Relationship between Urinary Parameters and Double-J Stent Encrustation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. Data Availability
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Finney, R. Experience with new double J ureteral catheter stent. J. Urol. 1978, 120, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Beysens, M.; Tailly, T.O. Ureteral stents in urolithiasis. Asian J. Urol. 2018, 5, 274–286. [Google Scholar] [CrossRef]
- Cepeda, M.; Mainez, J.A.; de la Cruz, B.; Amón, J.H. Indications and morbidity associated with double J. catheters. Arch. Esp. Urol. 2016, 69, 462–470. [Google Scholar] [PubMed]
- Skolarikos, A.; Jung, H.; Neisius, A.; Petrík, A.; Somani, B.; Tailly, T.; Gambaro, G. EAU Guidelines on Urolithiasis. In Proceedings of the EAU Annual Congress Amsterdam, Amsterdam, The Netherlands, 1–4 July 2022; EAU Guidelines Edition. ISBN 978-94-92671-16-5. [Google Scholar]
- Joshi, H.B.; Newns, N.; Stainthorpe, A.; MacDonagh, R.P.; Keeley, F.X.; Timoney, A.G. Ureteral stent symptom questionnaire: Development and validation of a multidimensional quality of life measure. J. Urol. 2003, 169, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.; Okeke, A.; Newns, N.; Keeley, F.; Timoney, A. Characterization of urinary symptoms in patients with ureteral stents. Urology 2002, 59, 511–516. [Google Scholar] [CrossRef]
- Pérez-Fentes, D. Complications of double j catheters and their endourological management. Arch. Esp. Urol. 2016, 69, 527–543. [Google Scholar]
- Singh, V.; Srinivastava, A.; Kapoor, R.; Kumar, A. Can the complicated forgotten indwelling ureteric stents be lethal? Int. Urol. Nephrol. 2005, 37, 541–546. [Google Scholar] [CrossRef]
- Sancaktutar, A.; Söylemez, H.; Bozkurt, Y.; Penbegül, N.; Atar, M. Treatment of forgotten ureteral stents: How much does it really cost? A cost-effectiveness study in 27 patients. Urol. Res. 2012, 40, 317–325. [Google Scholar] [CrossRef]
- Tsaturyan, A.; Faria-Costa, G.; Peteinaris, A.; Lattarulo, M.; Martinez, B.B.; Vrettos, T.; Liatsikos, E.; Kallidonis, P. Endoscopic management of encrusted ureteral stents: Outcomes and tips and tricks. World J. Urol. 2023, 41, 1415–1421. [Google Scholar] [CrossRef]
- Tomer, N.; Garden, E.; Small, A.; Palese, M. Ureteral Stent Encrustation: Epidemiology, Pathophysiology, Management and Current Technology. J. Urol. 2021, 205, 68–77. [Google Scholar] [CrossRef]
- Small, A.; Thorogood, S.; Shah, O.; Healy, K. Emerging Mobile Platforms to Aid in Stone Management. Urol. Clin. N. Am. 2019, 46, 287–301. [Google Scholar] [CrossRef]
- Ibilibor, C.; Grand, R.; Daneshfar, C.; DeRiese, W.; Smith, C. Impact of Retained Ureteral Stents on Long-Term Renal Function. Urol. Pract. 2019, 6, 107–111. [Google Scholar]
- Cao, Z.; Zhao, J.; Yang, K. Cu-bearing stainless steel reduces cytotoxicity and crystals adhesion after ureteral epithelial cells exposing to calcium oxalate monohydrate. Sci. Rep. 2018, 8, 14094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleeson, M.; Glueck, J.; Feldman, L.; Griffith, D.; Noon, G. Comparative in vitro encrustation studies of biomaterials in human urine. ASAIO Trans. 1989, 35, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Söhnel, O.; Costa-Bauzá, A.; Ramis, M.; Wang, Z. Study on concretions developed around urinary catheters and mechanisms of renal calculi development. Nephron 2001, 88, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Bouzidi, H.; Traxer, O.; Doré, B.; Amiel, J.; Hadjadj, H.; Conort, P.; Daudon, M. Characteristics of encrustation of ureteric stents in patients with urinary stones. Prog. Urol. 2008, 18, 230–237. [Google Scholar] [CrossRef]
- Rouprêt, M.; Hupertan, V.; Daudon, M.; Lebrun, S.; Sebe, P.; Gattegno, B.; Thibault, P.; Traxer, O. Value of infrared spectrophotometry morpho-constitutional analysis of double J stent encrustations for indirect determination of urinary stone composition. Prog. Urol. 2005, 15, 411–415. (In French) [Google Scholar]
- Grases, F.; Costa-Bauzá, A.; Ramis, M.; Montesinos, V.; Conte, A. Simple classification of renal calculi closely related to their micromorphology and etiology. Clin. Chim. Acta 2002, 322, 29–36. [Google Scholar] [CrossRef]
- Calvó, P.; Bauza, J.L.; Julià, F.; Guimerá, J.; Pieras, E.C.; Costa-Bauzá, A.; Grases, F. Characterization of deposits on double J stents. Comptes Rendus Chim. 2021, 24, 425–430. [Google Scholar] [CrossRef]
- Gauhar, V.; Pirola, G.M.; Scarcella, S.; Angelis, M.V.D.; Giulioni, C.; Rubilotta, E.; Gubbiotti, M.; Lim, E.J.; Law, Y.X.T.; Wroclawski, M.L.; et al. Nephrostomy tube versus double J ureteral stent in patients with malignant ureteric obstruction. A systematic review and meta-analysis of comparative studies. Int. Braz. J. Urol. 2022, 48, 903–914. [Google Scholar]
- Wilson, C.H.; Bhatti, A.B.; Rix, D.A.; Manas, D.M. Routine intraoperative ureteric stenting for kidney transplant recipients. Cochrane Database Syst. Rev. 2013, 6, CD004925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sali, G.M.; Joshi, H.B. Ureteric stents: Overview of current clinical applications and economic implications. Int. J. Urol. 2020, 27, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kram, W.; Buchholz, N.N.P.; Hakenberg, O.W. Ureteral stent encrustation. Pathophysiology. Arch. Esp. Urol. 2016, 69, 485–493. [Google Scholar]
- Bithelis, G.; Bouropoulos, N.; Liatsikos, E.N.; Perimenis, P.; Koutsoukos, P.G.; Barbalias, G.A. Assessment of encrustations on polyurethane ureteral stents. J. Endourol. 2004, 18, 550–556. [Google Scholar] [CrossRef] [PubMed]
- El-Faqih, S.R.; Shamsuddin, A.B.; Chakrabarti, A.; Atassi, R.; Kardar, A.H.; Osman, M.K.; Husain, I. Polyurethane internal ureteral stents in treatment of stone patients: Morbidity related to indwelling times. J. Urol. 1991, 146, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Ito, H.; Terao, H.; Yoshida, M.; Matsuzaki, J. Ureteral stent encrustation, incrustation, and coloring: Morbidity related to indwelling times. J. Endourol. 2012, 26, 178–182. [Google Scholar] [CrossRef]
- Legrand, F.; Saussez, T.; Ruffion, A.; Celia, A.; Djouhri, F.; Musi, G.; Kalakech, S.; Desriac, I.; Roumeguère, T. Double Loop Ureteral Stent Encrustation According to Indwelling Time: Results of a European Multicentric Study. J. Endourol. 2021, 35, 84–90. [Google Scholar] [CrossRef]
- Sighinolfi, M.C.; Sighinolfi, G.P.; Galli, E.; Micali, S.; Ferrari, N.; Mofferdin, A.; Bianchi, G. Chemical and Mineralogical Analysis of Ureteral Stent Encrustation and Associated Risk Factors. Urology 2015, 86, 703–706. [Google Scholar] [CrossRef]
- Torrecilla, C.; Fernández-Concha, J.; Cansino, J.R.; Mainez, J.A.; Amón, J.H.; Costas, S.; Angerri, O.; Emiliani, E.; Arrabal Martín, M.A.; Arrabal Polo, M.A.; et al. Reduction of ureteral stent encrustation by modulating the urine pH and inhibiting the crystal film with a new oral composition: A multicenter, placebo controlled, double blind, randomized clinical trial. BMC Urol. 2020, 20, 65. [Google Scholar] [CrossRef]
- Calvó, P.; Mateu-Borras, M.; Costa-Bauza, A.; Albertí, S.; Grases, F. Effect of phytate on crystallization on ureteral stents and bacterial attachment: An in vitro study. Urolithiasis 2022, 50, 737–742. [Google Scholar] [CrossRef]





| Gender | Male | 57.3% (43) |
| Female | 42.7% (32) | |
| Age | 55 (28–84) | |
| Comorbidities | Hypertension | 37.3% (28) |
| Type II diabetes | 10.6% (8) | |
| Dyslipidemia | 21.3% (16) | |
| Stent placement reason | Emergent | 65.6% (59) |
| Programmed | 34.4% (31) | |
| Stone episode | First | 37.7% (34) |
| Recurrent | 62.2% (56) | |
| Indwelling time (days) | 58 (26–102) | |
| Urine culture | Negative | 77.7% (70) |
| Positive | 22.2% (20) | |
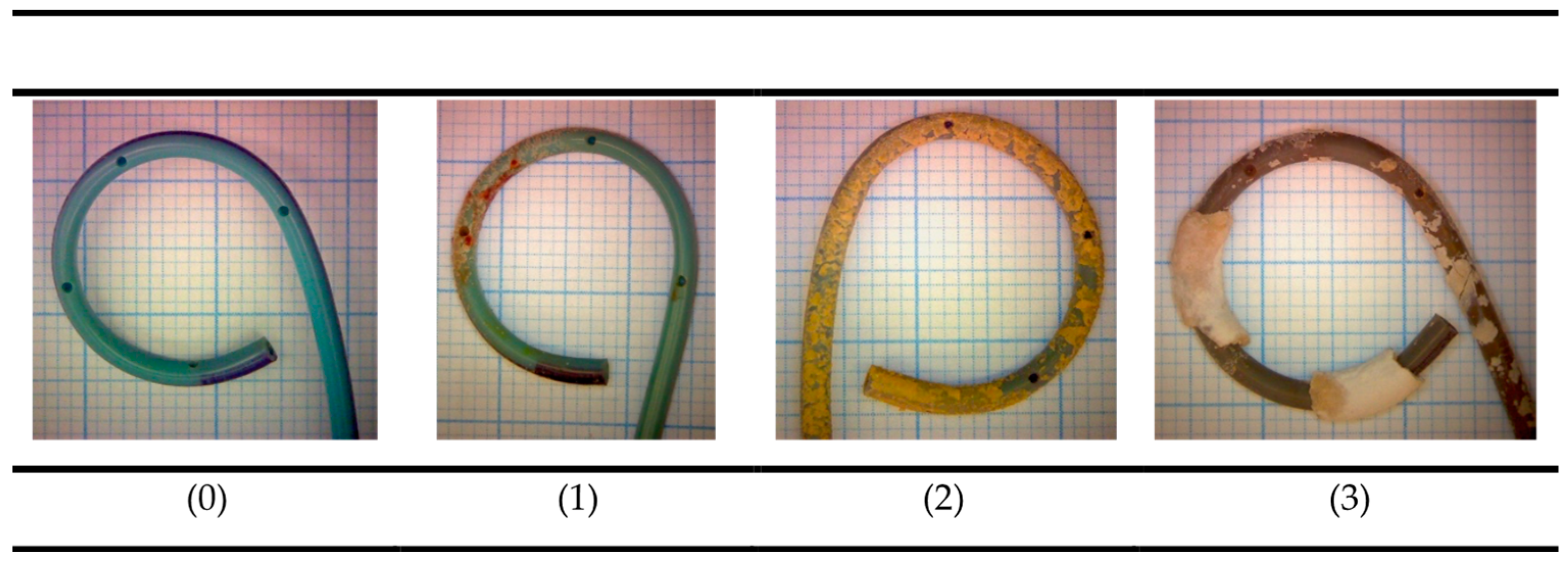
| Encrustation Type | Encrustation Grade | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Calcium (µmol) Median | Phosphorus (µmol) Median | Magnesium (µmol) Median | |||||||
| Low | Medium | High | Low | Medium | High | Low | Medium | High | |
| Calcium oxalate | 46.4 | 351 | 876 | 3.74 | 12.8 | 30.3 | 0.23 | 0.87 | 1.27 |
| Infectious phosphates | - | 486 | 1687 | - | 784 | 1202 | - | 366 | 235 |
| Non-infectious phosphates | 4.99 | 390 | 3607 | 8.1 | 298 | 2674 | 0.43 | 10.4 | 112 |
| Non-Encrusted (n = 25) | Encrusted (n = 65) | ||
|---|---|---|---|
| Urinary Parameters | Median (IQR) | Median (IQR) | p |
| pH | 5.6 (5.2–6.5) | 5.8 (5.5–6.7) | 0.255 |
| 24 h diuresis (mL) | 2000 (1450–2700) | 1800 (1225–2200) | 0.041 |
| Urine creatinine (mg/dL) | 57 (38–99) | 80 (48–99) | 0.178 |
| Urine creatinine (mg/24 h) | 1227 (782–1490) | 1199 (917–1708) | 0.377 |
| Urine urate (mg/dL) | 28 (13–43) | 34 (21–48) | 0.122 |
| Urine urate (mg/24 h) | 534 (376–730) | 551 (362–758) | 0.869 |
| Urine calcium (mg/dL) | 6.9 (3.9–10.5) | 8.3 (6.2–13.1) | 0.111 |
| Urine calcium (mg/24 h) | 132 (89–234) | 165 (100–227) | 0.472 |
| Urine phosphate (mg/dL) | - | 43 (30–68) | 0.054 |
| Urine phosphate (mg/24 h) | 669 (409–928) | 772 (594–945) | 0.283 |
| Urine magnesium (mg/dL) | 3.4 (2.0–6.2) | 4.3 (2.2–6.1) | 0.682 |
| Urine magnesium (mg/24 h) | 86 (56–117) | 85 (69–109) | 0.638 |
| Urine oxalate (mg/L) | 13.8 (9.6–19.8) | 18.0 (14.1–23.9) | 0.025 |
| Urine oxalate (mg/24 h) | 28 (16–39) | 26 (21–41) | 0.779 |
| Urine citrate (mg/L) | 153 (99–278) | 232 (117–409) | 0.154 |
| Urine citrate (mg/24 h) | 270 (221–608) | 476 (257–758) | 0.115 |
| Indwelling time (days) | 35 (16–92) | 67 (37–103) | 0.046 |
| Encrustation Grade | |||
|---|---|---|---|
| None–Low (n = 41) | Medium–High (n = 49) | ||
| Urinary Parameters | Median (IQR) | Median (IQR) | p |
| pH | 6.0 (5.3–6.7) | 5.7 (5.4–6.5) | 0.633 |
| 24 h diuresis (mL) | 2100 (1500–2525) | 1700 (1100–2200) | 0.029 |
| Urine creatinine (mg/dL) | 54 (37–90) | 83 (59–106) | 0.006 |
| Urine creatinine (mg/24 h) | 1106 (794–1484) | 1349 (990–1712) | 0.044 |
| Urine urate (mg/dL) | 24 (14–43) | 37 (22–49) | 0.015 |
| Urine urate (mg/24 h) | 500 (345–720) | 574 (396–778) | 0.262 |
| Urine calcium (mg/dL) | 7.1 (3.9–8.8) | 9.0 (6.6–16.7) | 0.018 |
| Urine calcium (mg/24 h) | 105 (82–214) | 176 (105–244) | 0.140 |
| Urine phosphate (mg/dL) | 35 (24–47) | 47 (30–70) | 0.021 |
| Urine phosphate (mg/24 h) | 701 (481–919) | 789 (576–951) | 0.268 |
| Urine magnesium (mg/dL) | 3.4 (2.1–5.2) | 4.5 (1.6–6.9) | 0.133 |
| Urine magnesium (mg/24 h) | 77 (57–105) | 93 (70–119) | 0.077 |
| Urine oxalate (mg/L) | 14 (10–20) | 19 (15–23) | 0.041 |
| Urine oxalate (mg/24 h) | 28 (21–41) | 26 (20–42) | 0.913 |
| Urine citrate (mg/L) | 158 (100–314) | 267 (151–446) | 0.049 |
| Urine citrate (mg/24 h) | 270 (226–590) | 495 (320–859) | 0.049 |
| Indwelling time (days) | 50 (16–93) | 66 (35–116) | 0.086 |
| Calcium Oxalate (n = 38) | Uric Acid (n = 10) | Infectious Phosphates (n = 5) | Non-Infectious Phosphates (n = 12) | ||
|---|---|---|---|---|---|
| Urinary Parameters | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | p |
| pH | 6.0 (5.7–6.7) | 5.3 (5.0–6.4) | 6.6 (6.2–6.8) | 5.9 (5.5–7.2) | 0.010 |
| 24 h diuresis (mL) | 1800 (1400–2200) | 1600 (1000–2600) | 1533 (1200–1830) | 2050 (1525–2650) | 0.382 |
| Urine creatinine (mg/dL) | 82 (42–99) | 89 (52–111) | 67 (39–90) | 58 (39.7–63.7) | 0.581 |
| Urine creatinine (mg/24 h) | 1067 (845–1712) | 1323 (829–1510) | 1045 (847–1623) | 1176 (919–1472) | 0.783 |
| Urine urate (mg/dL) | 34 (18–40) | 50 (29–420) | 28 (17–34) | 23(17–31) | 0.081 |
| Urine urate (mg/24 h) | 543 (339–749) | 733 (529–3434) | 417 (298–577) | 448 (414–692) | 0.019 |
| Urine calcium (mg/dL) | 8.1 (6.6–13.0) | 6.4 (3.6–12.6) | 10.5 (6.8–13.7) | 8.5 (7.2–11.1) | 0.313 |
| Urine calcium (mg/24 h) | 187 (80–273) | 142 (77–212) | 167 (78–253) | 227 (131–277) | 0.298 |
| Urine phosphate (mg/dL) | 43 (34–68) | 50 (32–75) | 36 (28–62) | 30 (26–39) | 0.651 |
| Urine phosphate (mg/24 h) | 725 (648–937) | 855 (657–1133) | 554 (345–737) | 773 (488–893) | 0.367 |
| Urine magnesium (mg/dL) | 3.5 (2.2–6.0) | 4.3 (2.4–6.0) | 3 (2.2–5.5) | 3.7 (0.8–5.6) | 0.541 |
| Urine magnesium (mg/24 h) | 82 (58–108) | 70 (68–78) | 52 (43–70) | 97 (77–127) | 0.841 |
| Urine oxalate (mg/L) | 18.8 (12.6–20.7) | 19.9 (14.3–23.5) | 5.6 (3.3–17.2) | 18.9 (14.8–25.4) | 0.490 |
| Urine oxalate (mg/24 h) | 28 (24–43) | 31 (24–42) | 24 (24–40) | 41 (32–45) | 0.671 |
| Urine citrate (mg/L) | 232 (102–344) | 255 (116–460) | 133 (101–312) | 120 (98–328) | 0.710 |
| Urine citrate (mg/24 h) | 516 (251–790) | 501 (215–705) | 233 (157–365) | 368 (243–956) | 0.861 |
| Indwelling time (days) | 67 (21–141) | 34 (26–40) | 55 (22–87) | 64 (26–78) | 0.043 |
| Encrustation Presence | |||
|---|---|---|---|
| No (n = 25) | Yes—Calcium Oxalate (n = 38) | ||
| Urinary Parameters | Median (IQR) | Median (IQR) | p |
| pH | 5.6 (5.2–6.5) | 6.00 (5.6–6.7) | 0.363 |
| 24 h diuresis (mL) | 2000 (1450–2700) | 1800 (1400–2200) | 0.01 |
| Urine creatinine (mg/dL) | 57 (38–99) | 82 (42–99) | 0.093 |
| Urine creatinine (mg/24 h) | 1227 (782–1490) | 1067 (845–1712) | 0.215 |
| Urine urate (mg/dL) | 28 (13–43) | 34 (18–40) | 0.100 |
| Urine urate (mg/24 h) | 534 (376–730) | 543 (339–749) | 0.829 |
| Urine calcium (mg/dL) | 6.9 (3.9–10.5) | 8.1 (6.6–13) | 0.045 |
| Urine calcium (mg/24 h) | 132 (89–234) | 187 (80–273) | 0.399 |
| Urine phosphate (mg/dL) | 33 (23–46) | 43 (34–68) | 0.022 |
| Urine phosphate (mg/24 h) | 669 (409–928) | 725 (648–937) | 0.207 |
| Urine magnesium (mg/dL) | 3.4 (2.0–6.2) | 3.5 (2.2–6.0) | 0.641 |
| Urine magnesium (mg/24 h) | 86 (56–117) | 82 (58–108) | 0.711 |
| Urine oxalate (mg/L) | 13.8 (9.6–19.8) | 18.8 (12.6–20.7) | 0.094 |
| Urine oxalate (mg/24 h) | 28 (16–39) | 28 (24–43) | 0.468 |
| Urine citrate (mg/L) | 153 (99–278) | 232 (102–344) | 0.042 |
| Urine citrate (mg/24 h) | 270 (221–608) | 516 (251–790) | 0.075 |
| Indwelling time (days) | 35 (16–92) | 67 (21–141) | 0.034 |
| Encrustation’s Presence | |||
|---|---|---|---|
| No (n = 25) | Yes—Uric Acid (n = 10) | ||
| Urinary Parameters | Median (IQR) | Median (IQR) | p |
| pH | 5.6 (5.2–6.5) | 5.3 (5.0–6.4) | 0.760 |
| 24 h diuresis (mL) | 2000 (1450–2700) | 1600 (1000–2600) | 0.047 |
| Urine creatinine (mg/dL) | 57 (38–99) | 89 (52–111) | 0.230 |
| Urine creatinine (mg/24 h) | 1227 (782–1490) | 1323 (829–1510) | 0.483 |
| Urine urate (mg/dL) | 28 (13–43) | 50 (29–420) | 0.037 |
| Urine urate (mg/24 h) | 534 (376–730) | 733 (529–3434) | 0.167 |
| Urine calcium (mg/dL) | 6.9 (3.9–10.5) | 6.4 (3.6–12.6) | 0.647 |
| Urine calcium (mg/24 h) | 132 (89–234) | 142 (77–212) | 0.184 |
| Urine phosphate (mg/dL) | 33 (23–46) | 50 (32–75) | 0.252 |
| Urine phosphate (mg/24 h) | 669 (409–928) | 855 (657–1133) | 0.492 |
| Urine magnesium (mg/dL) | 3.4 (2.0–6.2) | 4.3 (2.4–6.0) | 0.879 |
| Urine magnesium (mg/24 h) | 86 (56–117) | 70 (68–78) | 0.867 |
| Urine oxalate (mg/L) | 13.8 (9.6–19.8) | 19.9 (14.3–23.5) | 0.297 |
| Urine oxalate (mg/24 h) | 28 (16–39) | 31 (24–42) | 0.741 |
| Urine citrate (mg/L) | 153 (99–278) | 255 (116–460) | 0.859 |
| Urine citrate (mg/24 h) | 270 (221–608) | 501 (215–705) | 0.859 |
| Indwelling time (days) | 35 (16–92) | 34 (26–40) | 0.048 |
| Encrustation Presence | |||
|---|---|---|---|
| No (n = 25) | Yes—Infectious Phosphates (n = 8) | ||
| Urinary Parameters | Median (IQR) | Median (IQR) | p |
| pH | 5.6 (5.2–6.5) | 6.6 (6.2–6.8) | 0.043 |
| 24 h diuresis (mL) | 2000 (1450–2700) | 1533 (1200–1830) | 0.807 |
| Urine creatinine (mg/dL) | 57 (38–99) | 67 (39–90) | 0.312 |
| Urine creatinine (mg/24 h) | 1227 (782–1490) | 1045 (847–1623) | 0.224 |
| Urine urate (mg/dL) | 28 (13–43) | 28 (17–34) | 0.443 |
| Urine urate (mg/24 h) | 534 (376–730) | 417 (298–577) | 0.075 |
| Urine calcium (mg/dL) | 6.9 (3.9–10.5) | 10.5 (6.8–13.7) | 0.250 |
| Urine calcium (mg/24 h) | 132 (89–234) | 167 (78–253) | 0.201 |
| Urine phosphate (mg/dL) | 33 (23–46) | 36 (28–62) | 0.697 |
| Urine phosphate (mg/24 h) | 669 (409–928) | 554 (345–737) | 0.683 |
| Urine magnesium (mg/dL) | 3.4 (2.0–6.2) | 3 (2.2–5.5) | 0.876 |
| Urine magnesium (mg/24 h) | 86 (56–117) | 52 (43–70) | 0.554 |
| Urine oxalate (mg/L) | 13.8 (9.6–19.8) | 5.6 (3.3–17.2) | 0.107 |
| Urine oxalate (mg/24 h) | 28 (16–39) | 24 (24–40) | 0.745 |
| Urine citrate (mg/L) | 153 (99–278) | 133 (101–312) | 0.846 |
| Urine citrate (mg/24 h) | 270 (221–608) | 233 (157–365) | 0.344 |
| Indwelling time (days) | 35 (16–92) | 55 (22–87) | 0.068 |
| Encrustation Presence | |||
|---|---|---|---|
| No (n = 25) | Yes–Non-Infectious Phosphates (n = 9) | ||
| Urinary Parameters | Median (IQR) | Median (IQR) | p |
| pH | 5.6 (5.2–6.5) | 5.9 (5.5–7.2) | 0.048 |
| 24 h diuresis (mL) | 2000 (1450–2700) | 2050 (1525–2650) | 0.807 |
| Urine creatinine (mg/dL) | 57 (38–99) | 58 (40–64) | 0.312 |
| Urine creatinine (mg/24 h) | 1227 (782–1490) | 1176 (919–1472) | 0.224 |
| Urine urate (mg/dL) | 28 (13–43) | 23 (17–31) | 0.443 |
| Urine urate (mg/24 h) | 534 (376–730) | 448 (414–692) | 0.075 |
| Urine calcium (mg/dL) | 6.9 (3.9–10.5) | 8.5 (7.2–11.1) | 0.250 |
| Urine calcium (mg/24 h) | 132 (89–234) | 227 (131–277) | 0.201 |
| Urine phosphate (mg/dL) | 33 (23–46) | 30 (26–39) | 0.697 |
| Urine phosphate (mg/24 h) | 669 (409–928) | 773 (488–893) | 0.683 |
| Urine magnesium (mg/dL) | 3.4 (2.0–6.2) | 3.7 (0.8–5.6) | 0.876 |
| Urine magnesium (mg/24 h) | 86 (56–117) | 97 (77–127) | 0.554 |
| Urine oxalate (mg/L) | 14 (10–20) | 19 (15–25) | 0.317 |
| Urine oxalate (mg/24 h) | 28 (16–39) | 41 (32–45) | 0.453 |
| Urine citrate (mg/L) | 153 (99–278) | 120 (98–328) | 0.846 |
| Urine citrate (mg/24 h) | 270 (221–608) | 368 (243–956) | 0.344 |
| Indwelling time (days) | 35 (16–92) | 64 (26–78) | 0.068 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauzá, J.L.; Calvó, P.; Julià, F.; Guimerà, J.; Martínez, A.I.; Tienza, A.; Costa-Bauzá, A.; Sanchís, P.; Grases, F.; Pieras, E. Relationship between Urinary Parameters and Double-J Stent Encrustation. J. Clin. Med. 2023, 12, 5149. https://doi.org/10.3390/jcm12155149
Bauzá JL, Calvó P, Julià F, Guimerà J, Martínez AI, Tienza A, Costa-Bauzá A, Sanchís P, Grases F, Pieras E. Relationship between Urinary Parameters and Double-J Stent Encrustation. Journal of Clinical Medicine. 2023; 12(15):5149. https://doi.org/10.3390/jcm12155149
Chicago/Turabian StyleBauzá, Jose Luis, Paula Calvó, Francesca Julià, Jorge Guimerà, Ana Isabel Martínez, Antonio Tienza, Antonia Costa-Bauzá, Pilar Sanchís, Félix Grases, and Enrique Pieras. 2023. "Relationship between Urinary Parameters and Double-J Stent Encrustation" Journal of Clinical Medicine 12, no. 15: 5149. https://doi.org/10.3390/jcm12155149
APA StyleBauzá, J. L., Calvó, P., Julià, F., Guimerà, J., Martínez, A. I., Tienza, A., Costa-Bauzá, A., Sanchís, P., Grases, F., & Pieras, E. (2023). Relationship between Urinary Parameters and Double-J Stent Encrustation. Journal of Clinical Medicine, 12(15), 5149. https://doi.org/10.3390/jcm12155149






