Management of Spinal Metastasis by Minimally Invasive Surgical Techniques: Surgical Principles and Indications—A Literature Review
Abstract
1. Introduction
2. Material and Methods
2.1. Vertebral Augmentation Techniques: Vertebroplasty or Balloon Kyphoplasty
- -
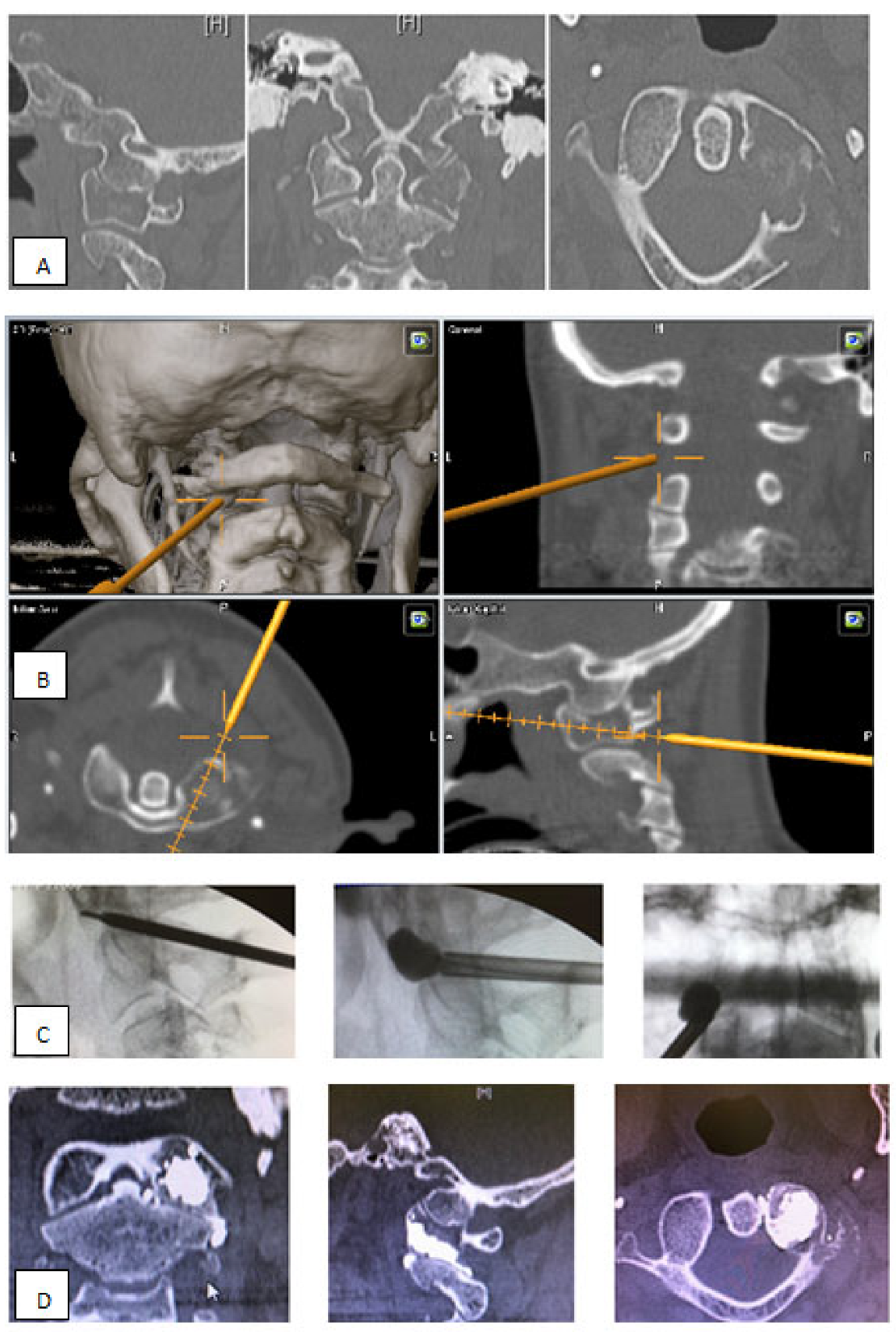
- Unrestricted reconstruction of datasets with views along the path of the needle with planning in 3D and 2D reconstructions (Figure 1).
- -
- Decreased X-ray exposure for the MISS technique. Navigation enables a reduction in X-ray exposure to the surgical team and the patient. Through a real time visualization of instruments, skin incisions and trajectories can be planned [21].
- -
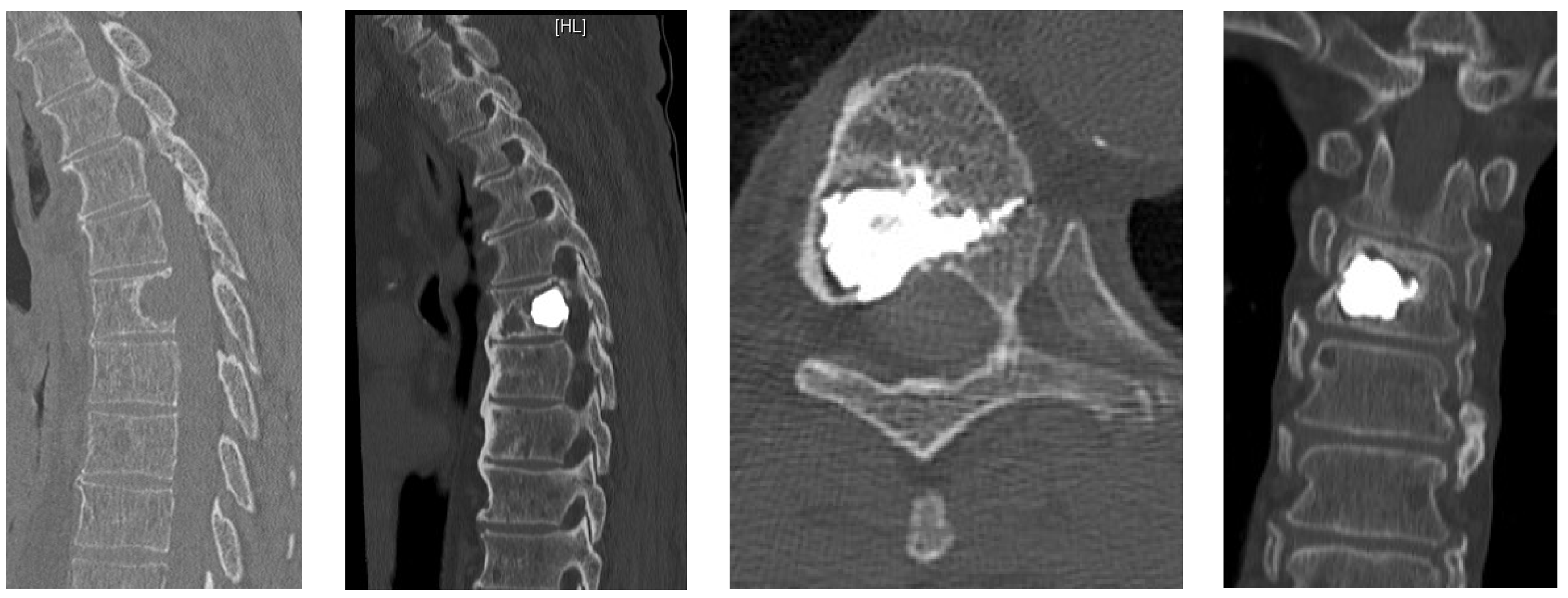
- Posterior percutaneous kyphoplasty for cervical spine metastases (Figure 2); case of C2-C3 kyphoplasty.
- -
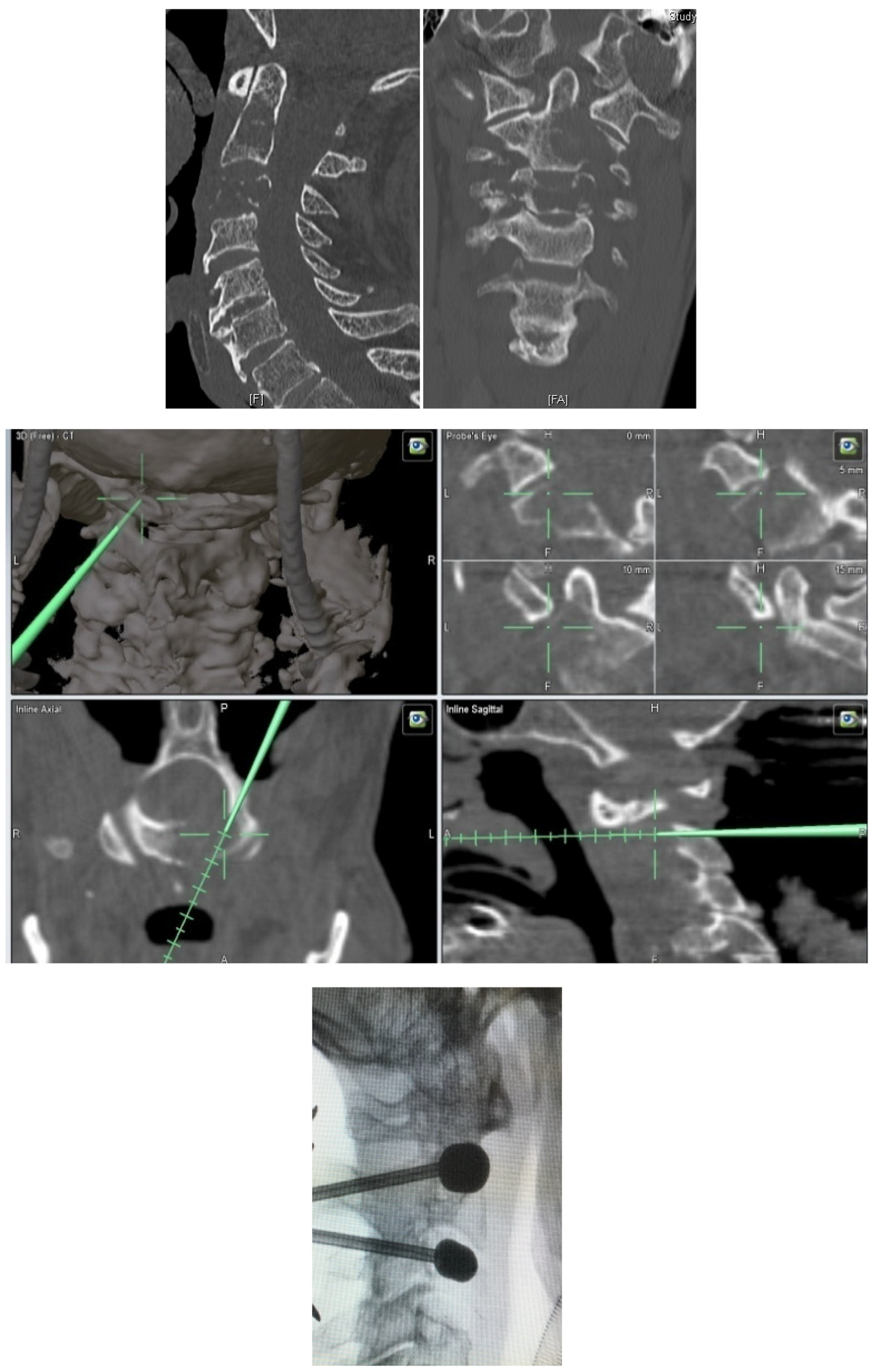
- Metastatic lesions of C1 are extremely rare, and their treatment by percutaneous cement augmentation is considered to be a technically challenging procedure due to complex anatomy. We also performed percutaneous kyphoplasty in a painful osteolytic lesion located on the left lateral mass of C1 through a posterolateral approach using a 3D CT scan intra-operative navigation system and fluoroscopy (Figure 3) [22].
- -
- Maintaining spinal stability;
- -
- Optimally reducing pathological fracture. The reduction by osteosynthesis will then allow for optimal vertebro/kyphoplasty.
2.2. Osteocool Radiofrequency
3. Osteocool
3.1. Osteosynthesis
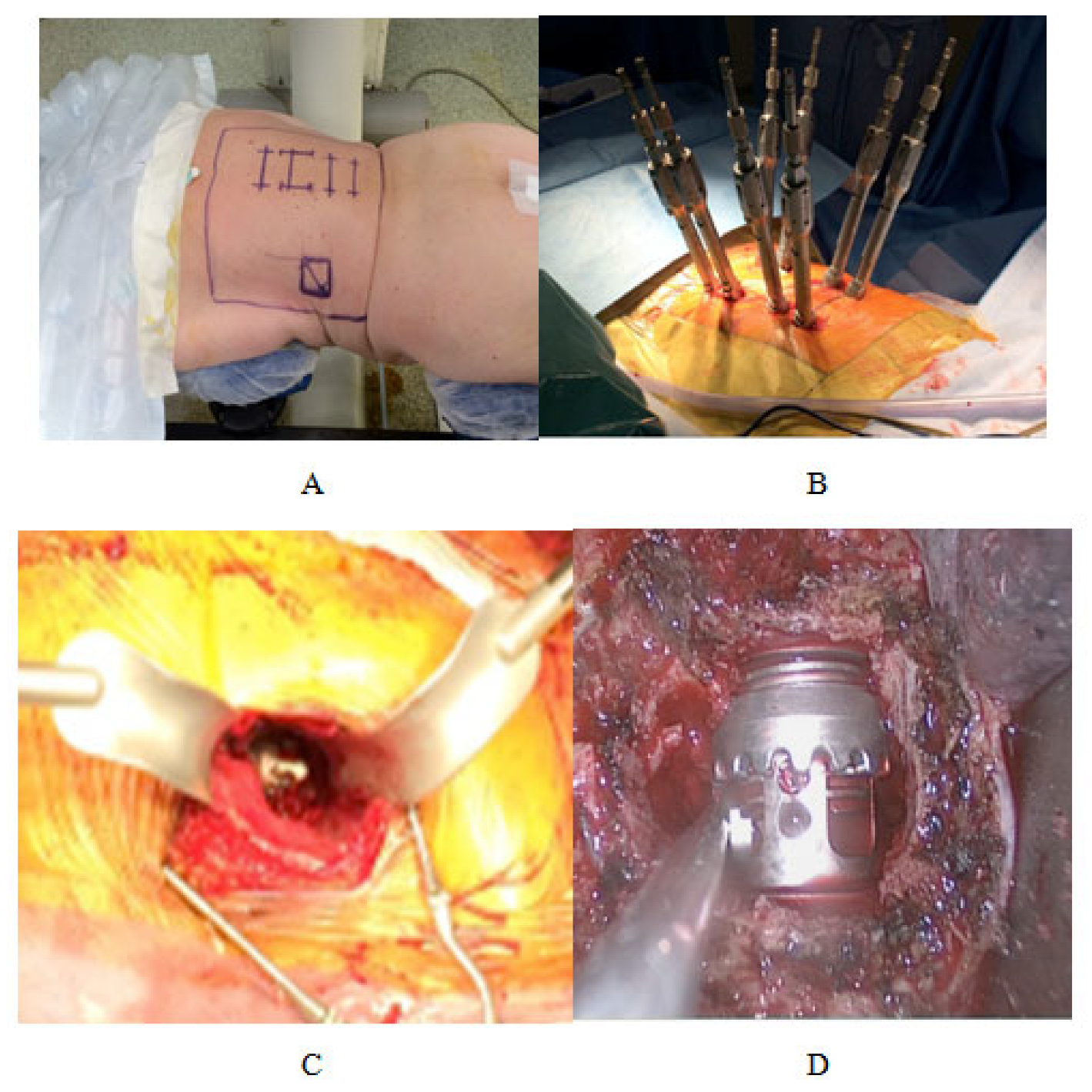
3.2. Open Ways Mini Invasives
3.2.1. Posterior Approach
3.2.2. Minimally Invasive Anterior Approaches
3.2.3. Thoracotomy
3.2.4. Retroperitoneal Retropleural Lumbotomy (Figure 5)

3.2.5. Classical Lobotomy
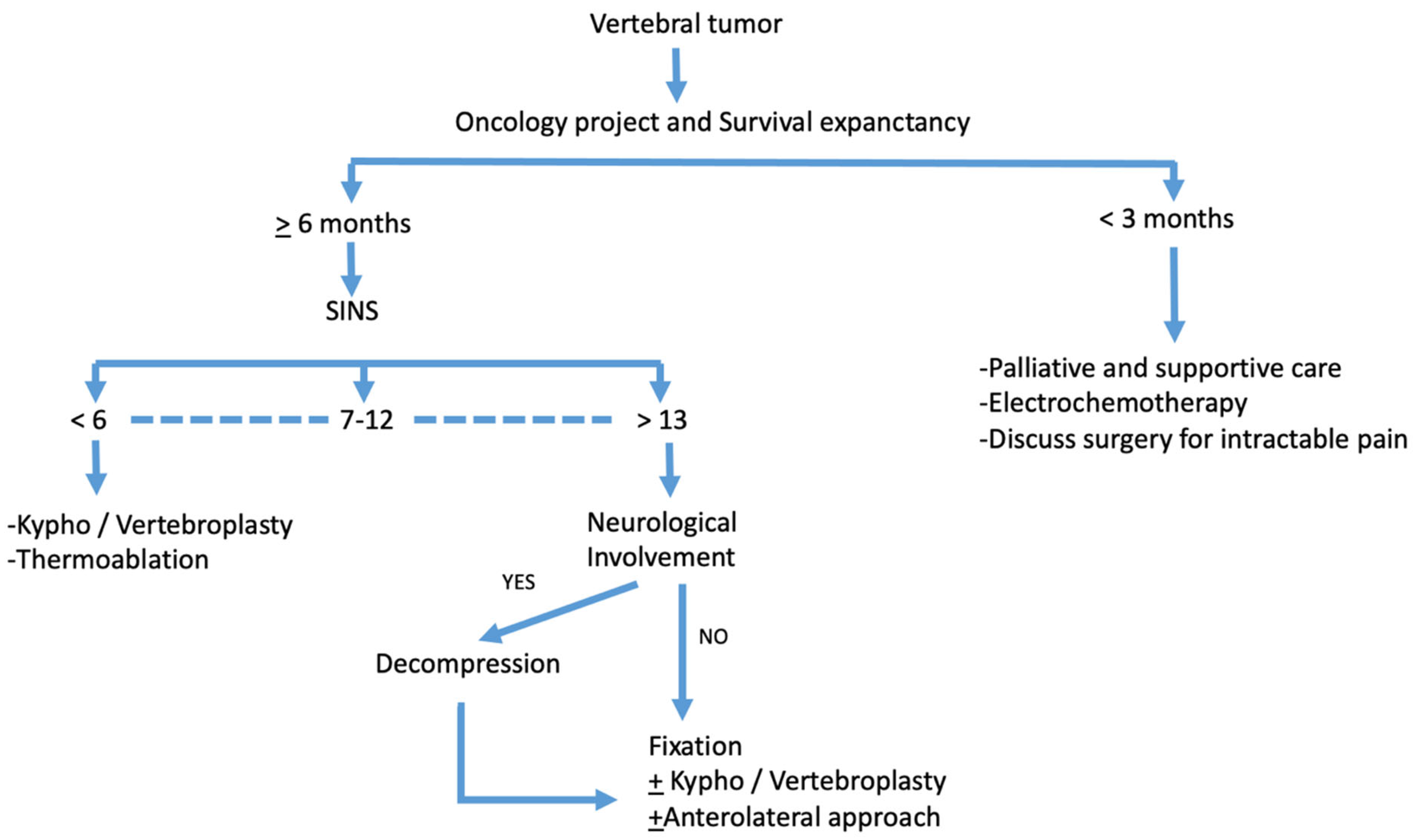
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tabouret, E.; Gravis, G.; Cauvin, C.; Loundou, A.; Adetchessi, T.; Fuentes, S. Long-term survivors after surgical management of metastatic spinal cord compression. Eur. Spine J. 2015, 24, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Pointillart, V.; Vital, J.M.; Salmi, R.; Diallo, A.; Quan, G.M. Survival prognostic factors and clinical outcomes in patients with spinal metastases. J. Cancer Res. Clin. Oncol. 2011, 137, 849–856. [Google Scholar] [CrossRef] [PubMed]
- La Combe, B.; Gaillard, S.; Bennis, S.; Chouaid, C. Management of spinal metastases of lung cancer. Rev. Mal. Respir. 2013, 30, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Crockard, A.; Bunger, C.; Harms, J.; Kawahara, N.; Mazel, C.; Melcher, R.; Tomita, K. Review of metastatic spine tumour classification and indications for surgery: The consensus statement of the Global Spine Tumour Study Group. Eur. Spine J. 2010, 19, 215–222. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chung, C.K.; Jahng, T.-A.; Kim, K.-J.; Kim, C.H.; Hyun, S.-J.; Kim, H.-J.; Jeon, S.R.; Chang, U.-K.; Lee, S.-H.; et al. Which one is a valuable surrogate for predicting survival between Tomita and Tokuhashi scores in patients with spinal metastases? A meta-analysis for diagnostic test accuracy and individual participant data analysis. J. Neurooncol. 2015, 123, 267–275. [Google Scholar] [CrossRef]
- Martz, G. Therapy of complications due to neoplasm metastases. Schweiz. Med. Wochenschr. 1978, 108, 1336–1337. [Google Scholar]
- Rodríguez-Vela, J.; Lobo-Escolar, A.; Joven-Aliaga, E.; Herrera, A.; Vicente, J.; Suñén, E.; Loste, A.; Tabuenca, A. Perioperative and short-term advantages of mini-open approach for lumbar spinal fusion. Eur. Spine J. 2009, 18, 1194–1201. [Google Scholar] [CrossRef]
- Brodano, G.B.; Martikos, K.; Lolli, F.; Gasbarrini, A.; Cioni, A.; Bandiera, S.; Silvestre, M.D.; Boriani, S.; Greggi, T. Transforaminal Lumbar Interbody Fusion in Degenerative Disk Disease and Spondylolisthesis Grade I: Minimally Invasive Versus Open Surgery. J. Spinal Disord. Tech. 2015, 28, E559–E564. [Google Scholar] [CrossRef]
- Villavicencio, A.T.; Burneikiene, S.; Roeca, C.M.; Nelson, E.L.; Mason, A. Minimally invasive versus open transforaminal lumbar interbody fusion. Surg. Neurol. Int. 2010, 1, 12. [Google Scholar]
- Archavlis, E.; Nievas, M.C.Y. Comparison of minimally invasive fusion and instrumentation versus open surgery for severe stenotic spondylolisthesis with high-grade facet joint osteoarthritis. Eur. Spine J. 2013, 22, 1731–1740. [Google Scholar] [CrossRef]
- Kotani, Y.; Abumi, K.; Ito, M.; Sudo, H.; Abe, Y.; Minami, A. Mid-term clinical results of minimally invasive decompression and posterolateral fusion with percutaneous pedicle screws versus conventional approach for degenerative spondylolisthesis with spinal stenosis. Eur. Spine J. 2012, 21, 1171–1177. [Google Scholar] [CrossRef]
- Campos, M.; Urrutia, J.; Zamora, T.; Román, J.; Canessa, V.; Borghero, Y.; Palma, A.; Molina, M. The Spine Instability Neoplastic Score: An independent reliability and reproducibility analysis. Spine J. 2014, 14, 1466–1469. [Google Scholar] [CrossRef]
- Galibert, P.; Deramond, H.; Rosat, P.; Le Gars, D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie 1987, 33, 166–168. [Google Scholar] [PubMed]
- Fuentes, S.; Blondel, B. Vertebroplasty and balloon kyphoplasty. Neurochirurgie 2010, 56, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Blondel, B.; Adetchessi, T.; Demakakos, J.; Pech-Gourg, G.; Dufour, H.; Fuentes, S. Anterolateral kyphoplasty in the management of cervical spinal metastasis. Orthop. Traumatol. Surg. Res. 2012, 98, 341–345. [Google Scholar] [CrossRef]
- Pesenti, S.; Blondel, B.; Peltier, E.; Adetchessi, T.; Dufour, H.; Fuentes, S. Percutaneous cement-augmented screws fixation in the fractures of the aging spine: Is it the solution? BioMed Res. Int. 2014, 2014, 610675. [Google Scholar] [CrossRef] [PubMed]
- Garfin, S.R.; Yuan, H.A.; Reiley, M.A. New technologies in spine: Kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine 2001, 26, 1511–1515. [Google Scholar] [CrossRef]
- Papanastassiou, I.D.; Phillips, F.M.; Meirhaeghe, J.; Berenson, J.R.; Andersson, G.B.J.; Chung, G.; Small, B.J.; Aghayev, K.; Vrionis, F.D. Comparing effects of kyphoplasty, vertebroplasty, and non-surgical management in a systematic review of randomized and non-randomized controlled studies. Eur. Spine J. 2012, 21, 1826–1843. [Google Scholar] [CrossRef]
- Farah, K.; Meyer, M.; Prost, S.; Dufour, H.; Blondel, B.; Fuentes, S. Cirq® Robotic Assistance for Minimally Invasive C1-C2 Posterior Instrumentation: Report on Feasibility and Safety. Oper. Neurosurg. 2020, 19, 730–734. [Google Scholar] [CrossRef]
- Florea, S.M.; Farah, K.; Meyer, M.; Dufour, H.; Graillon, T.; Fuentes, S. The interest of intraoperative scanner coupled to neuronavigation in traumatic or oncologic fractures of the cervical and upper thoracic spine requiring vertebral body height restoring procedures. Neurochirurgie 2020, 66, 240–246. [Google Scholar] [CrossRef]
- Farah, K.; Coudert, P.; Graillon, T.; Blondel, B.; Dufour, H.; Gille, O.; Fuentes, S. Prospective Comparative Study in Spine Surgery Between O-Arm and Airo Systems: Efficacy and Radiation Exposure. World Neurosurg. 2018, 118, e175–e184. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Farah, K.; Graillon, T.; Boissonneau, S.; Dufour, H.; Fuentes, S. Minimal Invasive Percutaneous Kyphoplasty of C1 Lytic Lesion Using an Intraoperative 3D Imaging-Based Navigation System and Fluoroscopy. J. Surg. Res. 2020, 3, 419–427. [Google Scholar] [CrossRef]
- Hoffmann, R.T.; Jakobs, T.F.; Trumm, C.; Weber, C.; Helmberger, T.K.; Reiser, M.F. Radiofrequency ablation in combination with osteoplasty in the treatment of painful metastatic bone disease. J. Vasc. Interv. Radiol. 2008, 19, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Callstrom, M.R.; Charboneau, J.W.; Farrell, M.A.; Maus, T.P.; Welch, T.J.; Wong, G.Y.; Sloan, J.A.; Novotny, P.J.; Petersen, I.A.; et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: A multicenter study. J. Clin. Oncol. 2004, 22, 300–306. [Google Scholar] [CrossRef]
- Dupuy, D.E.; Liu, D.; Hartfeil, D.; Hanna, L.; Blume, J.D.; Ahrar, K.; Lopez, R.; Safran, H.; DiPetrillo, T. Percutaneous radiofrequency ablation of painful osseous metastases: A multicenter American College of Radiology Imaging Network trial. Cancer 2010, 116, 989–997. [Google Scholar] [CrossRef]
- Guenette, J.P.; Lopez, M.J.; Kim, E.; Dupuy, D.E. Solitary painful osseous metastases: Correlation of imaging features with pain palliation after radiofrequency ablation—A multicenter american college of radiology imaging network study. Radiology 2013, 268, 907–915. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, Z.; Sun, M.; Zeng, H.; Zuo, D.; Hua, Y.; Cai, Z. A preliminary study of the safety and efficacy of radiofrequency ablation with percutaneous kyphoplasty for thoracolumbar vertebral metastatic tumor treatment. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2014, 20, 556–563. [Google Scholar]
- Anchala, P.R.; Irving, W.D.; Hillen, T.J.; Friedman, M.V.; A Georgy, B.; Coldwell, D.M.; Tran, N.D.; Vrionis, F.D.; Brook, A.; Jennings, J.W. Treatment of metastatic spinal lesions with a navigational bipolar radiofrequency ablation device: A multicenter retrospective study. Pain Physician 2014, 17, 317–327. [Google Scholar]
- Lee, D.Y.; Lee, S.H.; Maeng, D.H. Two-level anterior lumbar interbody fusion with percutaneous pedicle screw fixation: A minimum 3-year follow-up study. Neurol. Med.-Chir. 2010, 50, 645–650. [Google Scholar] [CrossRef][Green Version]
- Giorgi, H.; Blondel, B.; Adetchessi, T.; Dufour, H.; Tropiano, P.; Fuentes, S. Early percutaneous fixation of spinal thoracolumbar fractures in polytrauma patients. Orthop. Traumatol. Surg. Res. 2014, 100, 449–454. [Google Scholar] [CrossRef]
- Proietti, L.; Scaramuzzo, L.; Schirò, G.R.; Sessa, S.; D’Aurizio, G.; Tamburrelli, F.C. Posterior percutaneous reduction and fixation of thoraco-lumbar burst fractures. Orthop. Traumatol. Surg. Res. 2014, 100, 455–460. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Li, C.; Liu, J.; Xiang, L. Comparison of Open Versus Percutaneous Pedicle Screw Fixation Using the Sextant System in the Treatment of Traumatic Thoracolumbar Fractures. Clin. Spine Surgery 2017, 30, E239–E246. [Google Scholar] [CrossRef]
- Lee, J.K.; Jang, J.W.; Kim, T.W.; Kim, T.S.; Kim, S.H.; Moon, S.J. Percutaneous short-segment pedicle screw placement without fusion in the treatment of thoracolumbar burst fractures: Is it effective?: Comparative study with open short-segment pedicle screw fixation with posterolateral fusion. Acta Neurochir. 2013, 155, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Vanek, P.; Bradac, O.; Konopkova, R.; de Lacy, P.; Lacman, J.; Benes, V. Treatment of thoracolumbar trauma by short-segment percutaneous transpedicular screw instrumentation: Prospective comparative study with a minimum 2-year follow-up. J. Neurosurg. Spine 2014, 20, 150–156. [Google Scholar] [CrossRef]
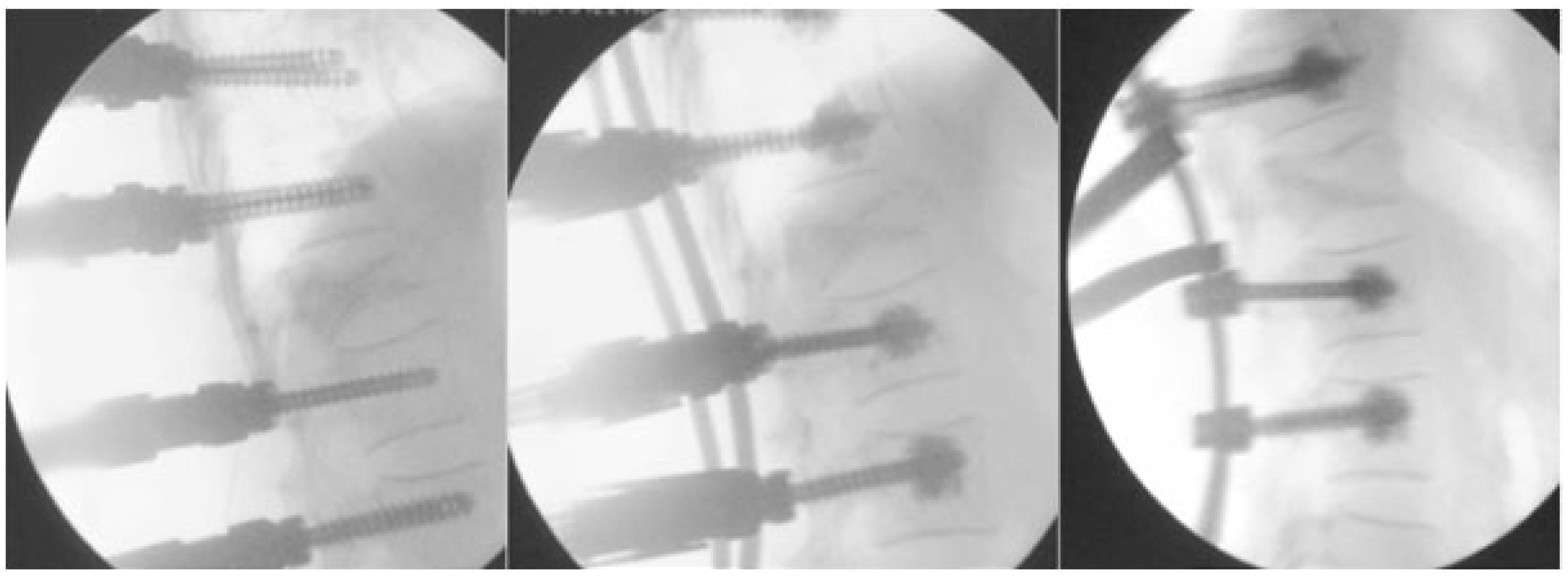
- Afathi, M.; Mansouri, N.; Farah, K.; Benichoux, V.; Blondel, B.; Fuentes, S. Use of Cement-Augmented Percutaneous Pedicular Screws in the Management of Multifocal Tumoral Spinal Fractures. Asian Spine J. 2019, 13, 305–312. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Payne, R.; Saris, S.; Kryscio, R.J.; Mohiuddin, M.; Young, B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet 2005, 366, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.; Zeng, G.; Du, L.; Fang, Y.; Gao, T.; Zhao, J. Treatment results in the different surgery of intradural extramedullary tumor of 122 cases. PLoS ONE 2014, 9, e111495. [Google Scholar] [CrossRef]
- Tan, L.A.; Kasliwal, M.K.; Wewel, J.; Fontes, R.B.V.; O’Toole, J.E. Minimally invasive surgery for synchronous, same-level lumbar intradural-extramedullary neoplasm and acute disc herniation. Neurosurg. Focus 2014, 37 (Suppl. S2), Video16. [Google Scholar] [CrossRef]
- Mobbs, R.J.; Li, J.; Sivabalan, P.; Raley, D.; Rao, P.J. Outcomes after decompressive laminectomy for lumbar spinal stenosis: Comparison between minimally invasive unilateral laminectomy for bilateral decompression and open laminectomy: Clinical article. J. Neurosurg. Spine 2014, 21, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Zairi, F.; Arikat, A.; Allaoui, M.; Marinho, P.; Assaker, R. Minimally invasive decompression and stabilization for the management of thoracolumbar spine metastasis. J. Neurosurg. Spine 2012, 17, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.H.; Gasbarrini, A.; Cappuccio, M.; Boriani, L.; De Iure, F.; Colangeli, S.; Boriani, S. Minimally Invasive Posterior Stabilization Improved Ambulation and Pain Scores in Patients with Plasmacytomas and/or Metastases of the Spine. Int. J. Surg. Oncol. 2011, 2011, 239230. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.P.; Drazin, D.; King, W.A.; Kim, T.T. Image-guided navigation and video-assisted thoracoscopic spine surgery: The second generation. Neurosurg. Focus 2014, 36, E8. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.; Ali, Z.; McEvoy, L.; Bolger, C. Mini-open retropleural transthoracic approach for the treatment of giant thoracic disc herniation. Spine 2012, 37, E1079–E1084. [Google Scholar] [CrossRef] [PubMed]
- Berjano, P.; Garbossa, D.; Damilano, M.; Pejrona, M.; Bassani, R.; Doria, C. Transthoracic lateral retropleural minimally invasive microdiscectomy for T9-T10 disc herniation. Eur Spine J. 2014, 23, 1376–1378. [Google Scholar] [CrossRef]
- Huang, T.J.; Hsu, R.W.W.; Li, Y.Y.; Cheng, C.C. Minimal access spinal surgery (MASS) in treating thoracic spine metastasis. Spine 2006, 31, 1860–1863. [Google Scholar] [CrossRef]
- Anterior Minimally Invasive Extrapleural Retroperitoneal Approach to the Thoraco-Lumbar Junction of the Spine—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/23246007/ (accessed on 23 July 2023).
- Mehren, C.; Mayer, H.M.; Siepe, C.; Grochulla, F.; Korge, A. The minimally invasive anterolateral approach to L2-L5. Oper. Orthopädie Traumatol. 2010, 22, 221–228. [Google Scholar] [CrossRef]
- Tomycz, L.; Parker, S.L.; McGirt, M.J. Minimally invasive transpsoas L2 corpectomy and percutaneous pedicle screw fixation for osteoporotic burst fracture in the elderly: A technical report. J Spinal Disord. Tech. 2015, 28, 53–60. [Google Scholar] [CrossRef]
- Fessler, R.G.; Khoo, L.T. Minimally invasive cervical microendoscopic foraminotomy: An initial clinical experience. Neurosurgery 2002, 51 (Suppl. S5), S37–S45. [Google Scholar] [CrossRef]
- Guiot, B.H.; Khoo, L.T.; Fessler, R.G. A minimally invasive technique for decompression of the lumbar spine. Spine 2002, 27, 432–438. [Google Scholar] [CrossRef]
- Isaacs, R.E.; Podichetty, V.K.; Santiago, P.; Sandhu, F.A.; Spears, J.; Kelly, K.; Rice, L.; Fessler, R.G. Minimally invasive microendoscopy-assisted transforaminal lumbar interbody fusion with instrumentation. J. Neurosurg. Spine 2005, 3, 98–105. [Google Scholar] [CrossRef]
- Kumar, N.; Malhotra, R.; Maharajan, K.; Zaw, A.S.; Wu, P.H.; Makandura, M.C.; Po Liu, G.K.; Thambiah, J.; Wong, H.K. Metastatic Spine Tumor Surgery: A Comparative Study of Minimally Invasive Approach Using Percutaneous Pedicle Screws Fixation Versus Open Approach. Clin. Spine Surg. 2017, 30, E1015–E1021. [Google Scholar] [CrossRef]
- Overley, S.C.; Cho, S.K.; Mehta, A.I.; Arnold, P.M. Navigation and Robotics in Spinal Surgery: Where Are We Now? Neurosurgery 2017, 80, S86–S99. [Google Scholar] [CrossRef]
- Kochanski, R.B.; Lombardi, J.M.; Laratta, J.L.; Lehman, R.A.; O’Toole, J.E. Image-Guided Navigation and Robotics in Spine Surgery. Neurosurgery 2019, 84, 1179–1189. [Google Scholar] [CrossRef]
- Barzilai, O.; Boriani, S.; Fisher, C.G.; Sahgal, A.; Verlaan, J.J.; Gokaslan, Z.L.; Lazary, A.; Bettegowda, C.; Rhines, L.D.; Laufer, I. Essential Concepts for the Management of Metastatic Spine Disease: What the Surgeon Should Know and Practice. Glob. Spine J. 2019, 9 (Suppl. S1), 98S–107S. [Google Scholar] [CrossRef]
- Barzilai, O.; McLaughlin, L.; Amato, M.-K.; Reiner, A.S.; Ogilvie, S.Q.; Lis, E.; Yamada, Y.; Bilsky, M.H.; Laufer, I. Minimal Access Surgery for Spinal Metastases: Prospective Evaluation of a Treatment Algorithm Using Patient-Reported Outcomes. World Neurosurg. 2018, 120, e889–e901. [Google Scholar] [CrossRef]
- Hansen-Algenstaedt, N.; Kwan, M.K.; Algenstaedt, P.; Chiu, C.K.; Viezens, L.; Chan, T.S.; Lee, C.K.; Wellbrock, J.; Chan, C.Y.W.; Schaefer, C. Comparison between Minimally Invasive Surgery and Conventional Open Surgery for Patients with Spinal Metastasis: A Prospective Propensity Score-Matched Study. Spine 2017, 42, 789–797. [Google Scholar] [CrossRef]
- Molina, C.A.; Gokaslan, Z.L.; Sciubba, D.M. A systematic review of the current role of minimally invasive spine surgery in the management of metastatic spine disease. Int. J. Surg. Oncol. 2011, 2011, 598148. [Google Scholar] [CrossRef][Green Version]
- Shahi, P.M.; Subramanian, T.B.; Araghi, K.B.; Singh, S.M.; Asada, T.; Maayan, O.B.; Korsun, M.B.; Singh, N.M.; Tuma, O.B.; Dowdell, J.; et al. Comparison of Robotics and Navigation for Clinical Outcomes Following Minimally Invasive Lumbar Fusion. Spine 2023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, M.; Farah, K.; Aurélie, T.; Graillon, T.; Dufour, H.; Fuentes, S. Management of Spinal Metastasis by Minimally Invasive Surgical Techniques: Surgical Principles and Indications—A Literature Review. J. Clin. Med. 2023, 12, 5165. https://doi.org/10.3390/jcm12165165
Meyer M, Farah K, Aurélie T, Graillon T, Dufour H, Fuentes S. Management of Spinal Metastasis by Minimally Invasive Surgical Techniques: Surgical Principles and Indications—A Literature Review. Journal of Clinical Medicine. 2023; 12(16):5165. https://doi.org/10.3390/jcm12165165
Chicago/Turabian StyleMeyer, Mikael, Kaissar Farah, Toquart Aurélie, Thomas Graillon, Henry Dufour, and Stephane Fuentes. 2023. "Management of Spinal Metastasis by Minimally Invasive Surgical Techniques: Surgical Principles and Indications—A Literature Review" Journal of Clinical Medicine 12, no. 16: 5165. https://doi.org/10.3390/jcm12165165
APA StyleMeyer, M., Farah, K., Aurélie, T., Graillon, T., Dufour, H., & Fuentes, S. (2023). Management of Spinal Metastasis by Minimally Invasive Surgical Techniques: Surgical Principles and Indications—A Literature Review. Journal of Clinical Medicine, 12(16), 5165. https://doi.org/10.3390/jcm12165165





