, L.; Gallo, G.; Mascagni, D. Stapled Hemorrhoidopexy: “Mucosectomy or Not Only Mucosectomy, This Is the Problem”. Front. Surg. 2021, 8, 655257. [Google Scholar] [CrossRef] [PubMed]
- Abegaz, S.B. Human ABO Blood Groups and Their Associations with Different Diseases. BioMed Res. Int. 2021, 2021, 6629060. [Google Scholar] [CrossRef] [PubMed]
- Groot, H.E.; Sierra, L.E.V.; Said, M.A.; Lipsic, E.; Karper, J.C.; van der Harst, P. Genetically Determined ABO Blood Group and its Associations with Health and Disease. Arter. Thromb. Vasc. Biol. 2020, 40, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Dahlén, T.; Clements, M.; Zhao, J.; Olsson, M.L.; Edgren, G. An agnostic study of associations between ABO and RhD blood group and phenome-wide disease risk. Elife 2021, 10, e65658. [Google Scholar] [CrossRef]
- Yu, H.; Xu, N.; Li, Z.-K.; Xia, H.; Ren, H.-T.; Li, N.; Wei, J.-B.; Bao, H.-Z. Association of ABO Blood Groups and Risk of Gastric Cancer. Scand. J. Surg. 2019, 109, 309–313. [Google Scholar] [CrossRef]
- Sardinas, C.; Díaz, D.; Oropeza, M.E.; Parrella, M.; Merheb, M. Rectal Prolapse due to Adenomatous Polyp: Case Report. J. Hepatol. Gastrointest. Disord. 2016, 2, 2. [Google Scholar] [CrossRef]
- Deng, Y.-J.; Li, S.-L.; Jing, F.-Y.; Ma, L.-L.; Guo, L.-L.; Na, F.; An, S.-L.; Ye, Y.; Yang, J.-M.; Bao, M.; et al. Myofibrotic malformation vessels: Unique angiodysplasia toward the progression of hemorrhoidal disease. Drug Des. Dev. Ther. 2015, 9, 4649–4656. [Google Scholar] [CrossRef][Green Version]
- Symeonidis, D.; Spyridakis, M.; Zacharoulis, D.; Tzovaras, G.; Samara, A.A.; Valaroutsos, A.; Diamantis, A.; Tepetes, K. Milligan–Morgan hemorrhoidectomy vs. hemorrhoid artery ligation and recto-anal repair: A comparative study. BMC Surg. 2022, 22, 416. [Google Scholar] [CrossRef]
- Matkovic, Z.; Zildzic, M. Colonoscopic Evaluation of Lower Gastrointestinal Bleeding (LGIB): Practical Approach. Med Arch. 2021, 75, 274–279. [Google Scholar] [CrossRef]
- Cafaro, D.D.; Sturiale, D.A.; Sinicropi, D.M.S.; Onofrio, D.L.; Catalano, A.; Naldini, G. Topical Treatment with Bergamot Flavonoid-Based Gel in Post-Surgical Wounds after Hemorrhoidectomy: Preliminary Results. Int. J. Innov. Res. Med. Sci. 2022, 7, 33–36. [Google Scholar] [CrossRef]
- Takahashi, Y.; Hayakawa, A.; Sano, R.; Fukuda, H.; Harada, M.; Kubo, R.; Okawa, T.; Kominato, Y. Histone deacetylase inhibitors suppress ACE2 and ABO simultaneously, suggesting a preventive potential against COVID-19. Sci. Rep. 2021, 11, 3379. [Google Scholar] [CrossRef]
- Marano, G.; Vaglio, S.; Catalano, L.; Pupella, S.; Liumbruno, G.M.; Franchini, M. The Role of ABO Blood Type in Thrombosis Scoring Systems. Semin. Thromb. Hemost. 2017, 43, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Bade, N.A.; Kazma, J.M.; Amdur, R.L.; Ellis-Kahana, J.; Ahmadzia, H.K. Blood type association with bleeding outcomes at delivery in a large multi-center study. J. Thromb. Thrombolysis 2020, 50, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Albánez, S.; Ogiwara, K.; Michels, A.; Hopman, W.; Grabell, J.; James, P.; Lillicrap, D. Aging and ABO blood type influence von Willebrand factor and factor VIII levels through interrelated mechanisms. J. Thromb. Haemost. 2016, 14, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Slade, R.; Alikhan, R.; Wise, M.P.; Germain, L.; Stanworth, S.; Morgan, M. Impact of blood group on survival following critical illness: A single-centre retrospective observational study. BMJ Open Respir. Res. 2019, 6, e000426. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, B.; Northup, P.G.; Gruber, A.B.; Semmler, G.; Leitner, G.; Quehenberger, P.; Thaler, J.; Ay, C.; Trauner, M.; Reiberger, T.; et al. The impact of ABO blood type on the prevalence of portal vein thrombosis in patients with advanced chronic liver disease. Liver Int. 2020, 40, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Danilo, C.; Felipe, C.; Alessandro, S.; Stefania, S.M. Innovative results in the treatment of inespecific anusitis-proctitis with the use of bergamot gel (Benebeo gel)®. Insights Clin. Cell. Immunol. 2019, 3, 020–024. [Google Scholar] [CrossRef]
- de Freitas, M.O.S.; Santos, J.A.D.; Figueiredo, M.F.S.; and Sampaio, C.A. Analysis of the main surgical techniques for hemorrhoids. J. Coloproctol. 2016, 36, 104–114. [Google Scholar] [CrossRef][Green Version]
- Naldini, G.; Caminati, F.; Sturiale, A.; Fabiani, B.; Cafaro, D.; Menconi, C.; Mascagni, D.; Celedon Porzio, F. Improvement in Hemorrhoidal Disease Surgery Outcomes Using a New Anatomical/Clinical–Therapeutic Classification (A/CTC). Surg. J. 2020, 6, e145–e152. [Google Scholar] [CrossRef]
- Taieb, S.; Atienza, P.; Zeitoun, J.D.; Taouk, M.; Bourguignon, J.; Thomas, C.; Rabahi, N.; Dahlouk, S.; Lesage, A.C.; Lobo, D.; et al. Frequency and risk factors of severe postoperative bleeding after proctological surgery: A retrospective case-control study. Ann. Coloproctol. 2022, 38, 370–375. [Google Scholar] [CrossRef]
- Yoo, B.E.; Kang, W.H.; Ko, Y.T.; Lee, Y.C.; Lim, C.H. The importance of compression time in stapled hemorrhoidopexy: Is patience a virtue? Ann. Coloproctol. 2022, 20, 1–6. [Google Scholar] [CrossRef]
- Ratto, C.; de Parades, V. Doppler-guided ligation of hemorrhoidal arteries with mucopexy: A technique for the future. J. Visc. Surg. 2015, 152, S15–S21. [Google Scholar] [CrossRef]
- Varut, P.S.; Shelygin, L.Y. Micronized Purified Flavonoid Fraction in Hemorrhoid Disease: A Systematic Review and Meta-Analysis. Adv. Ther. 2020, 37, 2792–2812. [Google Scholar] [CrossRef]
- Loste, M.A.; de la Peña, J.; Terán, A. Patología inflamatoria de recto y ano. Med.-Programa Form. Médica Contin. Acreditado 2012, 11, 413–419. [Google Scholar] [CrossRef]
- Li, Z.; Dong, S.; Huang, S.; Sun, Y.; Sun, Y.; Zhao, B.; Qi, Q.; Xiong, L.; Hong, F.; Jiang, Y. Role of CD34 in inflammatory bowel disease. Front. Physiol. 2023, 14, 1144980. [Google Scholar] [CrossRef] [PubMed]
- Bahardoust, M.; Naghshin, R.; Mokhtare, M.; Hejrati, A.; Namdar, P.; Talebi, A.; Tavakoli, T.; Amiri, H.; Kiapey, S.H. Association between ABO Blood Group and Clinical Outcomes in Patients with Gastrointestinal Bleeding. J. Clin. Cell. Immunol. 2018, 8. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fišere, I.; Groma, V.; Svirskis, Š.; Strautmane, E.; Gardovskis, A. Evaluation of Clinical Manifestations of Hemorrhoidal Disease, Carried Out Surgeries and Prolapsed Anorectal Tissues: Associations with ABO Blood Groups of Patients. J. Clin. Med. 2023, 12, 5119. https://doi.org/10.3390/jcm12155119
Fišere I, Groma V, Svirskis Š, Strautmane E, Gardovskis A. Evaluation of Clinical Manifestations of Hemorrhoidal Disease, Carried Out Surgeries and Prolapsed Anorectal Tissues: Associations with ABO Blood Groups of Patients. Journal of Clinical Medicine. 2023; 12(15):5119. https://doi.org/10.3390/jcm12155119
Chicago/Turabian StyleFišere, Inese, Valērija Groma, Šimons Svirskis, Estere Strautmane, and Andris Gardovskis. 2023. "Evaluation of Clinical Manifestations of Hemorrhoidal Disease, Carried Out Surgeries and Prolapsed Anorectal Tissues: Associations with ABO Blood Groups of Patients" Journal of Clinical Medicine 12, no. 15: 5119. https://doi.org/10.3390/jcm12155119
APA StyleFišere, I., Groma, V., Svirskis, Š., Strautmane, E., & Gardovskis, A. (2023). Evaluation of Clinical Manifestations of Hemorrhoidal Disease, Carried Out Surgeries and Prolapsed Anorectal Tissues: Associations with ABO Blood Groups of Patients. Journal of Clinical Medicine, 12(15), 5119. https://doi.org/10.3390/jcm12155119





