Supraglottic Airway Devices with Vision Guided Systems: Third Generation of Supraglottic Airway Devices
Abstract
:1. Introduction
- As the device of choice after failure of face mask ventilation.
- As an alternative approach to airway management after failed intubation as a ventilation device/intubation channel.
- To attempt fibre-optic-assisted (not blind) intubation via SAD.
- As an airway rescue device in non-ventilatory, non-intubatable situations, prior to the establishment of a surgical airway.
- -
- The distal tip of the SAD must rest on and block the upper oesophageal sphincter.
- -
- The cuff occupies the entire hypopharynx and is positioned behind the cricoid cartilage, anterior to the second to seventh cervical vertebrae.
- -
- The opening of the SAD opposes the glottis.
- -
- The sides of the cuff lie in the pyriform fossae.
- -
- The epiglottis is located external to the device, aligned with the proximal part of the laryngeal mask (The epiglottis determines the correct placement of any SAD.).
- -
- The superior border of the mask lies at the base of the tongue.
- -
- Two appropriate seals must be produced for the respiratory and digestive tract.
- -
- Lack of experience of the operator.
- -
- The technique used.
- -
- Inadequate plane of anaesthesia.
- -
- Inadequate choice of SAD size.
- -
- Patient anatomy: edentulous; patients with ogival palate or a history of temporomandibular joint dysfunction.
2. We Should No Longer Use SADs without Vision-Guided Systems
3. Assessment of SADs
- -
- Sign at placement: the presence of resistance at the end of insertion.
- -
- Outward movement of the LMA with inflation of the cuff.
- -
- The adverse suprasternal notch tap test (also known as the “Brimacombe bounce”; tapping the suprasternal notch or cricoid cartilage and observing simultaneous movement of a column of lubricant or a soap bubble membrane at the proximal end of the drain tube).
- -
- Appropriate chest rise with each breath.
- -
- Adequate oxygen saturation.
- -
- Capnography to corroborate a good capnography tracing.
- -
- The device pressure.
- -
- Insertion of an orogastric tube through the gastric channel without resistance.
4. Incorrect Positioning and Complications
- -
- Incorrect position of the epiglottis [57,58]. The epiglottis represents the most common cause of airway obstruction. Some have found that the epiglottis is deviated backwards in more than 80% of patients after blind insertion [22], while downfolding of the epiglottis occurs in 20–56% of patients [59]. The initial blind insertion resulted in epiglottis downfolding and positioning of epiglottis in the bowl of the device in >75% of cases [39]. It should be taken into consideration that in order not to encounter a high incidence of downward folded epiglottis, it is important to use the right size LMA.
- -
- Hyperinflated/hypoinflated cuff.
- -
- Cuff bent creating airway leaks.
- -
- If the SAD too small or too large, with insertion that is too deep or too shallow, it would produce an inadequate seal.
- -
- Rotation of the SAD cuff in a sagittal plane.
- -
- Ventilatory failure, including insufficient tidal volume due to an air leak.
- -
- Airway obstruction.
- -
- Twenty-six times greater likelihood gastric insufflation and subsequent aspiration.
- -
- -
- -
- Injury to the vocal cords. Transient bilateral vocal cord paralysis [61].
- -
- -
- -
5. Supraglottic Airway Devices with Vision Guided Systems Are Here, to Stay
6. Video Laryngeal Mask (VLMs)
- -
- The second-generation disposable SAD has separate gastric and ventilation channels allowing functional separation, a silicone cuff and a reinforced distal tip which allows a more compact seal and therefore a higher oropharyngeal sealing pressure.
- -
- It also has a reusable flexible videoscope which is placed into a specially designed blind-end channel terminating in the bowl of the SAD.
- -
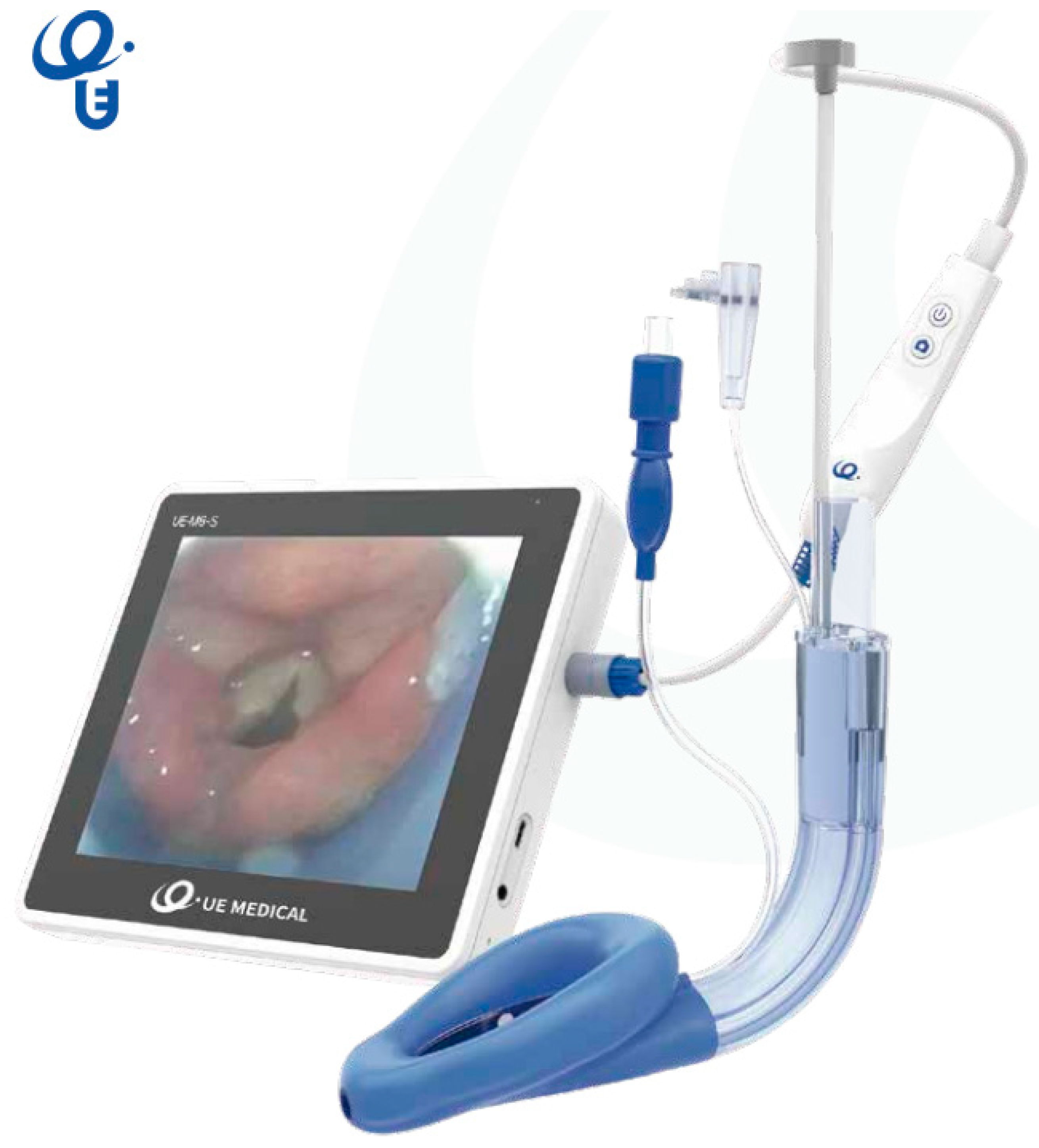
- The Video Laryngeal Mask VLMTM, UE Medical® (SaCoVLMTM).
- -
- The SafeLM®Video Laryngeal Mask System (SafeLMTMVLMS, Magill Medical Technology®).
- -
- Size 3 is suitable for patients with a body weight between 30 and 50 kg.
- -
- Size 4 is for patients between 50 and 70 kg.
- -
- Size 5 is for patients weighing 70–100 kg. They allow the passage through the ventilation channel of endotracheal tubes of 7.0, 7.5 and 8.0 mm. The size of the gastric tube is 16 French.
- An upper left-side access point which allows measurement with a manometer to determine the pressure inside the laryngeal mask in cm H2O, where the pressure ranges from 10 to 20 mmHg.
- A video stylus hole with connection. A left side access point is available for its camera stylet, which can be connected to a reusable 2.8-inch (Figure 6) and 7-inch (Figure 7) portable monitor with image/video recording capabilities. The channel is open at one end and closed at the other end, and never comes into contact with the patient.
- Its central access allows gas inlet and outlet, plus the introduction of an endotracheal tube (ETT) for rescue intubation or fibro or a video bronchoscope, with 15 mm connection.
- The lower right-side access has gastric access. It has an open channel at both ends. Suction catheter number 12 French or lower can be use in Vision Mask size 3 and suction catheter number 14 or lower can be use in Vision Mask size 4.
- An upper right-side access point for a free gas outlet from the interior when using CPAP ventilation.



7. Advantages and Limitations of New SAD with Integrated Video System
- -
- It facilitates the location of the glottis, optimising the laryngeal view.
- -
- It offers simple insertion like any other SAD but always visualises the airway. It allows confirmation of correct placement.
- -
- Manoeuvres can be performed to improve the anatomical view.
- -
- It has a better anatomical fit and airway function.
- -
- It provides confirmation that the epiglottis is seated on the outside of the device and avoids injuring it, as occurs in direct or indirect intubation, by seating the intubation device in the vallecula.
- -
- It allows rapid diagnosis of the problem if ventilation is inadequate.
- -
- It avoids damage and obstruction to the airway and damage to crowded airways.
- -
- It allows intubation through the SAD of ETT under direct vision, reducing the difficulty of advancing the ETT by overcoming anatomical obstacles.
- -
- It allows airway rescue in patients without obstructive problems due to difficulties with ventilation with face masks, poor apnoea tolerance and difficult intubation.
- -
- Vision-guided supraglottic airway devices insertion may further eliminate a need for fibreoptic checks and videolaringoscopy checks. It also reduces the extra space needed and increased complexity for the operator when the videolaryngoscope is inserted into the oropharynx external to an SAD [56].
- -
- It ensures that oro-pharyngeal leak pressure recordings are unaffected by spurious inaccuracy because of minor or gross SAD misalignment or incorrect positioning [56].
- -
- The device has a single chamber that allows the entrance of the glottis to be seen, but the entrance of the oesophagus cannot be seen after it is in place.
- -
- No information is obtained on the position of the distal cuff.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brain, F.A.I.J. The Laryngeal Mask—A New Concept in Airway Management. Br. J. Anaesth. 1983, 55, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Zoremba, M.; Aust, H.; Eberhart, L.; Braunecker, S.; Wulf, H. Comparison between intubation and the laryngeal mask airway in moderately obese adults. Acta Anaesthesiol. Scand. 2009, 53, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Abdi, W.; Dhonneur, G.; Amathieu, R.; Adhoum, A.; Kamoun, W.; Slavov, V.; Barrat, C.; Combes, X. LMA Supreme™ Versus Facemask Ventilation Performed by Novices: A Comparative Study in Morbidly Obese Patients Showing Difficult Ventilation Predictors. Obes. Surg. 2009, 19, 1624–1630. [Google Scholar] [CrossRef]
- Combes, X.; Sauvat, S.; Leroux, B.; Dumerat, M.; Sherrer, E.; Motamed, C.; Brain, A.; D’Honneur, G. Intubating laryngeal mask airway in morbidly obese and lean patients: A comparative study. Anesthesiology 2005, 102, 1106–1109. [Google Scholar] [CrossRef]
- Yao, W.Y.; Li, S.Y.; Sng, B.L.; Lim, Y.; Sia, A.T.H. The LMA Supreme™ in 700 parturients undergoing Cesarean delivery: An observational study. Can. J. Anaesth. 2012, 59, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Goel, S.; Brimacombe, J.R. The ProSeal™ Laryngeal Mask Airway is an Effective Alternative to Laryngoscope-Guided Tracheal Intubation for Gynaecological Laparoscopy. Anaesth. Intensiv. Care 2007, 35, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Abrishami, A.; Zilberman, P.; Chung, F. Brief review: Airway rescue with insertion of laryngeal mask airway devices with patients in the prone position. Can. J. Anaesth. 2010, 57, 1014–1020. [Google Scholar] [CrossRef]
- López, A.M.; Valero, R.; Brimacombe, J. Insertion and use of the LMA Supreme™ in the prone position. Anaesthesia 2010, 65, 154–157. [Google Scholar] [CrossRef]
- Sharma, V.; Verghese, C.; McKenna, P. Prospective audit on the use of the LMA-Supreme™ for airway management of adult patients undergoing elective orthopaedic surgery in prone position. Br. J. Anaesth. 2010, 105, 228–232. [Google Scholar] [CrossRef]
- Länkimäki, S.; Alahuhta, S.; Silfvast, T.; Kurola, J. Feasibility of LMA Supreme for airway management in unconscious patients by ALS paramedics. Scand. J. Trauma Resusc. Emerg. Med. 2015, 23, 24. [Google Scholar] [CrossRef]
- Woodall, N.M.; Cook, T.M. National census of airway management techniques used for anaesthesia in the UK: First phase of the Fourth National Audit Project at the Royal College of Anaesthetists. Br. J. Anaesth. 2011, 106, 266–271. [Google Scholar] [CrossRef]
- Marshall, S.D.; Pandit, J.J. Radical evolution: The 2015 Difficult Airway Society guidelines for managing unanticipated difficult or failed tracheal intubation. Anaesthesia 2016, 71, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.M. Strengths and Limitations of Airway Techniques. Anesthesiol. Clin. 2015, 33, 241–255. [Google Scholar] [CrossRef]
- van Zundert, T.C.R.V.; Brimacombe, J.R.; Ferson, D.Z.; Bacon, D.R.; Wilkinson, D.J. Archie Brain: Celebrating 30 years of development in laryngeal mask airways. Anaesthesia 2012, 67, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Van Zundert, A.A.J.; Kumar, C.M.; Van Zundert, T.C.R.V. Malpositioning of supraglottic airway devices: Preventive and corrective strategies. Br. J. Anaesth. 2016, 116, 579–582. [Google Scholar] [CrossRef]
- Joshi, S.; Sciacca, R.R.; Solanki, D.; Young, W.L.; Mathru, M.M. A Prospective Evaluation of Clinical Tests for Placement of Laryngeal Mask Airways. Anesthesiology 1998, 89, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Nandi, P.R.; Nunn, J.F.; Charlesworth, C.H.; Taylor, S.J. Radiological study of the Laryngeal Mask. Eur. J. Anaesthesiol. Suppl. 1991, 4, 33–39. [Google Scholar]
- Goudsouzian, N.G.; Denman, W.; Cleveland, R.; Shorten, G. Radiologic Localization of the Laryngeal Mask Airway in Children. Anesthesiology 1992, 77, 1085–1089. [Google Scholar] [CrossRef]
- Aoyama, K.; Takenaka, I.; Sata, T.; Shigematsu, A. The triple airway manoeuvre for insertion of the laryngeal mask airway in paralyzed patients. Can. J. Anaesth. 1995, 42, 1010–1016. [Google Scholar] [CrossRef]
- Timmermann, A. Supraglottic airways in difficult airway management: Successes, failures, use and misuse. Anaesthesia 2011, 66, 45–56. [Google Scholar] [CrossRef]
- Van Zundert, A.A.J.; Kumar, C.M.; Van Zundert, T.C.R.V.; Gatt, S.P.; Pandit, J.J. The case for a 3rd generation supraglottic airway device facilitating direct vision placement. J. Clin. Monit. Comput. 2021, 35, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Van Zundert, A.A.J.; Gatt, S.P.; Kumar, C.M.; Van Zundert, T.C.R.V.; Pandit, J.J. ‘Failed supraglottic airway’: An algorithm for suboptimally placed supraglottic airway devices based on videolaryngoscopy. Br. J. Anaesth. 2017, 118, 645–649. [Google Scholar] [CrossRef]
- Kim, G.W.; Kim, J.Y.; Kim, S.J.; Moon, Y.R.; Park, E.J.; Park, S.Y. Conditions for laryngeal mask airway placement in terms of oropharyngeal leak pressure: A comparison between blind insertion and laryngoscope-guided insertion. BMC Anesthesiol. 2019, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Yu, J.; Hong, J.H.; Hwang, J.; Kim, Y. Head elevation and laryngeal mask airway Supreme insertion: A randomized controlled trial. Acta Anaesthesiol. Scand. 2021, 65, 343–350. [Google Scholar] [CrossRef]
- Brimacombe, J. Analysis of 1500 laryngeal mask uses by one anaesthetist in adults undergoing routine anaesthesia. Anaesthesia 1996, 51, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Brimacombe, J.; Berry, A. Insertion of the Laryngeal Mask Airway—A Prospective Study of Four Techniques. Anaesth. Intensiv. Care 1993, 21, 89–92. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, J.S.; Nam, S.B.; Ju, J.W.; Kim, M.-S. Standard versus Rotation Technique for Insertion of Supraglottic Airway Devices: Systematic Review and Meta-Analysis. Yonsei Med. J. 2016, 57, 987–997. [Google Scholar] [CrossRef]
- Campbell, R.L.; Biddle, C.; Assudmi, N.; Campbell, J.R.; Hotchkiss, M. Fiberoptic assessment of laryngeal mask airway placement: Blind insertion versus direct visual epiglottoscopy. J. Oral Maxillofac. Surg. 2004, 62, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Monzón, C.G.; Marroquín-Valz, H.A.; Gigante, S.M. Manejo de la Vía Aérea ¿Qué Sabemos? Madrid. Editorial Ergon 2016. Password: castillo2015. Available online: www.ergoncreacion.com/manejodelaviaaerea/ (accessed on 5 May 2017).
- Greenland, K.; Edwards, M.; Hutton, N.; Challis, V.; Irwin, M.; Sleigh, J. Changes in airway configuration with different head and neck positions using magnetic resonance imaging of normal airways: A new concept with possible clinical applications. Br. J. Anaesth. 2010, 105, 683–690. [Google Scholar] [CrossRef]
- Cui, X.L.; Xue, F.S.; Liao, X.; Cheng, Y. Correct use of a proper sniff position for laryngoscopy. J. Clin. Anesth. 2013, 25, 675. [Google Scholar] [CrossRef]
- Collins, J.S.; Lemmens, H.J.; Brodsky, J.B.; Brock-Utne, J.G.; Levitan, R.M. Laryngoscopy and Morbid Obesity: A Comparison of the “Sniff” and “Ramped” Positions. Obes. Surg. 2004, 14, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Rich, J.M. Use of an Elevation Pillow to Produce the Head-Elevated Laryngoscopy Position for Airway Management in Morbidly Obese and Large-Framed Patients. Obstet. Anesth. Dig. 2004, 98, 264–265. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Park, J.H.; Lee, K.-Y.; Choi, S.H.; Jung, H.H.; Kim, J.-H.; Lee, B. Influence of head and neck position on the performance of supraglottic airway devices: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0216673. [Google Scholar] [CrossRef]
- Jain, D.; Banerjee, G.; Bala, I.; Gandhi, K.; Samujh, R. Comparison of the ProSeal laryngeal mask airway with the I-Gel™ in the different head-and-neck positions in anaesthetised paralysed children: A randomised controlled trial. Indian J. Anaesth. 2018, 62, 103–108. [Google Scholar] [CrossRef]
- Okuda, K.; Inagawa, G.; Miwa, T.; Hiroki, K. Influence of head and neck position on cuff position and oropharyngeal sealing pressure with the laryngeal mask airway in children. Br. J. Anaesth. 2001, 86, 122–124. [Google Scholar] [CrossRef]
- Van Zundert, T.C.; Hendrickx, J.F.; De Witte, J.L.; Wong, D.T.; Cattano, D.; Brimacombe, J.R. Do mask aperture bars of extraglottic airway devices prevent prolapse of epiglottis causing airway obstruction? A randomized crossover trial in anesthetized adult patients. J. Clin. Anesth. 2016, 31, 231–237. [Google Scholar] [CrossRef]
- Norii, T.; Makino, Y.; Unuma, K.; Hatch, G.M.; Adolphi, N.L.; Dallo, S.; Albright, D.; Sklar, D.P.; Braude, D. Extraglottic Airway Device Misplacement: A Novel Classification System and Findings in Postmortem Computed Tomography. Ann. Emerg. Med. 2021, 77, 285–295. [Google Scholar] [CrossRef] [PubMed]
- van Zundert, A.A.J.; Wyssusek, K.H.; Pelecanos, A.; Roets, M.; Kumar, C.M. A prospective randomized comparison of airway seal using the novel vision-guided insertion of LMA-Supreme® and LMA-Protector®. J. Clin. Monit. Comput. 2020, 34, 285–294. [Google Scholar] [CrossRef]
- Keller, C.; Brimacombe, J.R.; Keller, K.; Morris, R. Comparison of four methods for assessing airway sealing pressure with the laryngeal mask airway in adult patients. Br. J. Anaesth. 1999, 82, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Füllekrug, B.; Pothmann, W.; Werner, C.; Schulte am Esch, J. The laryngeal mask airway: Anesthetic gas leakage and fiberoptic control of positioning. J. Clin. Anesth. 1993, 5, 357–363. [Google Scholar] [CrossRef]
- Van Zundert, A.A.J.; Gatt, S.P.; Kumar, C.; Van Zundert, T.C.R.V. Vision-guided placement of supraglottic airway device prevents airway obstruction: A prospective audit. Br. J. Anaesth. 2017, 118, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, A.; Bergner, U.A.; Russo, S.G. Laryngeal mask airway indications: New frontiers for second-generation supraglottic airways. Curr. Opin. Anaesthesiol. 2015, 28, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Chandan, S.N.; Sharma, S.M.; Raveendra, U.S.; Prasad, B.R. Fiberoptic assessment of laryngeal mask airway placement: A comparison of blind insertion and insertion with the use of a laryngoscope. J. Maxillofac. Oral Surg. 2009, 8, 95–98. [Google Scholar] [CrossRef]
- Song, K.; Yi, J.; Liu, W.; Huang, S.; Huang, Y. Confirmation of laryngeal mask airway placement by ultrasound examination: A pilot study. J. Clin. Anesth. 2016, 34, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.C.; Ajithan, S.; Puri, A. Comparison of leakage test and ultrasound imaging to validate ProSeal supraglottic airway device placement. J. Anaesthesiol. Clin. Pharmacol. 2020, 36, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Arican, S.; Pekcan, S.; Hacibeyoglu, G.; Yusifov, M.; Yuce, S.; Uzun, S.T. The place of ultrasonography in confirming the position of the laryngeal mask airway in pediatric patients: An observational study. Braz. J. Anesthesiol. 2021, 71, 523–529. [Google Scholar] [CrossRef]
- Shorten, G.D.; Opie, N.J.; Graziotti, P.; Morris, I.; Khangure, M. Assessment of Upper Airway Anatomy in Awake, Sedated and Anaesthetised Patients Using Magnetic Resonance Imaging. Anaesth. Intensiv. Care 1994, 22, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, F.; Antenucci, G.; Piervincenzi, E.; Buonopane, C.; Bellucci, R.; Andreoli, C.; Fegatelli, D.A.; Ranieri, M.V.; Bilotta, F. Ultrasound as a new tool in the assessment of airway difficulties. Eur. J. Anaesthesiol. 2019, 36, 509–515. [Google Scholar] [CrossRef]
- Zhou, Z.-F.; Xia, C.-Z.; Wu, M.; Yu, L.-N.; Yan, G.-Z.; Ren, Q.-S.; Hu, C.-X.; Yan, M. Comparison of Three Methods for the Confirmation of Laryngeal Mask Airway Placement in Female Patients Undergoing Gynecologic Surgery. Ultrasound Med. Biol. 2015, 41, 1212–1220. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.Y.; Kim, W.O.; Kil, H.K. An Ultrasound Evaluation of Laryngeal Mask Airway Position in Pediatric Patients: An observational study. Obstet. Anesth. Dig. 2015, 120, 427–432. [Google Scholar] [CrossRef]
- Prasad, A.; Yu, E.; Wong, D.T.; Karkhanis, R.; Gullane, P.; Chan, V.W.S.; Mbbs, D.A.P.; Mbbs, R.K.; Gullane, F.P.; Chan, F.V.W.S. Comparison of Sonography and Computed Tomography as Imaging Tools for Assessment of Airway Structures. J. Ultrasound Med. 2011, 30, 965–972. [Google Scholar] [CrossRef]
- Warner, M.A.; Warner, M.E.; Weber, J.G. Clinical Significance of Pulmonary Aspiration during the Perioperative Period. Anesthesiology 1993, 78, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Michalek, P.; Donaldson, W.; Vobrubova, E.; Hakl, M. Complications Associated with the Use of Supraglottic Airway Devices in Perioperative Medicine. BioMed Res. Int. 2015, 2015, 746560. [Google Scholar] [CrossRef]
- Brimacombe, J.R. Pathophysiology. In Laryngeal Mask Anesth. Principles and Practice; Brimacombe, J.R., Ed.; WB Saunders: Philadelphia, PA, USA, 2005; pp. 105–136. [Google Scholar]
- Van Zundert, A.A.J.; Gatt, S.P.; Van Zundert, T.C.R.V.; Kumar, C.M.; Pandit, J.J. Features of new vision-incorporated third-generation video laryngeal mask airways. J. Clin. Monit. Comput. 2022, 36, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Van Zundert, A.; Brimacombe, J.; Kamphuis, R.; Haanschoten, M. The anatomical position of three extraglottic airway devices in patients with clear airways. Anaesthesia 2006, 61, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Benumof, J.L. Function of the aperture bars on the LMA. Can. J. Anaesth. 2003, 50, 968. [Google Scholar] [CrossRef]
- Keller, C.; Brimacombe, J. Pharyngeal Mucosal Pressures, Airway Sealing Pressures, and Fiberoptic Position with the Intubating versus the Standard Laryngeal Mask Airway. Anesthesiology 1999, 90, 1001–1006. [Google Scholar] [CrossRef]
- L’hermite, J.; Dubout, E.; Bouvet, S.; Bracoud, L.-H.; Cuvillon, P.; de La Coussaye, J.-E.; Ripart, J. Sore throat following three adult supraglottic airway devices. Eur. J. Anaesthesiol. 2017, 34, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Inomata, S.; Nishikawa, T.; Suga, A.; Yamashita, S. Transient Bilateral Vocal Cord Paralysis after Insertion of a Laryngeal Mask Airway. Anesthesiology 1995, 82, 787–788. [Google Scholar] [CrossRef]
- Geng, Z.Y.; Gao, W.H.; Li, Y.W. A case of arytenoid cartilage dislocation following insertion of a supreme laryngeal mask. J. Clin. Anesth. 2020, 61, 109642. [Google Scholar] [CrossRef]
- Emmett, S.R.; Lloyd, S.D.; Johnston, M.N. Uvular trauma from a laryngeal mask. Br. J. Anaesth. 2012, 109, 468–469. [Google Scholar] [CrossRef]
- Arigliani, M.; Dolcemascolo, V.; Passone, E.; Vergine, M.; Cogo, P. Uvular Trauma after Laryngeal Mask Airway Use. J. Pediatr. 2016, 176, 217. [Google Scholar] [CrossRef]
- Li, C.; Lou, Y.; Shen, Y.; Jiang, S.; Xu, H. Unilateral lingual nerve and hypoglossal nerve injury caused by a novel laryngeal mask airway: A case report. Braz. J. Anesthesiol. 2022, 72, 666–668. [Google Scholar] [CrossRef]
- Stewart, A.; Lindsay, W.A. Bilateral hypoglossal nerve injury following the use of the laryngeal mask airway. Anaesthesia 2002, 57, 264–265. [Google Scholar] [CrossRef]
- Takahoko, K.; Iwasaki, H.; Sasakawa, T.; Suzuki, A.; Matsumoto, H.; Iwasaki, H. Unilateral Hypoglossal Nerve Palsy after Use of the Laryngeal Mask Airway Supreme. Case Rep. Anesthesiol. 2014, 2014, 369563. [Google Scholar] [CrossRef]
- Nagai, K.; Sakuramoto, C.; Goto, F. Unilateral hypoglossal nerve paralysis following the use of the laryngeal mask airway. Anaesthesia 1994, 49, 603–604. [Google Scholar] [CrossRef]
- Brimacombe, J.; Clarke, G.; Keller, C. Lingual nerve injury associated with the ProSeal laryngeal mask airway: A case report and review of the literature. Br. J. Anaesth. 2005, 95, 420–423. [Google Scholar] [CrossRef]
- Brain, A.I.J.; Howard, D. Lingual nerve injury associated with laryngeal mask use. Anaesthesia 1998, 53, 713–714. [Google Scholar] [CrossRef]
- Lowinger, D.; Benjamin, B.; Gadd, L. Recurrent Laryngeal Nerve Injury Caused by a Laryngeal Mask Airway. Anaesth. Intensiv. Care 1999, 27, 202–205. [Google Scholar] [CrossRef]
- Akhtar, N.; Ungureanu, N.; Cakir, S.; Ansari, U.; Mohamed, T.; Brown, K.; Stocker, J.; Mendonca, C. Temporomandibular joint dysfunction following the use of a supraglottic airway device during general anaesthesia: A prospective observational study. Anaesthesia 2021, 76, 1511–1517. [Google Scholar] [CrossRef]
- Sia, S.-L.; Chang, Y.-L.; Lee, T.-M.; Lai, Y.-Y. Temporomandibular Joint Dislocation After Laryngeal Mask Airway Insertion. Acta Anaesthesiol. Taiwanica 2008, 46, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Brimacombe, J.; Berry, A. A proposed fiber-optic scoring system to standardize the assessment of laryngeal mask airway position (Letter). Anesth. Analg. 1993, 76, 457. [Google Scholar] [PubMed]
- Brimacombe, J.; Berry, A. The laryngeal mask airway—Anatomical and physiological implications. Acta Anaesthesiol. Scand. 1996, 40, 201–209. [Google Scholar] [CrossRef]
- Brimacombe, J.; Berry, A. Neuromuscular Block and Insertion of the Laryngeal Mask Airway. Br. J. Anaesth. 1993, 71, 166–167. [Google Scholar] [CrossRef]
- Gómez-Ríos, M.; López, T.; Sastre, J.A.; Gaszyński, T.; Van Zundert, A.A.J. Video laryngeal masks in airway management. Expert Rev. Med. Devices 2022, 19, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.-L.; Chen, Y.; Sun, P.; Qv, Z.-Y.; Zuo, M.-Z. Preliminary evaluation of SaCoVLM™ video laryngeal mask airway in airway management for general anesthesia. BMC Anesthesiol. 2022, 22, 3. [Google Scholar] [CrossRef]
- Zhi, J.; Deng, X.-M.; Zhang, Y.-M.; Wei, L.-X.; Wang, Q.-Y.; Yang, D. Preliminary evaluation of SaCoVLM video laryngeal mask-guided intubation in airway management for anesthetized children. BMC Anesthesiol. 2023, 23, 49. [Google Scholar] [CrossRef]
- Gaszynski, T. New vision-incorporated third-generation video laryngeal mask airways for intubation of patients in prone position. J. Clin. Monit Comput. 2023, 37, 705–707. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, L.; Xu, L.; Zhang, M.; Guo, Y.; Wang, Y. The Application of a SaCoVLMTM Visual Intubation Laryngeal Mask for the Management of Difficult Airways in Morbidly Obese Patients: Case Report. Front. Med. 2021, 8, 763103. [Google Scholar] [CrossRef]


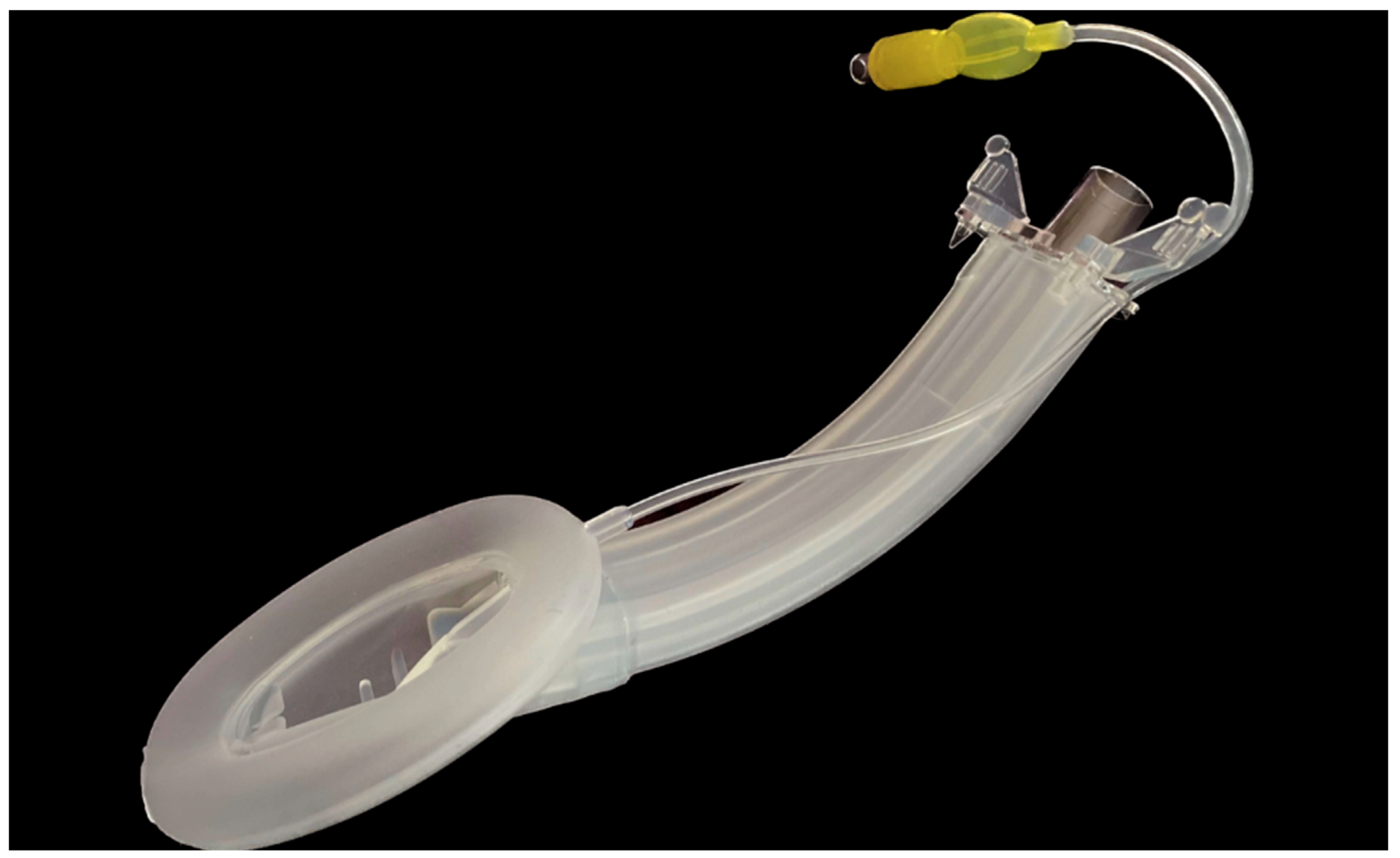
| Size | Patient Weight | MAX Inflation Volume | Identifying Colour |
|---|---|---|---|
| 3 | 30–70 kg | 20 mL | Green |
| 4 | 70–90 kg | 30 mL | Yellow |
| 5 | Coming soon | 40 mL | Pink |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Monzón, C.G.; Gaszyński, T.; Marroquín-Valz, H.A.; Orozco-Montes, J.; Ratajczyk, P. Supraglottic Airway Devices with Vision Guided Systems: Third Generation of Supraglottic Airway Devices. J. Clin. Med. 2023, 12, 5197. https://doi.org/10.3390/jcm12165197
Castillo-Monzón CG, Gaszyński T, Marroquín-Valz HA, Orozco-Montes J, Ratajczyk P. Supraglottic Airway Devices with Vision Guided Systems: Third Generation of Supraglottic Airway Devices. Journal of Clinical Medicine. 2023; 12(16):5197. https://doi.org/10.3390/jcm12165197
Chicago/Turabian StyleCastillo-Monzón, Caridad G., Tomasz Gaszyński, Hugo A. Marroquín-Valz, Javier Orozco-Montes, and Pawel Ratajczyk. 2023. "Supraglottic Airway Devices with Vision Guided Systems: Third Generation of Supraglottic Airway Devices" Journal of Clinical Medicine 12, no. 16: 5197. https://doi.org/10.3390/jcm12165197
APA StyleCastillo-Monzón, C. G., Gaszyński, T., Marroquín-Valz, H. A., Orozco-Montes, J., & Ratajczyk, P. (2023). Supraglottic Airway Devices with Vision Guided Systems: Third Generation of Supraglottic Airway Devices. Journal of Clinical Medicine, 12(16), 5197. https://doi.org/10.3390/jcm12165197






