The Influence of Age, Gender and Education on Neuropsychological Test Scores: Updated Clinical Norms for Five Widely Used Cognitive Assessments
Abstract
:1. Introduction
1.1. Mini-Mental State Examination
1.2. F-A-S Test
1.3. Rey–Osterrieth Complex Figure Test
1.4. Trail Making Test, Part A and B
1.5. Aims of the Present Study
2. Methods
2.1. Participants
2.2. Statistical Analysis
3. Results
3.1. Sociodemographics, Neuropsychological Test Results and Correlations
3.2. Sociodemographic Effects on Test Scores
3.3. Percentile Rank Generation and z-Score Transformation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shulman, K.I.; Herrmann, N.; Brodaty, H.; Chiu, H.; Lawlor, B.; Ritchie, K.; Scanlan, J. IPA survey of brief cognitive screening instruments. Int. Psychogeriatr. 2006, 18, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Creavin, S.; Wisniewski, S.; Noel-Storr, A.; Trevelyan, C.; Hampton, T.; Rayment, D.; Thom, V.; Nash, K.; Elhamoui, H. Mini-Mental State Examination (MMSE) for the detection of dementia in people aged over 65. Cochrane Database Syst. Rev. 2016, 1, 1–185. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini mental state”: Practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Kessler, J.; Markowitsch, H.J.; Denzler, P. Mini-Mental-Status-Test (MMST); Beltz: Weinheim, Germany, 2000. [Google Scholar]
- Anstey, K.; Christensen, H. Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: A review. Gerontology 2000, 46, 163–177. [Google Scholar] [CrossRef]
- Crum, R.M.; Anthony, J.C.; Bassett, S.S.; Folstein, M.F. Population-Based Norms for the Mini-Mental State Examination by Age and Educational Level. JAMA J. Am. Med. Assoc. 1993, 269, 2386–2391. [Google Scholar] [CrossRef]
- Freitas, S.; Simões, M.R.; Alves, L.; Santana, I. The Relevance of Sociodemographic and Health Variables on MMSE Normative Data. Appl. Neuropsychol. Adult 2015, 22, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Piccinin, A.M.; Muniz-Terrera, G.; Clouston, S.; Reynolds, C.A.; Thorvaldsson, V.; Deary, I.J.; Deeg DJ, H.; Johansson, B.; MacKinnon, A.; Spiro, A.; et al. Coordinated analysis of age, sex, and education effects on change in MMSE scores. J. Gerontol.-Ser. B Psychol. Sci. Soc. Sci. 2013, 68, 374–390. [Google Scholar] [CrossRef] [Green Version]
- Scheffels, J.F.; Fröhlich, L.; Kalbe, E.; Kessler, J. Concordance of Mini-Mental State Examination, Montreal Cognitive Assessment and Parkinson Neuropsychometric Dementia Assessment in the classification of cognitive performance in Parkinson’s disease. J. Neurol. Sci. 2020, 412, 116735. [Google Scholar] [CrossRef]
- Kochhann, R.; Cerveira, M.O.; Godinho, C.; Camozzato, A.; Chaves, M.L.F. Evaluation of Mini-Mental State Examination scores according to different age and education strata, and sex, in a large Brazilian healthy sample. Dement. Neuropsychol. 2009, 3, 88–93. [Google Scholar] [CrossRef]
- Tombaugh, T.N.; McIntyre, N.J. The mini-mental state examination: A comprehensive review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef]
- Benton, A.L.; Hamsher, K. Multilingual Aphasia Examination; AJA Associates: Iowa City, IA, USA, 1976. [Google Scholar]
- Whiteside, D.M.; Kealey, T.; Semla, M.; Luu, H.; Rice, L.; Basso, M.R.; Roper, B. Verbal Fluency: Language or Executive Function Measure? Appl. Neuropsychol. Adult 2016, 23, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Tallberg, I.M.; Ivachova, E.; Jones Tinghag, K.; Östberg, P. Swedish norms for word fluency tests: FAS, animals and verbs. Scand. J. Psychol. 2008, 49, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.A.; Caramelli, P. Normative data for middle-aged brazilians in verbal fluency (Animals and fas), trail making test (TMT) and clock drawing test (CDT). Dement. E Neuropsychol. 2020, 14, 14–23. [Google Scholar] [CrossRef]
- Steinberg, B.A.; Bieliauskas, L.A.; Smith, G.E.; Ivnik, R.J. Mayo’s Older Americans Normative Studies: Age- and IQ-adjusted norms for the Trail-Making Test, the Stroop Test, and MAE Controlled Oral Word Association Test. Clin. Neuropsychol. 2005, 19, 329–377. [Google Scholar] [CrossRef] [PubMed]
- Bolla, K.; Lindgrend, K.; Bonaccorsy, C.; Bleecker, M. Predictors of verbal fluency (FAS) in the healthy elderly. J. Clin. Psychol. 1990, 46, 623–628. [Google Scholar] [CrossRef]
- Loonstra, A.S.; Tarlow, A.R.; Sellers, A.H. COWAT metanorms across age, education, and gender. Appl. Neuropsychol. 2001, 8, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Tombaugh, T.N.; Kozak, J.; Rees, L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch. Clin. Neuropsychol. 1999, 14, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Yeudall, L.F.; Reddon, J.; Stefanyk, W. Normative data stratified by age and sex for 12 neuropsychological tests. J. Clin. Psychol. 1986, 42, 918–946. [Google Scholar] [CrossRef]
- Osterrieth, P.A. Le test de copie d’une figure complexe; contribution a l’etude de la perception et de la memoire. Arch. Psychol. 1944, 30, 206–356. [Google Scholar]
- Rey, A. L’examen psychologique dans les cas d’encéphalopathie traumatique. Arch. Psychol. 1941, 28, 215–285. [Google Scholar]
- Shin, M.S.; Kim, M.S.; Park, S.J.; Lee, Y.H.; Ha, T.H.; Kwon, J.S. Deficits of organizational strategy and visual memory in obsessive-compulsive disorder. Neuropsychology 2004, 18, 665–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, M.S.; Park, S.Y.; Park, S.R.; Seol, S.H.; Kwon, J.S. Clinical and empirical applications of the Rey-Osterrieth Complex Figure Test. Nat. Protoc. 2006, 1, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Peña-Casanova, J.; Gramunt-Fombuena, N.; Quiñones-Úbeda, S.; Sánchez-Benavides, G.; Aguilar, M.; Badenes, D.; Molinuevo, J.L.; Robles, A.; Barquero, M.S.; Payno, M.; et al. Spanish multicenter normative studies (NEURONORMA project): Norms for the rey-osterrieth complex figure (copy and memory), and free and cued selective reminding test. Arch. Clin. Neuropsychol. 2009, 24, 371–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicente, S.G.; Ramos-Usuga, D.; Barbosa, F.; Gaspar, N.; Dores, A.R.; Rivera, D.; Arango-Lasprilla, J.C. Regression-Based Norms for the Hopkins Verbal Learning Test-Revised and the Rey-Osterrieth Complex Figure in a Portuguese Adult Population. Arch. Clin. Neuropsychol. 2021, 36, 587–596. [Google Scholar] [CrossRef]
- Berry, D.T.R.; Allen, R.S.; Schmitt, F.A. Rey-Osterrieth Complex Figure: Psychometric characteristics in a geriatric sample. Clin. Neuropsychol. 1991, 5, 143–153. [Google Scholar] [CrossRef]
- Brauer Boone, K.; Lesser, I.M.; Hill-Gutierrez, E.; Berman, N.G.; D’Elia, L.F. Rey-Osterrieth complex figure performance in healthy, older adults: Relationship to age, education, sex, and IQ. Clin. Neuropsychol. 1993, 7, 22–28. [Google Scholar] [CrossRef]
- Reitan, R.M. Trail Making Test; Reitan Neuropsychology Laboratory: Tucson, AZ, USA, 1992. [Google Scholar]
- Lucas, J.A.; Ivnik, R.J.; Smith, G.E.; Ferman, T.J.; Willis, F.B.; Petersen, R.C.; Graff-Radford, N.R. Mayo’s Older African Americans Normative Studies: Norms for Boston Naming Test, Controlled Oral Word Association, Category Fluency, Animal Naming, Token Test, WRAT-3 Reading, Trail Making Test, Stroop Test, and Judgment of Line Orientation. Clin. Neuropsychol. 2005, 19, 243–269. [Google Scholar] [CrossRef]
- Heaton, R.K. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychologicalnorms for African American and Caucasian Adults; Psychological Assessment Resources: Odessa, FL, USA, 2004. [Google Scholar]
- Lu, L.; Bigler, E.D. Performance on original and a Chinese version of Trail Making Test Part B: A normative bilingual sample. Appl. Neuropsychol. 2000, 7, 243–246. [Google Scholar] [CrossRef]
- Bowie, C.R.; Harvey, P.D. Administration and interpretation of the Trail Making Test. Nat. Protoc. 2006, 1, 2277–2281. [Google Scholar] [CrossRef]
- Fernández, A.L.; Marcopulos, B.A. A comparison of normative data for the Trail Making Test from several countries: Equivalence of norms and considerations for interpretation: Cognition and Neurosciences. Scand. J. Psychol. 2008, 49, 239–246. [Google Scholar] [CrossRef]
- Sánchez-Cubillo, I.; Periáñez, J.A.; Arover-Roig, D.; Rodríguez-Sánchez, J.M.; Ríos-Lago, M.; Tirapu, J.; Barceló, F. Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J. Int. Neuropsychol. Soc. 2009, 15, 438–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbuthnott, K.; Frank, J. Trail Making Test, Part B as a measure of executive control: Validation using a set-switching paradigm. J. Clin. Exp. Neuropsychol. 2000, 22, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Drane, D.L.; Yuspeh, R.L.; Huthwaite, J.S.; Klingler, L.K. Demographic characteristics and normative observations for derived-trail making test indices. Cogn. Behav. Neurol. 2002, 15, 39–43. [Google Scholar]
- Lamberty, G.J.; Putnam, S.H.; Chatel, D.M.; Bieliauskas, L.A. Derived Trail Making Test indices: A preliminary report. Neuropsychiatry Neuropsychol. Behav. Neurol. 1994, 7, 230–234. [Google Scholar]
- Martin, T.A.; Hoffman, N.M.; Donders, J. Clinical utility of the Trail Making Test ratio score. Appl. Neuropsychol. 2003, 10, 163–169. [Google Scholar] [CrossRef]
- Hamdan, A.C.; Hamdan, E.M.L.R. Effects of age and education level on the Trail Making Test in a healthy Brazilian sample. Psychol. Neurosci. 2009, 2, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, R.; Meguro, K.; Lee, E.; Kasai, M.; Ishii, H.; Yamaguchi, S. Effect of age and education on the Trail Making Test and determination of normative data for Japanese elderly people: The Tajiri Project. Psychiatry Clin. Neurosci. 2006, 60, 422–428. [Google Scholar] [CrossRef]
- Mitrushina, M.; Boone, K.B.; Razani, J.; D’Elia, L.F. Handbook of Normative Data for Neuropsychological Assessment, 2nd ed.; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Rodewald, K.; Bartolovic, M.; Debelak, R.; Aschenbrenner, S.; Weisbrod, M.; Roesch-Ely, D. Eine Normierungsstudie eines modifizierten Trail Making Tests im deutschsprachigen Raum. Z. Fur Neuropsychol. 2012, 23, 37–48. [Google Scholar] [CrossRef]
- Tombaugh, T.N. Trail Making Test A and B: Normative data stratified by age and education. Arch. Clin. Neuropsychol. 2004, 19, 203–214. [Google Scholar] [CrossRef]
- Periáñez, J.A.; Ríos-Lago, M.; Rodríguez-Sánchez, J.M.; Adrover-Roig, D.; Sánchez-Cubillo, I.; Crespo-Facorro, B.; Quemada, J.I.; Barceló, F. Trail Making Test in traumatic brain injury, schizophrenia, and normal ageing: Sample comparisons and normative data. Arch. Clin. Neuropsychol. 2007, 22, 433–447. [Google Scholar] [CrossRef] [Green Version]
- Zalonis, I.; Kararizou, E.; Triantafyllou, N.I.; Kapaki, E.; Papageorgiou, S.; Sgouropoulos, P.; Vassilopoulos, D. A normative study of the trail making test A and B in Greek adults. Clin. Neuropsychol. 2007, 22, 842–850. [Google Scholar] [CrossRef]
- Morlett Paredes, A.; Gooding, A.; Artiola i Fortuny, L.; Rivera Mindt, M.; Suárez, P.; Scott, T.M.; Heaton, A.; Heaton, R.K.; Cherner, M.; Marquine, M.J. The state of neuropsychological test norms for Spanish-speaking adults in the United States. Clin. Neuropsychol. 2021, 35, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Kalbe, E.; Kessler, J.; Calabrese, P.; Smith, R.; Passmore, A.P.; Brand, M.; Bullock, R. DemTect: A new, sensitive cognitive screening test to support the diagnosis of mild cognitive impairment and early dementia. Int. J. Geriatr. Psychiatry 2004, 19, 136–143. [Google Scholar] [CrossRef]
- JASP Team. JASP (0.16). Computer Software. 2021. Available online: https://jasp-stats.org/ (accessed on 4 July 2023).
- Sunderland, K.M.; Beaton, D.; Fraser, J.; Kwan, D.; McLaughlin, P.M.; Montero-Odasso, M.; Peltsch, A.J.; Pieruccini-Faria, F.; Sahlas, D.J.; Swartz, R.H.; et al. The utility of multivariate outlier detection techniques for data quality evaluation in large studies: An application within the ONDRI project. BMC Med. Res. Methodol. 2019, 19, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eck, D.J. Bootstrapping for multivariate linear regression models. Stat. Probab. Lett. 2018, 134, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Cleveland, W.S. Robust Locally Weighted Regression and Smoothing Scatterplotts. J. Am. Stat. Assoc. 1979, 74, 829–836. [Google Scholar] [CrossRef]
- Larner, A.J. Cognitive Screening Instruments: A Practical Approach; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Lienert, G.A.; Raatz, U. Testaufbau Und Testanalyse; Beltz: Weinheim, Germany, 1998. [Google Scholar]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 115–159. [Google Scholar] [CrossRef]
- Lezak, M.D.; Howieson, D.B.; Bigler, E.D.; Tranel, D. Neuropsychological Assessment, 5th ed.; O. U. Press: Guangzhou, China, 2012. [Google Scholar]
- Kim, R.; Chung, W. Effect of Aging on Educational Differences in the Risk of Cognitive Impairment: A Gender-Specific Analysis Using Korean Longitudinal Study of Aging (2006–2016). Healthcare 2022, 10, 1062. [Google Scholar] [CrossRef]
- Lin, L.; Xiong, M.; Jin, Y.; Kang, W.; Wu, S.; Sun, S.; Fu, Z. Quantifying Brain and Cognitive Maintenance as Key Indicators for Sustainable Cognitive Aging: Insights from the UK Biobank. Sustainability 2023, 15, 9620. [Google Scholar] [CrossRef]
- Gur, R.E.; Gur, R.C. Gender differences in aging: Cognition, emotions, and neuroimaging studies. Dialogues Clin. Neurosci. 2002, 4, 197–210. [Google Scholar] [CrossRef]
- Bugg, J.M.; Zook, N.A.; DeLosh, E.L.; Davalos, D.B.; Davis, H.P. Age differences in fluid intelligence: Contributions of general slowing and frontal decline. Brain Cogn. 2006, 62, 9–16. [Google Scholar] [CrossRef]
- Etienne, V.; Marin-Lamellet, C.; Laurent, B. Executive functioning in normal aging. Rev. Neurol. 2008, 164, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Helmchen, H.; Reischies, F.M. Normales und pathologisches kognitives Altern. Nervenarzt 1998, 69, 369–378. [Google Scholar] [CrossRef]
- Miller, E.M. Intelligence and brain myelination: A hypothesis. Personal. Individ. Differ. 1994, 17, 803–832. [Google Scholar] [CrossRef]
- Barrick, T.R.; Charlton, R.A.; Clark, C.A.; Markus, H.S. White matter structural decline in normal ageing: A prospective longitudinal study using tract-based spatial statistics. NeuroImage 2010, 51, 565–577. [Google Scholar] [CrossRef]
- Pfefferbaum, A.; Sullivan, E.V. Increased brain white matter diffusivity in normal adult aging: Relationship to anisotropy and partial voluming. Magn. Reson. Med. 2003, 49, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R.; Nyberg, L.; Park, D.C. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Laws, K.R.; Irvine, K.; Gale, T.M. Sex differences in cognitive impairment in Alzheimer’s disease. World J. Psychiatry 2016, 6, 54. [Google Scholar] [CrossRef] [Green Version]
- Levine, D.A.; Gross, A.L.; Briceño, E.M.; Tilton, N.; Giordani, B.J.; Sussman, J.B.; Hayward, R.A.; Burke, J.F.; Hingtgen, S.; Elkind MS, V.; et al. Sex Differences in Cognitive Decline among US Adults. JAMA Netw. Open 2021, 4, e210169. [Google Scholar] [CrossRef]
- Proust-Lima, C.; Amieva, H.; Letenneur, L.; Orgogozo, J.M.; Jacqmin-Gadda, H.; Dartigues, J.F. Gender and Education Impact on Brain Aging: A General Cognitive Factor Approach. Psychol. Aging 2008, 23, 608–620. [Google Scholar] [CrossRef] [Green Version]
- Cauthen, N.R. Verbal fluency: Normative data. J. Clin. Psychol. 1978, 34, 126–129. [Google Scholar] [CrossRef]
- Borkowski, J.G.; Benton, A.L.; Spreen, O. Word fluency and brain damage. Neuropsychologia 1967, 5, 135–140. [Google Scholar] [CrossRef]
- Invitto, S.; Accogli, G.; Leucci, M.; Salonna, M.; Serio, T.; Fancello, F.; Ciccarese, V.; Lankford, D. Spatial Olfactory Memory and Spatial Olfactory Navigation, Assessed with a Variant of Corsi Test, Is Modulated by Gender and Sporty Activity. Brain Sci. 2022, 12, 1108. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.C.; Huttenlocher, J.; Taylor, A.; Langrock, A. Early sex differences in spatial skill. Dev. Psychol. 1999, 35, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Lewin, C.; Wolgers, G.; Herlitz, A. Sex Differences Favoring Women in Verbal but not in Visuospatial Episodic Memory. Neuropsychology 2001, 15, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Liepelt-Scarfone, I.; Gräber, S.; Kalbe, E.; Riedel, O.; Ringendahl, H.; Schmidt, N.; Witt, K.; Roeske, S. Empfehlungen zur neuropsychologischen Diagnostik beim Morbus Parkinson. Fortschritte Der Neurol. Psychiatr. 2021, 89, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Roebuck-Spencer, T.M.; Glen, T.; Puente, A.E.; Denney, R.L.; Ruff, R.M.; Hostetter, G.; Bianchini, K.J. Cognitive Screening Tests Versus Comprehensive Neuropsychological Test Batteries: A National Academy of Neuropsychology Education Paper. Arch. Clin. Neuropsychol. 2017, 32, 491–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
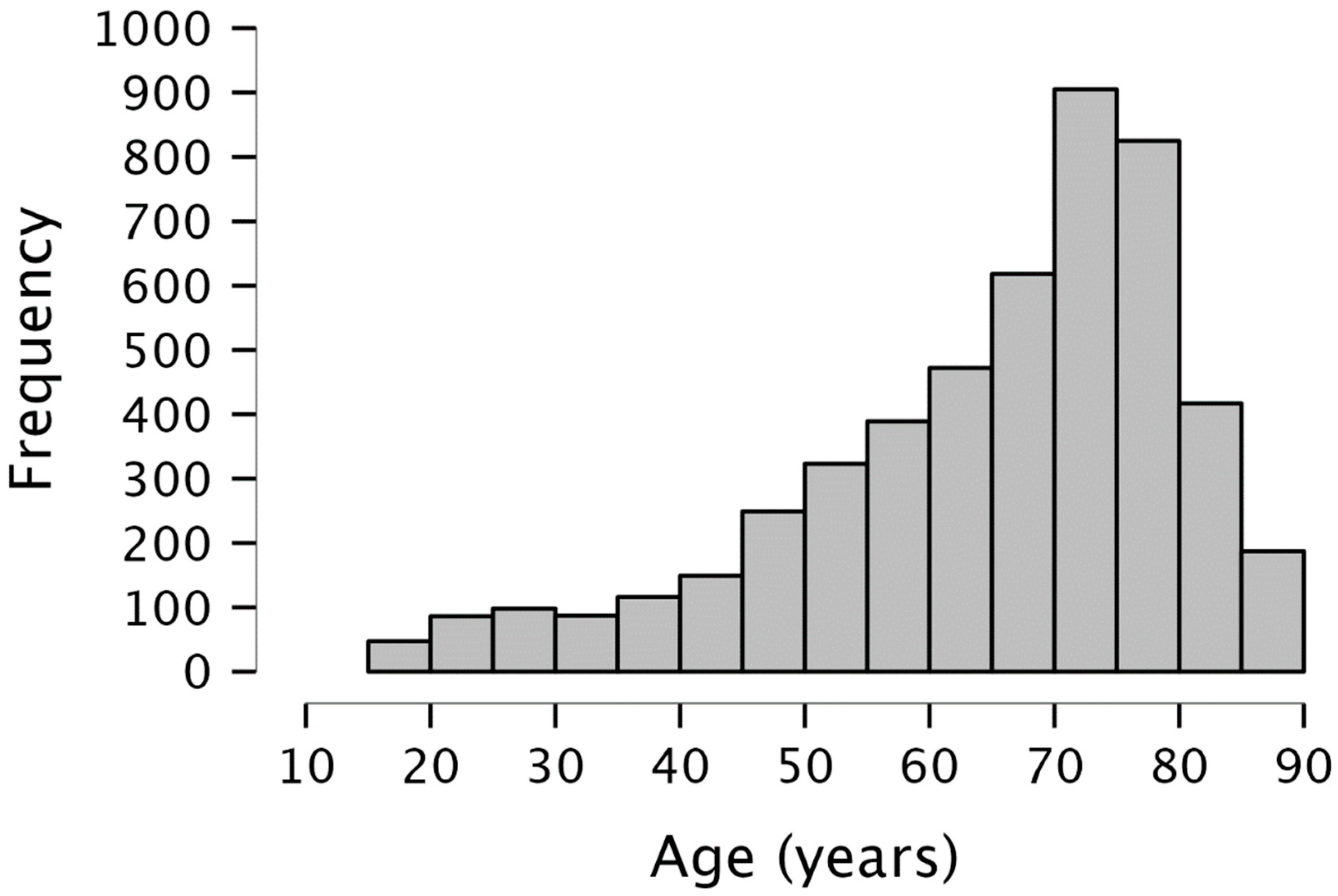
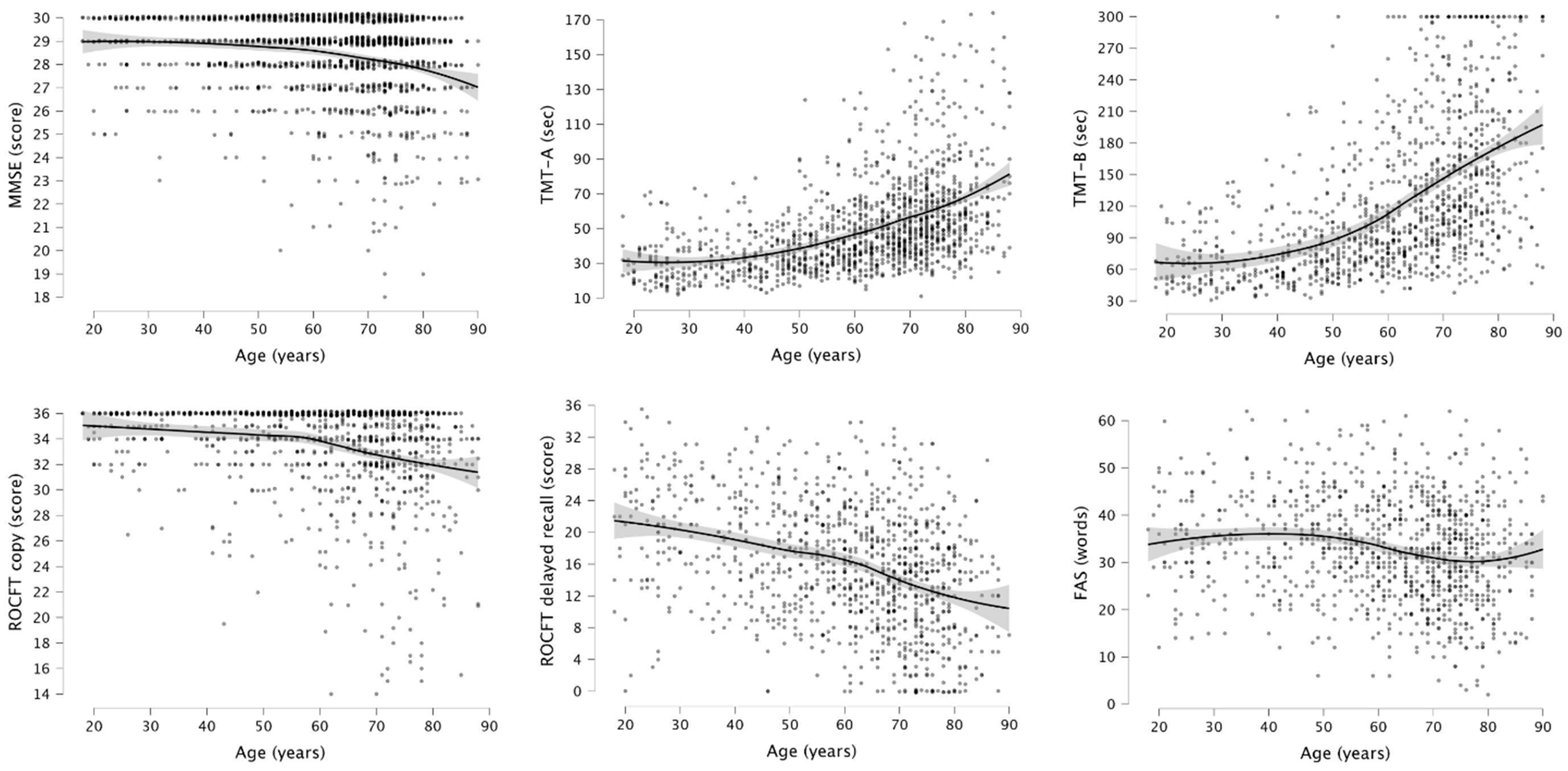
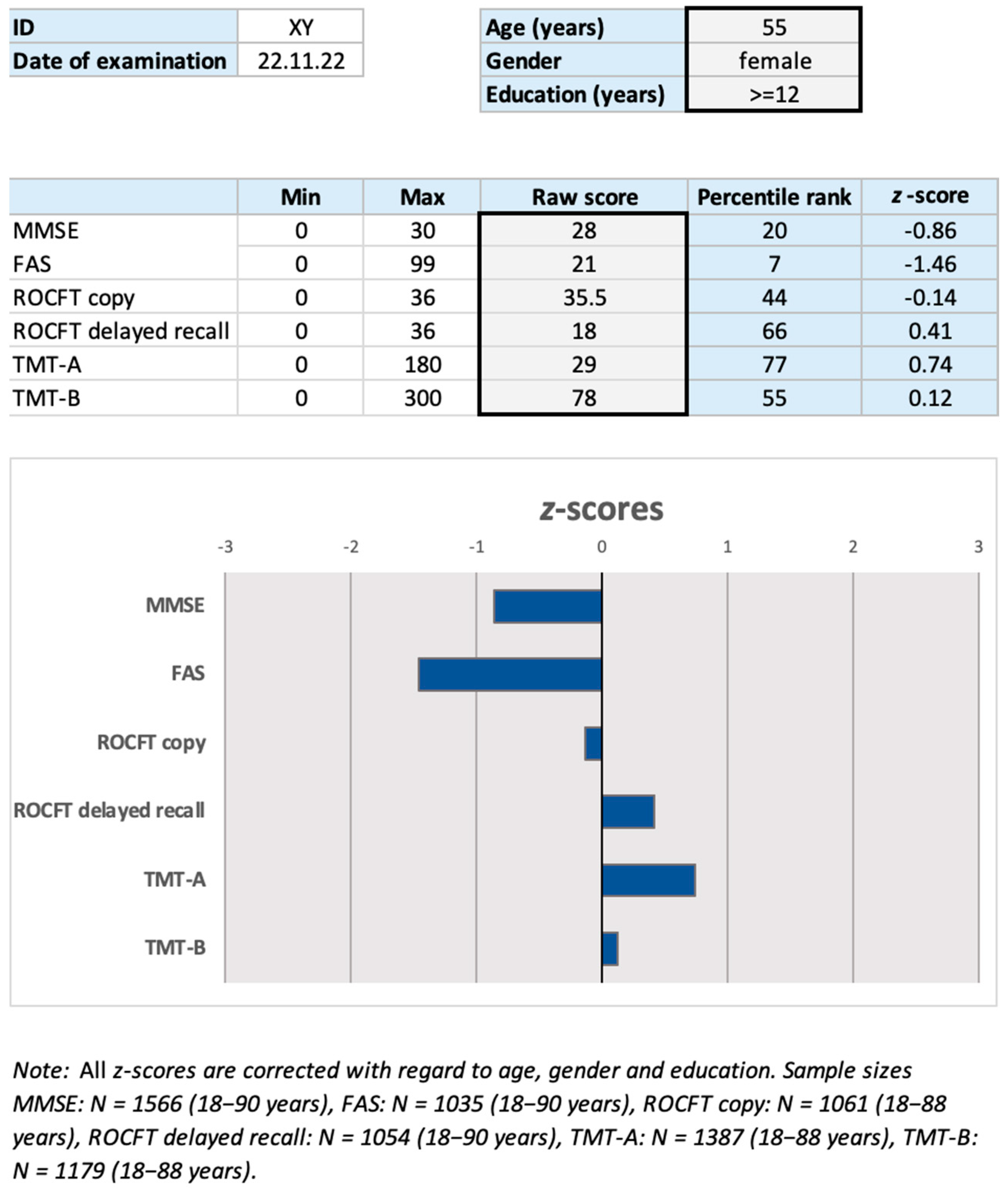
| Entire Sample (n = 4968) | DemTect ≥ 13 (n = 1603) | DemTect < 13 (n = 2518) | |
|---|---|---|---|
| Age (Years) [range] | 65.3 ± 15.6 [18–90] | 61.2 ± 16.3 [18–90] | 68 ± 14.6 [18–90] |
| Gender (% female) | 43.6 | 47.1 | 42.9 |
| Education ≥ 12 years | 36.8 | 49.5 | 29.6 |
| Education < 12 years | 63.2 | 50.5 | 70.4 |
| Cognition | |||
| DemTect [range] | 10.8 ± 4.5 [0–18] | - | - |
| MMSE [range] | 26.4 ± 3.3 [17–30] | 28.4 ± 1.8 [18–30] | 25.2 ± 3.4 [17–30] |
| FAS [range] | 24.9 ± 12.7 [0–62] | 32.7 ± 10.8 [2–62] | 19.4 ± 11 [0–61] |
| ROCFT copy [range] | 31.6 ± 5.4 [13.5–36] | 33.4 ± 3.8 [14–36] | 29.8 ± 6.1 [13.5–36] |
| ROCFT delayed recall + [range] | 11.8 ± 8.3 [0–35.5] | 15.7 ± 7.7 [0–35.5] | 8.1 ± 7.3 [0–32] |
| TMT-A [range] ++ | 64.4 ± 35.2 [11–175] | 50 ± 25.9 [11–174] | 76.6 ± 36.9 [11–175] |
| TMT-B [range] +++ | 154.3 ± 80.6 [31–300] | 123.7 ± 68.9 [31–300] | 190 ± 78.1 [32–300] |
| Test | MMSE | FAS | ROCFT Copy | RCOFT Delayed Recall + | TMT-A | TMT-B |
|---|---|---|---|---|---|---|
| MMSE | - | |||||
| FAS | 0.48 * | - | ||||
| ROCFT copy | 0.37 * | 0.37 * | - | |||
| ROCFT delayed recall + | 0.54 * | 0.41 * | 0.53 * | - | ||
| TMT-A | −0.45 * | −0.49 * | −0.46 * | −0.48 * | - | |
| TMT-B | −0.47 * | −0.50 * | −0.51 * | −0.52 * | 0.76 * | - |
| Variables | Without Bootstrapping | With Bootstrapping (5000 Iterations) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| b | SE | β | t | p | 95% CI | |||||
| b | SE | p | Lower | Upper | ||||||
| Dependent variable: MMSE (n = 4302) | ||||||||||
| Age | −0.06 | 0.003 | −0.26 | −17.98 | <0.001 ** | −0.06 | 0.003 | <0.001 ** | −0.06 | −0.05 |
| Gender a | −0.08 | 0.10 | −0.80 | 0.42 | −0.08 | 0.10 | 0.44 | −0.27 | 0.11 | |
| Education b | −1.02 | 0.10 | −10.32 | <0.001 ** | −1.02 | 0.10 | <0.001 ** | −1.21 | −0.83 | |
| Dependent variable: FAS (n = 2746) | ||||||||||
| Age | −0.17 | 0.02 | −0.22 | −11.93 | <0.001 ** | −0.17 | 0.01 | <0.001 ** | −0.20 | −0.15 |
| Gender a | 1.58 | 0.47 | 3.39 | <0.001 ** | 1.58 | 0.47 | 0.003 * | 0.65 | 2.48 | |
| Education b | −5.98 | 0.48 | −12.56 | <0.001 ** | −5.99 | 0.49 | <0.001 ** | −6.92 | −5.0 | |
| Dependent variable: ROCF copy (n = 2367) | ||||||||||
| Age | −0.09 | 0.01 | −0.29 | −14.70 | <0.001 ** | −0.09 | 0.006 | <0.001 ** | −0.11 | −0.08 |
| Gender a | −0.09 | 0.21 | −0.42 | 0.43 | −0.09 | 0.21 | 0.66 | −0.50 | 0.31 | |
| Education b | −1.71 | 0.21 | −7.98 | <0.001 ** | −1.72 | 0.21 | <0.001 ** | −2.10 | −1.29 | |
| Dependent variable: ROCF delayed recall + (n = 2524) | ||||||||||
| Age | −0.19 | 0.01 | −0.37 | −20.27 | <0.001 ** | −0.19 | 0.009 | <0.001 ** | −0.20 | −0.17 |
| Gender a | −1.56 | 0.30 | −5.15 | <0.001 ** | −1.56 | 0.30 | <0.001 ** | −2.15 | −0.98 | |
| Education b | −2.56 | 0.31 | −8.26 | <0.001 ** | −2.56 | 0.31 | <0.001 ** | −3.15 | −1.95 | |
| Dependent variable: TMT-A (n = 3345) | ||||||||||
| Age | 0.94 | 0.03 | 0.43 | 27.61 | <0.001 ** | 0.94 | 0.03 | <0.001 ** | 0.88 | 1.00 |
| Gender a | 0.20 | 1.10 | 0.18 | 0.86 | 0.20 | 1.11 | 0.86 | −1.99 | 2.36 | |
| Education b | 8.47 | 1.12 | 7.58 | <0.001 ** | 8.46 | 1.10 | <0.001 ** | 6.34 | 10.66 | |
| Dependent variable: TMT-B (n = 2506) | ||||||||||
| Age | 2.42 | 0.08 | 0.50 | 29.26 | <0.001 ** | 2.42 | 0.08 | <0.001 ** | 2.28 | 2.58 |
| Gender a | −3.17 | 2.74 | −1.16 | 0.25 | −3.18 | 2.74 | 0.24 | −8.66 | 2.21 | |
| Education b | 27.86 | 2.76 | 10.09 | <0.001 ** | 27.96 | 2.72 | <0.001 ** | 22.37 | 33.16 | |
| Test | Sociodemographic Effect | Interpretation According to Cohen [55] | |||
|---|---|---|---|---|---|
| Age | Gender | Education | |||
| MMSE | − | 0 | + | 0.11 | Small |
| FAS | − | ♀↑ | + | 0.13 | Small |
| ROCFT copy | − | 0 | + | 0.14 | Small |
| ROCFT delayed recall | − | ♂↑ | + | 0.23 | Medium |
| TMT-A | − | 0 | + | 0.26 | Medium |
| TMT-B | − | 0 | + | 0.43 | Large |
| Test | Education (Years) | Age (Years) | |||||
|---|---|---|---|---|---|---|---|
| 18–29 | 30–49 | 50–59 | 60–69 | 70–79 | ≥80 | ||
| MMSE | ≥12 | 29.2 ± 1.1 (n = 65) | 29.2 ± 1.2 (n = 146) | 29 ± 1.2 (n = 127) | 28.5 ± 1.8 (n = 180) | 28.4 ± 1.7 (n = 195) | 27.7 ± 2 (n = 62) |
| <12 | 28.7 ± 1.5 (n = 40) | 28.4 ± 1.5 (n = 100) | 28.5 ± 1.7 (n = 109) | 28.5 ± 1.5 (n = 205) | 27.7 ± 2.3 (n = 268) | 27.6 ± 2.2 (n = 69) | |
| TMT-A | ≥12 | 29.9 ± 13.5 (n = 64) | 32.1 ± 12.2 (n =133) | 40.7 ± 16.4 (n = 118) | 47.6 ± 21 (n = 166) | 59.4 ± 26.1 (n = 171) | 68.6 ± 36.7 (n = 53) |
| <12 | 30.5 ± 13.6 (n = 38) | 36.4 ± 12.9 (n = 87) | 45 ± 20.2 (n = 91) | 53.1 ± 22.8 (n = 180) | 63.2 ± 27.7 (n = 226) | 74 ± 32.9 (n = 60) | |
| TMT-B | ≥12 | 61.8 ± 22.5 (n = 59) | 72.5 ± 38.3 (n = 123) | 93.4 ± 44.2 (n = 103) | 120.3 ± 62.13 (n = 142) | 146.7 ± 63.6 (n = 140) | 170.2 ± 84.3 (n = 44) |
| <12 | 74.7 ± 25.1 (n = 29) | 84.8 ± 37.8 (n = 73) | 102.2 ± 43.7 (n = 82) | 140.1 ± 68 (n = 146) | 167.2 ± 68.4 (n = 186) | 198.4 ± 70.1 (n = 52) | |
| Test | Gender | Education (Years) | Age (Years) | ||||
|---|---|---|---|---|---|---|---|
| 18–29 | 30–49 | 50–64 | 65–74 | ≥75 | |||
| FAS | Male | ≥12 | 35.2 ± 10.8 (n = 19) | 37.5 ± 8.5 (n = 60) | 37.5 ± 9.6 (n = 77) | 31.2 ± 11.1 (n = 93) | 32.7 ± 10.9 (n = 60) |
| <12 | 31.5 ± 9.7 (n = 17) | 30.8 ± 10.5 (n = 31) | 32.8 ± 10.5 (n = 66) | 29.1 ± 8.6 (n = 82) | 27.3 ± 10.3 (n = 64) | ||
| Female | ≥12 | 35.2 ± 11.6 (n = 26) | 36.3 ± 11.5 (n = 42) | 35 ± 10.4 (n = 65) | 36.4 ± 9 (n = 43) | 35.2 ± 11 (n = 35) | |
| <12 | 34.8 ± 8.2 (n = 10) | 36.2 ± 11.8 (n = 34) | 32 ± 9.9 (n = 63) | 29.4 ± 10.6 (n = 75) | 28.5 ± 11.6 (n = 73) | ||
| ROCFT copy | - | ≥12 | 34.9 ± 1.6 (n = 56) | 34.8 ± 2.3 (n = 110) | 34.3 ± 2.8 (n = 153) | 33.4 ± 3.8 (n = 150) | 32.6 ± 3.9 (n = 82) |
| <12 | 34.7 ± 2.1 (n = 32) | 33.6 ± 3.6 (n = 70) | 33.6 ± 3.6 (n = 124) | 32.5 ± 4.3 (n = 159) | 31.6 ± 5.1 (n = 125) | ||
| ROCFT delayed recall + | Male | ≥12 | 22.7 ± 6.9 (n = 26) | 19.7 ± 7.2 (n = 63) | 19.2 ± 6.3 (n = 80) | 15.6 ± 7.8 (n = 96) | 13.5 ± 7.2 (n = 51) |
| <12 | 18.9 ± 5.8 (n = 17) | 19.9 ± 8.2 (n = 36) | 16.7 ± 7.5 (n = 66) | 14.6 ± 7.2 (n = 79) | 12.7 ± 7.7 (n = 64) | ||
| Female | ≥12 | 21.6 ± 9.1 (n = 28) | 18.5 ± 6.9 (n = 46) | 15.8 ± 5.9 (n = 72) | 12 ± 5.7 (n = 51) | 12.6 ± 6.5 (n = 32) | |
| <12 | 18 ± 8.5 (n = 12) | 16.5 ± 5.5 (n = 33) | 15 ± 6.7 (n = 59) | 12.7 ± 7.3 (n = 78) | 9.9 ± 6.4 (n = 65) | ||
| Test Score | Age (Years) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18–29 (n = 65) | 30–49 (n = 146) | 50–59 (n = 127) | 60–69 (n = 180) | 70–79 (n = 195) | ≥80 (n = 62) | |||||||
| PR | z-Score | PR | z-Score | PR | z-Score | PR | z-Score | PR | z-Score | PR | z-Score | |
| ≤20 | 0 | −3 | 0 | −3 | 0 | −3 | 0 | −3 | 0 | −3 | 0 | −3 |
| 21 | 0 | −3 | 0 | −3 | 0 | −3 | 1 | −2.54 | 1 | −2.57 | 0 | −3 |
| 22 | 0 | −3 | 0 | −3 | 0 | −3 | 1 | −2.2 | 1 | −2.32 | 1 | −2.41 |
| 23 | 0 | −3 | 0 | −3 | 0 | −3 | 2 | −2.01 | 1 | −2.24 | 4 | −1.75 |
| 24 | 0 | −3 | 0 | −2.71 | 0 | −2.66 | 3 | −1.84 | 2 | −2 | 8 | −1.41 |
| 25 | 1 | −2.43 | 1 | −2.47 | 1 | −2.42 | 5 | −1.62 | 4 | −1.71 | 11 | −1.22 |
| 26 | 3 | −1.87 | 3 | −1.92 | 3 | −1.86 | 8 | −1.39 | 10 | −1.3 | 18 | −0.93 |
| 27 | 5 | −1.61 | 7 | −1.52 | 8 | −1.39 | 15 | −1.03 | 18 | −0.9 | 29 | −0.56 |
| 28 | 11 | −1.24 | 14 | −1.07 | 20 | −0.86 | 30 | −0.54 | 33 | −0.46 | 46 | −0.11 |
| 29 | 32 | −0.46 | 33 | −0.44 | 43 | −0.19 | 53 | 0.07 | 57 | 0.16 | 69 | 0.48 |
| 30 | 75 | 0.66 | 73 | 0.61 | 78 | 0.78 | 84 | 0.97 | 86 | 1.06 | 90 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheffels, J.F.; Ballasch, I.; Scheichel, N.; Voracek, M.; Kalbe, E.; Kessler, J. The Influence of Age, Gender and Education on Neuropsychological Test Scores: Updated Clinical Norms for Five Widely Used Cognitive Assessments. J. Clin. Med. 2023, 12, 5170. https://doi.org/10.3390/jcm12165170
Scheffels JF, Ballasch I, Scheichel N, Voracek M, Kalbe E, Kessler J. The Influence of Age, Gender and Education on Neuropsychological Test Scores: Updated Clinical Norms for Five Widely Used Cognitive Assessments. Journal of Clinical Medicine. 2023; 12(16):5170. https://doi.org/10.3390/jcm12165170
Chicago/Turabian StyleScheffels, Jannik F., Isabell Ballasch, Nadine Scheichel, Martin Voracek, Elke Kalbe, and Josef Kessler. 2023. "The Influence of Age, Gender and Education on Neuropsychological Test Scores: Updated Clinical Norms for Five Widely Used Cognitive Assessments" Journal of Clinical Medicine 12, no. 16: 5170. https://doi.org/10.3390/jcm12165170
APA StyleScheffels, J. F., Ballasch, I., Scheichel, N., Voracek, M., Kalbe, E., & Kessler, J. (2023). The Influence of Age, Gender and Education on Neuropsychological Test Scores: Updated Clinical Norms for Five Widely Used Cognitive Assessments. Journal of Clinical Medicine, 12(16), 5170. https://doi.org/10.3390/jcm12165170






