Safety of Citrate Anticoagulation in CKRT: Monocentric Experience of a Dynamic Protocol of Calcium Monitoring
Abstract
1. Introduction
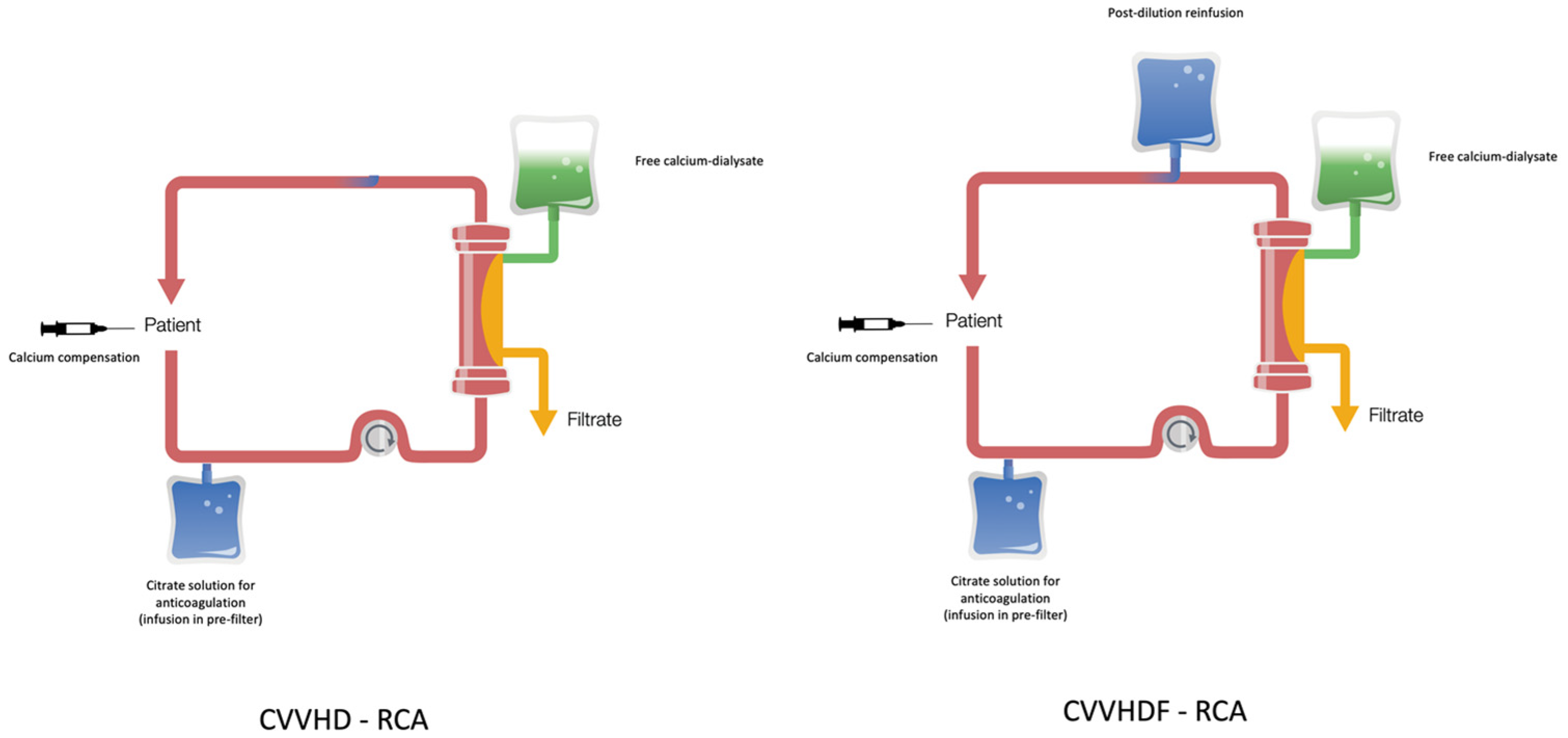
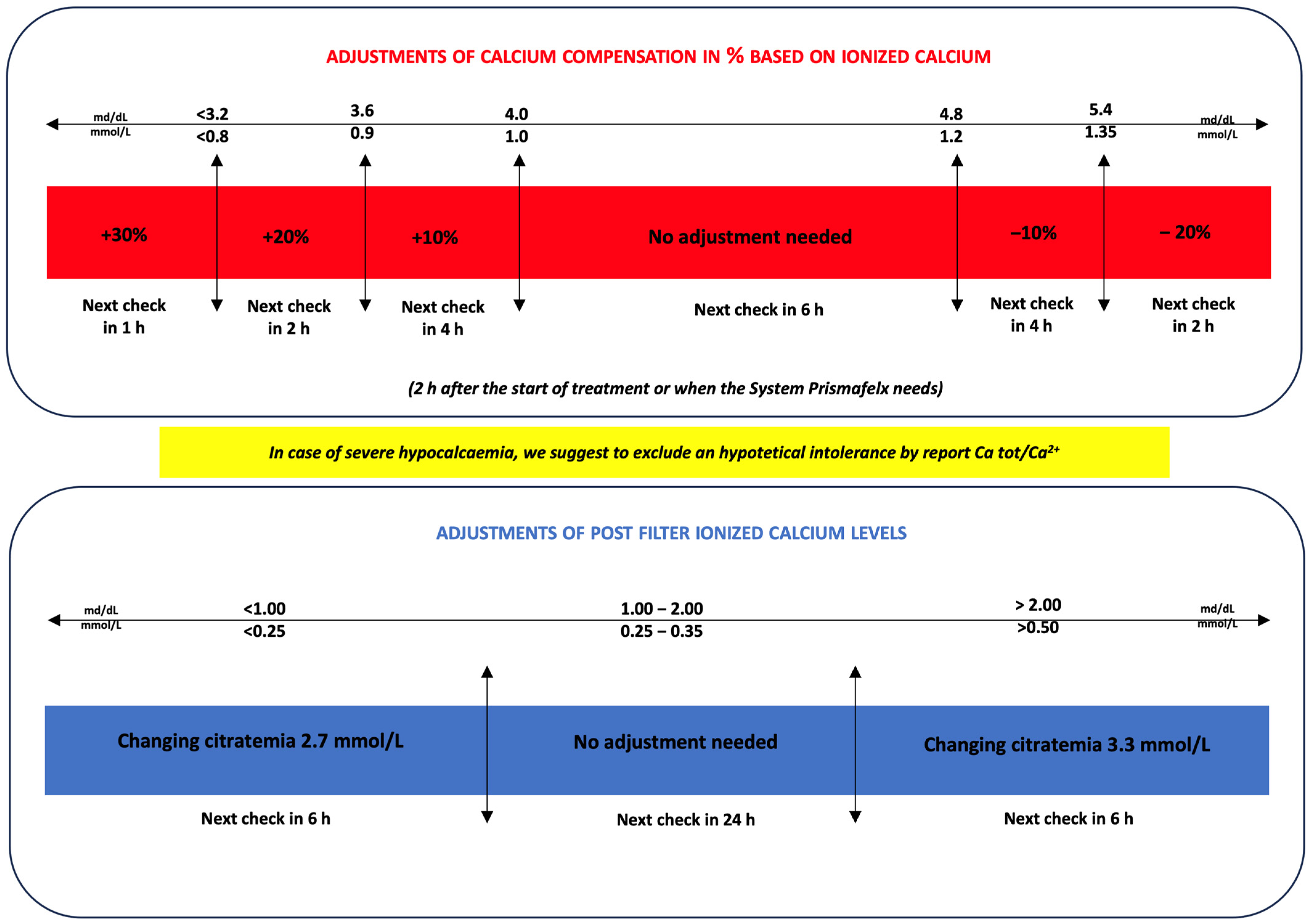
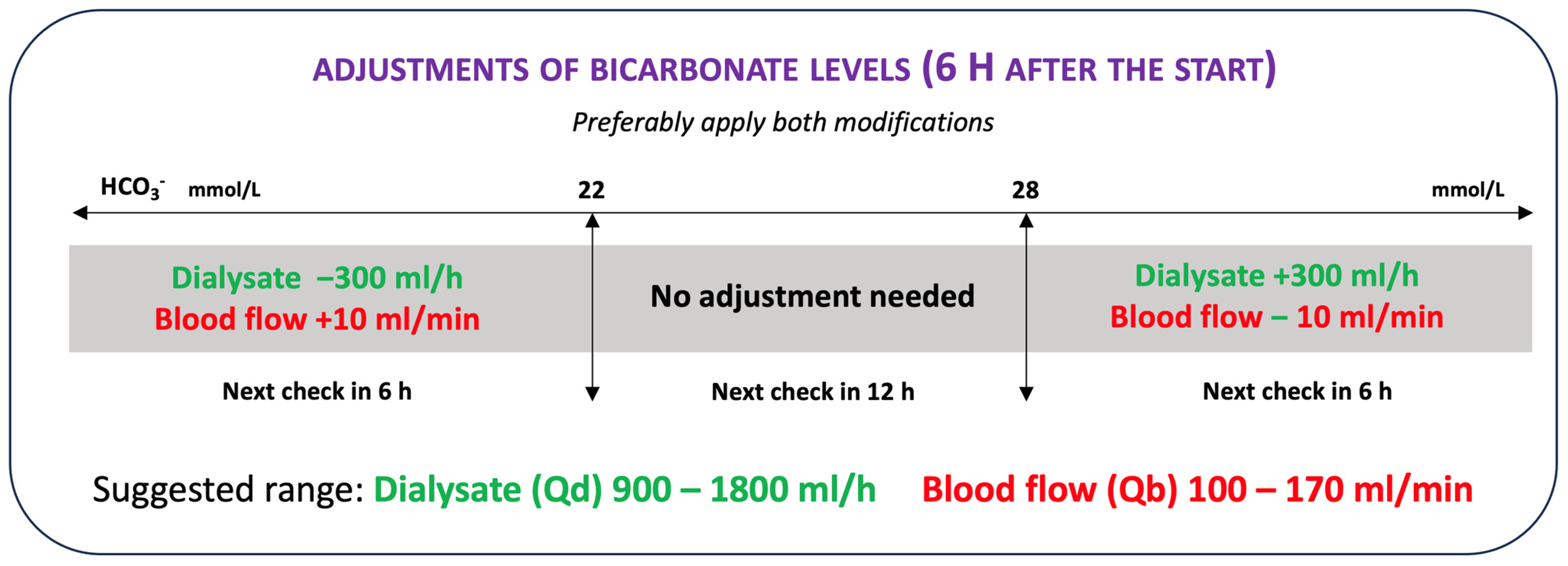
2. Materials and Methods
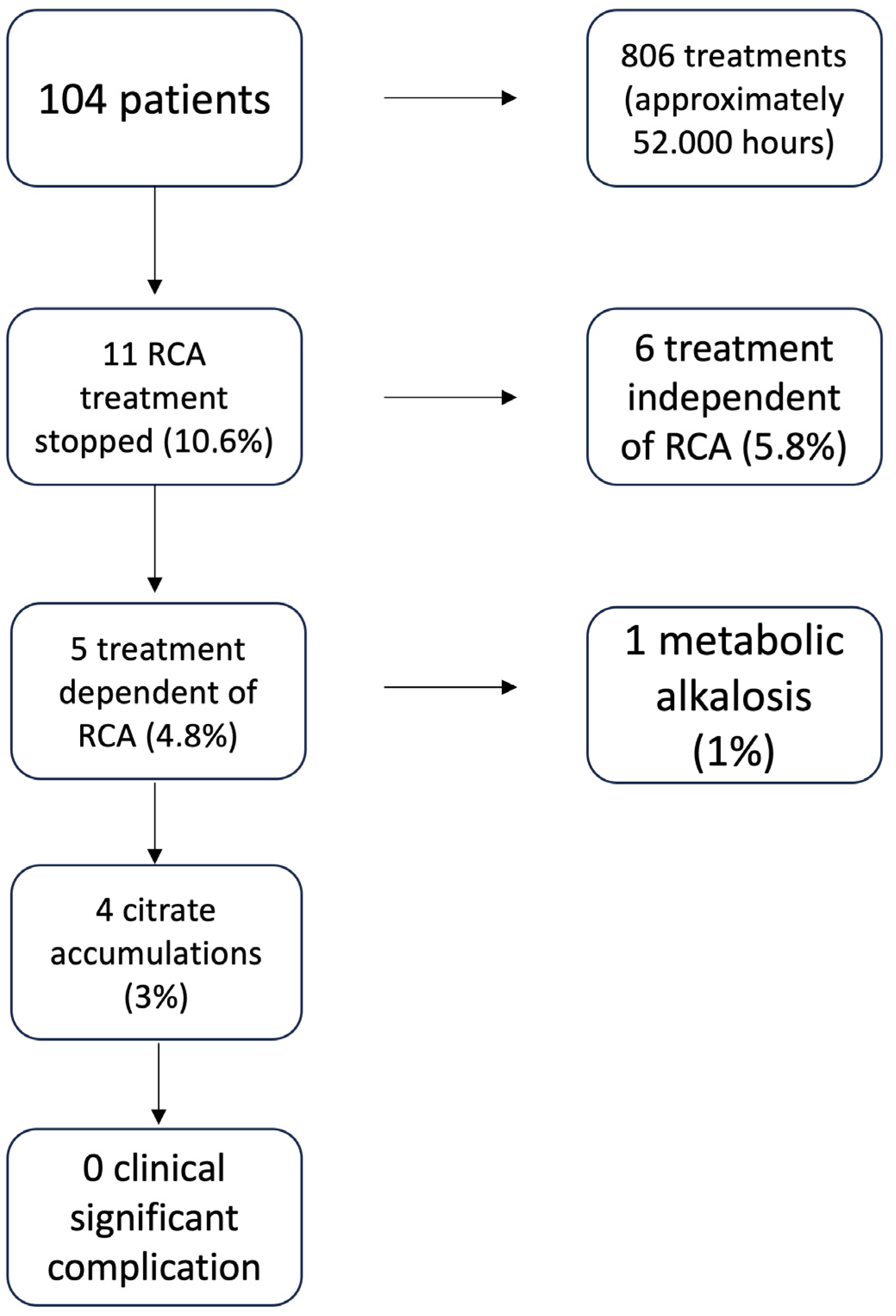
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uchino, S.; Bellomo, R.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; Gibney, N.; Tolwani, A.; et al. Continuous renal replacement therapy: A worldwide practice survey. The beginning and ending supportive therapy for the kidney (B.E.S.T. kidney) investigators. Intensive Care Med. 2007, 33, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Ricci, D.; Panicali, L.; Facchini, M.G.; Mancini, E. Citrate Anticoagulation during Continuous Renal Replacement Therapy. Contrib. Nephrol. 2017, 190, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Joannidis, M.; Oudemans-van Straaten, H.M. Clinical review: Patency of the circuit in continuous renal replacement therapy. Crit. Care 2007, 11, 218. [Google Scholar] [CrossRef]
- Zarbock, A.; Küllmar, M.; Kindgen-Milles, D.; Wempe, C.; Gerss, J.; Brandenburger, T.; Dimski, T.; Tyczynski, B.; Jahn, M.; Mülling, N.; et al. Effect of Regional Citrate Anticoagulation vs Systemic Heparin Anticoagulation During Continuous Kidney Replacement Therapy on Dialysis Filter Life Span and Mortality Among Critically Ill Patients With Acute Kidney Injury: A Randomized Clinical Trial. JAMA 2020, 324, 1629–1639. [Google Scholar] [CrossRef]
- Stucker, F.; Ponte, B.; Tataw, J.; Martin, P.Y.; Wozniak, H.; Pugin, J.; Saudan, P. Efficacy and safety of citrate-based anticoagulation compared to heparin in patients with acute kidney injury requiring continuous renal replacement therapy: A randomized controlled trial. Crit. Care 2015, 19, 91. [Google Scholar] [CrossRef]
- Gattas, D.J.; Rajbhandari, D.; Bradford, C.; Buhr, H.; Lo, S.; Bellomo, R. A Randomized Controlled Trial of Regional Citrate Versus Regional Heparin Anticoagulation for Continuous Renal Replacement Therapy in Critically Ill Adults. Crit. Care Med. 2015, 43, 1622–1629. [Google Scholar] [CrossRef]
- Schilder, L.; Nurmohamed, S.A.; Bosch, F.H.; Purmer, I.M.; den Boer, S.S.; Kleppe, C.G.; Vervloet, M.G.; Beishuizen, A.; Girbes, A.R.; Ter Wee, P.M.; et al. Citrate anticoagulation versus systemic heparinisation in continuous venovenous hemofiltration in critically ill patients with acute kidney injury: A multi-center randomized clinical trial. Crit. Care 2014, 18, 472. [Google Scholar] [CrossRef]
- Zhang, Z.; Hongying, N. Efficacy and safety of regional citrate anticoagulation in critically ill patients undergoing continuous renal replacement therapy. Intensive Care Med. 2012, 38, 20–28. [Google Scholar] [CrossRef]
- Bai, M.; Zhou, M.; He, L.; Ma, F.; Li, Y.; Yu, Y.; Wang, P.; Li, L.; Jing, R.; Zhao, L.; et al. Citrate versus heparin anticoagulation for continuous renal replacement therapy: An updated meta-analysis of RCTs. Intensive Care Med. 2015, 41, 2098–2110. [Google Scholar] [CrossRef]
- Liu, C.; Mao, Z.; Kang, H.; Hu, J.; Zhou, F. Regional citrate versus heparin anticoagulation for continuous renal replacement therapy in critically ill patients: A meta-analysis with trial sequential analysis of randomized controlled trials. Crit. Care 2016, 20, 144. [Google Scholar] [CrossRef]
- Kośka, A.; Kirwan, C.J.; Kowalik, M.M.; Lango-Maziarz, A.; Szymanowicz, W.; Jagielak, D.; Lango, R. Filter life span in postoperative cardiovascular surgery patients requiring continuous renal replacement therapy, using a postdilution regional citrate anticoagulation continuous hemofiltration circuit. Cardiol. J. 2022, 29, 53–61. [Google Scholar] [CrossRef]
- Honore, P.M.; De Bels, D.; Preseau, T.; Redant, S.; Spapen, H.D. Citrate: How to Get Started and What, When, and How to Monitor? J. Transl. Int. Med. 2018, 6, 115–127. [Google Scholar] [CrossRef]
- James, M.F.; Roche, A.M. Dose-response relationship between plasma ionized calcium concentration and thrombelastography. J. Cardiothorac. Vasc. Anesth. 2004, 18, 581–586. [Google Scholar] [CrossRef]
- Calatzis, A.; Toepfer, M.; Schramm, W.; Spannagl, M.; Schiffl, H. Citrate anticoagulation for extracorporeal circuits: Effects on whole blood coagulation activation and clot formation. Nephron 2001, 89, 233–236. [Google Scholar] [CrossRef]
- Meier-Kriesche, H.U.; Gitomer, J.; Finkel, K.; DuBose, T. Increased total to ionized calcium ratio during continuous venovenous hemodialysis with regional citrate anticoagulation. Crit. Care Med. 2001, 29, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Nalesso, F.; Garzotto, F.; Rossi, B.; Cattarin, L.; Simioni, F.; Carretta, G.; Brunori, G.; Gambaro, G.; Calo, L.A. Proactive approach for the patient safety in the extracorporeal blood purification treatments in nephrology. G. Ital. Di Nefrol. 2018, 35, 42–49. [Google Scholar]
- Anstey, C.M.; Russell, F.D. Measurement of the Concentration of Citrate in Human Biofluids in Patients Undergoing Continuous Renal Replacement Therapy Using Regional Citrate Anticoagulation: Application of a Two-Step Enzymatic Assay. Blood Purif. 2021, 50, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Anstey, C.M.; Venkatesh, B. A Comparison of the Commonly Used Surrogate Markers for Citrate Accumulation and Toxicity during Continuous Renal Replacement Therapy with Regional Citrate Anticoagulation. Blood Purif. 2022, 51, 997–1005. [Google Scholar] [CrossRef]
- Link, A.; Klingele, M.; Speer, T.; Rbah, R.; Pöss, J.; Lerner-Gräber, A.; Fliser, D.; Böhm, M. Total-to-ionized calcium ratio predicts mortality in continuous renal replacement therapy with citrate anticoagulation in critically ill patients. Crit. Care 2012, 16, R97. [Google Scholar] [CrossRef]
- Karkar, A.; Ronco, C. Prescription of CRRT: A pathway to optimize therapy. Ann. Intensive Care 2020, 10, 32. [Google Scholar] [CrossRef]
- Schultheiß, C.; Saugel, B.; Phillip, V.; Thies, P.; Noe, S.; Mayr, U.; Haller, B.; Einwächter, H.; Schmid, R.M.; Huber, W. Continuous venovenous hemodialysis with regional citrate anticoagulation in patients with liver failure: A prospective observational study. Crit. Care 2012, 16, R162. [Google Scholar] [CrossRef]
- Slowinski, T.; Morgera, S.; Joannidis, M.; Henneberg, T.; Stocker, R.; Helset, E.; Andersson, K.; Wehner, M.; Kozik-Jaromin, J.; Brett, S.; et al. Safety and efficacy of regional citrate anticoagulation in continuous venovenous hemodialysis in the presence of liver failure: The Liver Citrate Anticoagulation Threshold (L-CAT) observational study. Crit. Care 2015, 19, 349. [Google Scholar] [CrossRef]
- De Vico, P.; Messino, V.; Tartaglione, A.; Beccaris, C.; Buonomo, C.; Talarico, D.; Prati, P.; Sabato, A.F.; Colella, D.F. Safety and efficacy of citrate anti-coagulation continuous renal replacement therapies in post-cardiac surgery patients with liver dysfunction. Ther. Apher. Dial. 2015, 19, 272–278. [Google Scholar] [CrossRef]
- Oudemans-van Straaten, H.M.; Kellum, J.A.; Bellomo, R. Clinical review: Anticoagulation for continuous renal replacement therapy--heparin or citrate? Crit. Care 2011, 15, 202. [Google Scholar] [CrossRef]
- Szamosfalvi, B.; Puri, V.; Sohaney, R.; Wagner, B.; Riddle, A.; Dickinson, S.; Napolitano, L.; Heung, M.; Humes, D.; Yessayan, L. Regional Citrate Anticoagulation Protocol for Patients with Presumed Absent Citrate Metabolism. Kidney360 2021, 2, 192–204. [Google Scholar] [CrossRef]
- Morabito, S.; Pistolesi, V.; Tritapepe, L.; Vitaliano, E.; Zeppilli, L.; Polistena, F.; Fiaccadori, E.; Pierucci, A. Continuous venovenous hemodiafiltration with a low citrate dose regional anticoagulation protocol and a phosphate-containing solution: Effects on acid-base status and phosphate supplementation needs. BMC Nephrol. 2013, 14, 232. [Google Scholar] [CrossRef]
- Rhee, H.; Berenger, B.; Mehta, R.L.; Macedo, E. Regional Citrate Anticoagulation for Continuous Kidney Replacement Therapy With Calcium-Containing Solutions: A Cohort Study. Am. J. Kidney Dis. 2021, 78, 550–559. [Google Scholar] [CrossRef]
- Kindgen-Milles, D.; Brandenburger, T.; Dimski, T. Regional citrate anticoagulation for continuous renal replacement therapy. Curr. Opin. Crit. Care 2018, 24, 450–454. [Google Scholar] [CrossRef]
- Nalesso, F.; Cattarin, L.; Gobbi, L.; Fragasso, A.; Garzotto, F.; Calo, L.A. Evaluating Nephrocheck((R)) as a Predictive Tool for Acute Kidney Injury. Int. J. Nephrol. Renov. Dis. 2020, 13, 85–96. [Google Scholar] [CrossRef]
- Di Leo, L.; Nalesso, F.; Garzotto, F.; Xie, Y.; Yang, B.; Virzì, G.M.; Passannante, A.; Bonato, R.; Carta, M.; Giavarina, D.; et al. Predicting Acute Kidney Injury in Intensive Care Unit Patients: The Role of Tissue Inhibitor of Metalloproteinases-2 and Insulin-Like Growth Factor-Binding Protein-7 Biomarkers. Blood Purif. 2018, 45, 270–277. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Uchino, S.; Bellomo, R.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; Gibney, N.; et al. Timing of renal replacement therapy and clinical outcomes in critically ill patients with severe acute kidney injury. J. Crit. Care 2009, 24, 129–140. [Google Scholar] [CrossRef]
- Alexander, M.P.; Mangalaparthi, K.K.; Madugundu, A.K.; Moyer, A.M.; Adam, B.A.; Mengel, M.; Singh, S.; Herrmann, S.M.; Rule, A.D.; Cheek, E.H.; et al. Acute Kidney Injury in Severe COVID-19 Has Similarities to Sepsis-Associated Kidney Injury: A Multi-Omics Study. Mayo Clin. Proc. 2021, 96, 2561–2575. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; on behalf of the KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit. Care 2013, 17, 204. [Google Scholar] [CrossRef]
- Neri, M.; Villa, G.; Garzotto, F.; Bagshaw, S.; Bellomo, R.; Cerda, J.; Ferrari, F.; Guggia, S.; Joannidis, M.; Kellum, J.; et al. Nomenclature for renal replacement therapy in acute kidney injury: Basic principles. Crit. Care 2016, 20, 318. [Google Scholar] [CrossRef]
- Schneider, A.G.; Journois, D.; Rimmelé, T. Complications of regional citrate anticoagulation: Accumulation or overload? Crit. Care 2017, 21, 281. [Google Scholar] [CrossRef]
- Pistolesi, V.; Morabito, S.; Pota, V.; Valente, F.; Di Mario, F.; Fiaccadori, E.; Grasselli, G.; Brienza, N.; Cantaluppi, V.; De Rosa, S.; et al. Regional citrate anticoagulation (RCA) in critically ill patients undergoing renal replacement therapy (RRT): Expert opinion from the SIAARTI-SIN joint commission. J. Anesth. Analg. Crit. Care 2023, 3, 7. [Google Scholar] [CrossRef]
- Hoste, E.A.; De Corte, W. Implementing the Kidney Disease: Improving Global Outcomes/acute kidney injury guidelines in ICU patients. Curr. Opin. Crit. Care 2013, 19, 544–553. [Google Scholar] [CrossRef]
- Guo, L.; Hu, Y.; Zeng, Q.; Yang, X. Factors affecting continuous renal replacement therapy duration in critically ill patients: A retrospective study. Ther. Apher. Dial. 2023. [Google Scholar] [CrossRef]
- Morabito, S.; Pistolesi, V.; Tritapepe, L.; Fiaccadori, E. Regional citrate anticoagulation for RRTs in critically ill patients with AKI. Clin. J. Am. Soc. Nephrol. 2014, 9, 2173–2188. [Google Scholar] [CrossRef]
- Assefi, M.; Leurent, A.; Blanchard, F.; Quemeneur, C.; Deransy, R.; Monsel, A.; Constantin, J.M. Impact of increasing post-filter ionized calcium target on filter lifespan in renal replacement therapy with regional citrate anticoagulation: A before-and-after study. J. Crit. Care 2023, 78, 154364. [Google Scholar] [CrossRef]
| Time from the Beginning of the CVVHDF-RCA | Total Calcium in the Patient | Ionized Calcium in the Patient | Ionized Calcium Post-Filter | CaTot/Ca2+ |
|---|---|---|---|---|
| 0 | X | X | ||
| 30′ | X | X | ||
| 3 h | X | X | ||
| 6 h | X | X | X | |
| Every 6 h | X | X | ||
| Every 24 h | X | X | ||
| (*) Every 12 h | X | X |
| 104 Patients 80 Males (77.7%) 24 Females (22.3%) | Mean | Standard Deviation | Minimun | Maximum |
|---|---|---|---|---|
| Age (years) | 67.0 | 11.3 | 19 | 87 |
| Weight (Kg) | 82.0 | 19.6 | 30 | 130 |
| Urea (mmol/L) | 26.53 | 13.29 | 4.80 | 59.00 |
| Creatinine (µmol/L) | 321.0 | 205.2 | 43.0 | 1191.0 |
| Potassium (mmol/L) | 4.44 | 0.73 | 3.00 | 7.20 |
| Qb (mL/min) | 123.6 | 80 | 80 | 200 |
| PBP (mL/h) | 1203.6 | 212.7 | 800 | 1800 |
| Qd (mL/h) | 1190.6 | 223.7 | 800 | 2000 |
| Qr (mL/h) | 597.1 | 235.0 | 100 | 1600 |
| Effluent dose (mL/Kg/h) | 37.9 | 9.3 | 16.9 | 74.3 |
| Albumin (g/L) | 26.5 | 6.1 | 21 | 50 |
| Mg (mmol/L) | 0.89 | 0.129 | 0.67 | 1.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nalesso, F.; Bettin, E.; Bogo, M.; Cacciapuoti, M.; Cattarin, L.; Scaparrotta, G.; Calò, L.A. Safety of Citrate Anticoagulation in CKRT: Monocentric Experience of a Dynamic Protocol of Calcium Monitoring. J. Clin. Med. 2023, 12, 5201. https://doi.org/10.3390/jcm12165201
Nalesso F, Bettin E, Bogo M, Cacciapuoti M, Cattarin L, Scaparrotta G, Calò LA. Safety of Citrate Anticoagulation in CKRT: Monocentric Experience of a Dynamic Protocol of Calcium Monitoring. Journal of Clinical Medicine. 2023; 12(16):5201. https://doi.org/10.3390/jcm12165201
Chicago/Turabian StyleNalesso, Federico, Elisabetta Bettin, Marco Bogo, Martina Cacciapuoti, Leda Cattarin, Giuseppe Scaparrotta, and Lorenzo A. Calò. 2023. "Safety of Citrate Anticoagulation in CKRT: Monocentric Experience of a Dynamic Protocol of Calcium Monitoring" Journal of Clinical Medicine 12, no. 16: 5201. https://doi.org/10.3390/jcm12165201
APA StyleNalesso, F., Bettin, E., Bogo, M., Cacciapuoti, M., Cattarin, L., Scaparrotta, G., & Calò, L. A. (2023). Safety of Citrate Anticoagulation in CKRT: Monocentric Experience of a Dynamic Protocol of Calcium Monitoring. Journal of Clinical Medicine, 12(16), 5201. https://doi.org/10.3390/jcm12165201






