The Evolving Role of Surgical Aortic Valve Replacement in the Era of Transcatheter Valvular Procedures
Abstract
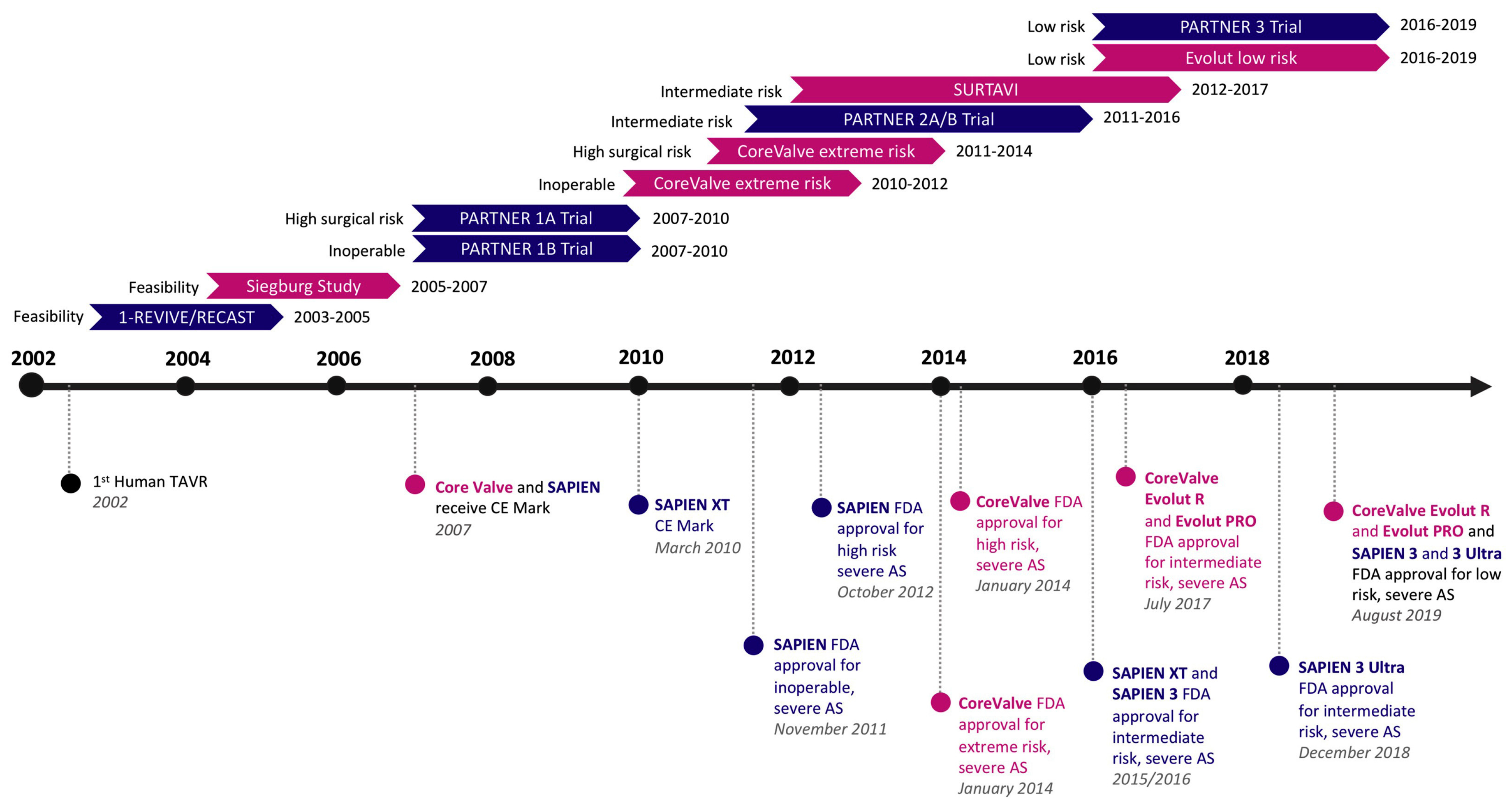
:1. Introduction
2. Young Low-Risk Patients
2.1. Prosthesis Durability
2.2. Long-Term Complications
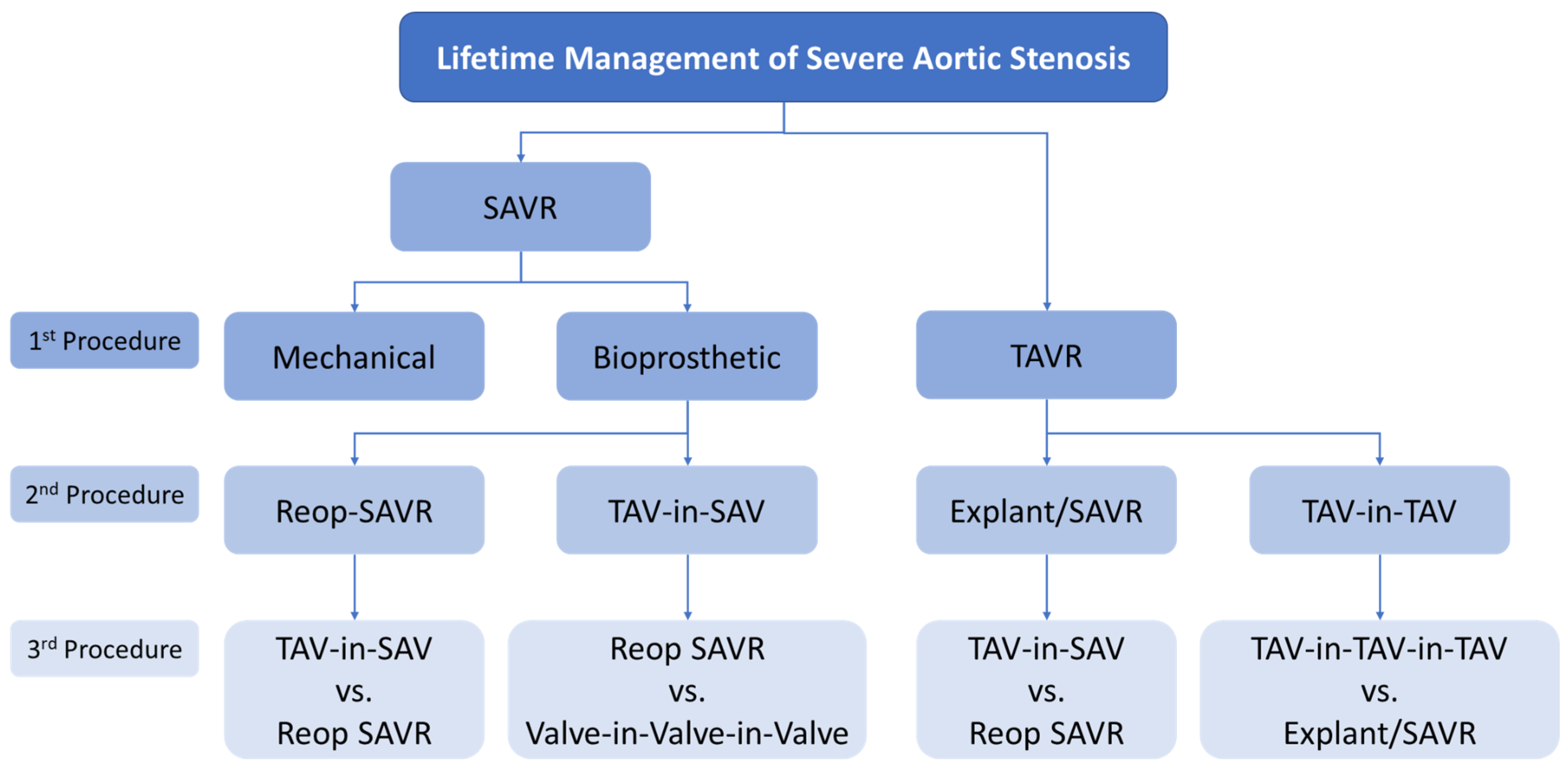
2.3. Lifetime Management
3. Anatomical Challenges
3.1. Bicuspid Aortic Valve
3.2. Annular and LVOT Calcification
3.3. Low Coronary Height
3.4. Isolated Aortic Regurgitation
3.5. Small Aortic Annulus
4. Need for Concomitant Procedures
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otto, C.M.; Kumbhani, D.J.; Alexander, K.P.; Calhoon, J.H.; Desai, M.Y.; Kaul, S.; Lee, J.C.; Ruiz, C.E.; Vassileva, C.M. 2017 ACC Expert Consensus Decision Pathway for Transcatheter Aortic Valve Replacement in the Management of Adults with Aortic Stenosis. J. Am. Coll. Cardiol. 2017, 69, 1313–1346. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary. Circulation 2014, 129, 2440–2492. [Google Scholar] [CrossRef]
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B.; et al. Percutaneous Transcatheter Implantation of an Aortic Valve Prosthesis for Calcific Aortic Stenosis. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J., Jr.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Prosthesis. N. Engl. J. Med. 2014, 370, 1790–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mack, M.J.; Leon, M.B.; Smith, C.R.; Miller, D.C.; Moses, J.W.; Tuzcu, E.M.; Webb, J.G.; Douglas, P.S.; Anderson, W.N.; Blackstone, E.H.; et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2477–2484. [Google Scholar] [CrossRef]
- Makkar, R.R.; Thourani, V.H.; Mack, M.J.; Kodali, S.K.; Kapadia, S.; Webb, J.G.; Yoon, S.-H.; Trento, A.; Svensson, L.G.; Herrmann, H.C.; et al. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N. Engl. J. Med. 2020, 382, 799–809. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Thourani, V.H.; Suri, R.M.; Gunter, R.L.; Sheng, S.; O’Brien, S.M.; Ailawadi, G.; Szeto, W.Y.; Dewey, T.M.; Guyton, R.A.; Bavaria, J.E.; et al. Contemporary Real-World Outcomes of Surgical Aortic Valve Replacement in 141,905 Low-Risk, Intermediate-Risk, and High-Risk Patients. Ann. Thorac. Surg. 2015, 99, 55–61. [Google Scholar] [CrossRef]
- Thyregod, H.G.H.; Steinbrüchel, D.A.; Ihlemann, N.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients with Severe Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2015, 65, 2184–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Sundt, T.M.; Jneid, H. Guideline Update on Indications for Transcatheter Aortic Valve Implantation Based on the 2020 American College of Cardiology/American Heart Association Guidelines for Management of Valvular Heart Disease. JAMA Cardiol. 2021, 6, 1088. [Google Scholar] [CrossRef] [PubMed]
- Percy, E.D.; Hirji, S.A.; Yazdchi, F.; Pelletier, M.P. The Sky Is Not Falling: Surgical Perspectives on a New Transcatheter Paradigm. Can. J. Cardiol. 2021, 37, 22–26. [Google Scholar] [CrossRef]
- Kapadia, S.R.; Leon, M.B.; Makkar, R.R.; Tuzcu, E.M.; Svensson, L.G.; Kodali, S.; Webb, J.G.; Mack, M.J.; Douglas, P.S.; Thourani, V.H.; et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.K.; Deeb, G.M.; Yakubov, S.J.; Gada, H.; Mumtaz, M.A.; Ramlawi, B.; Bajwa, T.; Teirstein, P.S.; DeFrain, M.; Muppala, M.; et al. 3-Year Outcomes after Transcatheter or Surgical Aortic Valve Replacement in Low-Risk Patients with Aortic Stenosis. J. Am. Coll. Cardiol. 2023, 81, 1663–1674. [Google Scholar] [CrossRef]
- Leon, M.B.; Mack, M.J.; Hahn, R.T.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Alu, M.C.; Madhavan, M.V.; Chau, K.H.; Russo, M.; et al. Outcomes 2 Years after Transcatheter Aortic Valve Replacement in Patients at Low Surgical Risk. J. Am. Coll. Cardiol. 2021, 77, 1149–1161. [Google Scholar] [CrossRef]
- Johnston, D.R.; Mahboubi, R.; Soltesz, E.G.; Artis, A.S.; Roselli, E.E.; Blackstone, E.H.; Svensson, L.G.; Kakavand, M.; Gillinov, A.M.; Kapadia, S.; et al. Redefining “low risk”: Outcomes of surgical aortic valve replacement in low-risk patients in the transcatheter aortic valve replacement era. J. Thorac. Cardiovasc. Surg. 2022, 165, 591–604.e3. [Google Scholar] [CrossRef]
- Aboud, A.; Charitos, E.I.; Fujita, B.; Stierle, U.; Reil, J.-C.; Voth, V.; Liebrich, M.; Andreas, M.; Holubec, T.; Bening, C.; et al. Long-Term Outcomes of Patients Undergoing the Ross Procedure. J. Am. Coll. Cardiol. 2021, 77, 1412–1422. [Google Scholar] [CrossRef]
- David, T.E.; Ouzounian, M.; David, C.M.; Lafreniere-Roula, M.; Manlhiot, C. Late results of the Ross procedure. J. Thorac. Cardiovasc. Surg. 2018, 157, 201–208. [Google Scholar] [CrossRef] [PubMed]
- El-Hamamsy, I.; Toyoda, N.; Itagaki, S.; Stelzer, P.; Varghese, R.; Williams, E.E.; Egorova, N.; Adams, D.H. Propensity-Matched Comparison of the Ross Procedure and Prosthetic Aortic Valve Replacement in Adults. J. Am. Coll. Cardiol. 2022, 79, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Kataruka, A.; Otto, C.M. Valve durability after transcatheter aortic valve implantation. J. Thorac. Dis. 2018, 10 (Suppl. S30), S3629–S3636. [Google Scholar] [CrossRef]
- Dvir, D.; Bourguignon, T.; Otto, C.M.; Hahn, R.T.; Rosenhek, R.; Webb, J.G.; Treede, H.; Sarano, M.E.; Feldman, T.; Wijeysundera, H.; et al. Standardized Definition of Structural Valve Degeneration for Surgical and Transcatheter Bioprosthetic Aortic Valves. Circulation 2018, 137, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Capodanno, D.; Petronio, A.S.; Prendergast, B.; Eltchaninoff, H.; Vahanian, A.; Modine, T.; Lancellotti, P.; Sondergaard, L.; Ludman, P.F.; Tamburino, C.; et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: A consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2017, 38, 3382–3390. [Google Scholar] [CrossRef] [Green Version]
- VARC-3 WRITING COMMITTEE; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef]
- Rahimtoola, S.H. Choice of Prosthetic Heart Valve in Adults: An Update. J. Am. Coll. Cardiol. 2010, 55, 2413–2426. [Google Scholar] [CrossRef] [Green Version]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef]
- Goldstone, A.B.; Chiu, P.; Baiocchi, M.; Lingala, B.; Patrick, W.L.; Fischbein, M.P.; Woo, Y.J. Mechanical or Biologic Prostheses for Aortic-Valve and Mitral-Valve Replacement. N. Engl. J. Med. 2017, 377, 1847–1857. [Google Scholar] [CrossRef]
- O’Hair, D.; Yakubov, S.J.; Grubb, K.J.; Oh, J.K.; Ito, S.; Deeb, G.M.; Van Mieghem, N.M.; Adams, D.H.; Bajwa, T.; Kleiman, N.S.; et al. Structural Valve Deterioration after Self-Expanding Transcatheter or Surgical Aortic Valve Implantation in Patients at Intermediate or High Risk. JAMA Cardiol. 2023, 8, 111. [Google Scholar] [CrossRef]
- Rodés-Cabau, J.; Ellenbogen, K.A.; Krahn, A.D.; Latib, A.; Mack, M.; Mittal, S.; Muntané-Carol, G.; Nazif, T.M.; Sondergaard, L.; Urena, M.; et al. Management of Conduction Disturbances Associated with Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2019, 74, 1086–1106. [Google Scholar] [CrossRef] [PubMed]
- Siontis, G.C.M.; Overtchouk, P.; Cahill, T.J.; Modine, T.; Prendergast, B.; Praz, F.; Pilgrim, T.; Petrinic, T.; Nikolakopoulou, A.; Salanti, G.; et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: An updated meta-analysis. Eur. Heart J. 2019, 40, 3143–3153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazif, T.M.; Dizon, J.M.; Hahn, R.T.; Xu, K.; Babaliaros, V.; Douglas, P.S.; El-Chami, M.F.; Herrmann, H.C.; Mack, M.; Makkar, R.R.; et al. Predictors and Clinical Outcomes of Permanent Pacemaker Implantation after Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2015, 8 Pt A, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Aljabbary, T.; Qiu, F.; Masih, S.; Fang, J.; Elbaz-Greener, G.; Austin, P.C.; Rodés-Cabau, J.; Ko, D.; Singh, S.; Wijeysundera, H.C. Association of Clinical and Economic Outcomes with Permanent Pacemaker Implantation after Transcatheter Aortic Valve Replacement. JAMA Netw. Open 2018, 1, e180088. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, E.; Qian, Z.; Sun, J.; Zou, F.; Wang, Y.; Hou, X.; Zou, J. Mid- to Long-Term Clinical and Echocardiographic Effects of Post-procedural Permanent Pacemaker Implantation after Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 911234. [Google Scholar] [CrossRef]
- Okuno, T.; Tomii, D.; Heg, D.; Lanz, J.; Praz, F.; Stortecky, S.; Reineke, D.; Windecker, S.; Pilgrim, T. Five-year outcomes of mild paravalvular regurgitation after transcatheter aortic valve implantation. Eurointervention 2022, 18, 33–42. [Google Scholar] [CrossRef]
- Ternacle, J.; Pibarot, P.; Herrmann, H.C.; Kodali, S.; Leipsic, J.; Blanke, P.; Jaber, W.; Mack, M.J.; Clavel, M.-A.; Salaun, E.; et al. Prosthesis-Patient Mismatch after Aortic Valve Replacement in the PARTNER 2 Trial and Registry. JACC Cardiovasc. Interv. 2021, 14, 1466–1477. [Google Scholar] [CrossRef]
- Wang, N.; Tsai, Y.-C.; Niles, N.; Tchantchaleishvili, V.; Di Eusanio, M.; Yan, T.D.; Phan, K. Transcatheter aortic valve implantation (TAVI) versus sutureless aortic valve replacement (SUAVR) for aortic stenosis: A systematic review and meta-analysis of matched studies. J. Thorac. Dis. 2016, 8, 3283–3293. [Google Scholar] [CrossRef] [Green Version]
- Naji, P.; Griffin, B.P.; Sabik, J.F.; Kusunose, K.; Asfahan, F.; Popovic, Z.; Rodriguez, L.L.; Lytle, B.W.; Grimm, R.A.; Svensson, L.G.; et al. Characteristics and Outcomes of Patients with Severe Bioprosthetic Aortic Valve Stenosis Undergoing Redo Surgical Aortic Valve Replacement. Circulation 2015, 132, 1953–1960. [Google Scholar] [CrossRef]
- Stulak, J.M.; Tchantchaleishvili, V.; Daly, R.C.; Eleid, M.F.; Greason, K.L.; Dearani, J.A.; Joyce, L.D.; Pochettino, A.; Schaff, H.V.; Maltais, S. Conventional redo biological valve replacement over 20 years: Surgical benchmarks should guide patient selection for transcatheter valve-in-valve therapy. J. Thorac. Cardiovasc. Surg. 2018, 156, 1380–1390. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, L.D.F.C.; Urena, M.; Wijeysundera, H.C.; Muñoz-García, A.; Serra, V.; Benitez, L.M.; Auffret, V.; Cheema, A.N.; Amat-Santos, I.J.; Fisher, Q.; et al. Long-Term Outcomes after Transcatheter Aortic Valve-in-Valve Replacement. Circ. Cardiovasc. Interv. 2018, 11, e007038. [Google Scholar] [CrossRef] [PubMed]
- Raschpichler, M.; de Waha, S.; Holzhey, D.; Schwarzer, G.; Flint, N.; Kaewkes, D.; Bräuchle, P.T.; Dvir, D.; Makkar, R.; Ailawadi, G.; et al. Valve-in-Valve Transcatheter Aortic Valve Replacement Versus Redo Surgical Aortic Valve Replacement for Failed Surgical Aortic Bioprostheses: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2022, 11, e7965. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S.; Kim, K.M.; Yang, B.; Romano, M.; Ailawadi, G.; Patel, H.J.; Deeb, G.M. Reoperation following transcatheter aortic valve replacement: Insights from 10 years’ experience. J. Thorac. Cardiovasc. Surg. 2023. [Google Scholar] [CrossRef]
- Bapat, V.N.; Zaid, S.; Fukuhara, S.; Saha, S.; Vitanova, K.; Kiefer, P.; Squiers, J.J.; Voisine, P.; Pirelli, L.; von Ballmoos, M.W.; et al. Surgical Explantation after TAVR Failure. JACC Cardiovasc. Interv. 2021, 14, 1978–1991. [Google Scholar] [CrossRef]
- Gallo, M.; Fovino, L.N.; Blitzer, D.; Doulamis, I.P.; Guariento, A.; Salvador, L.; Tagliari, A.P.; Ferrari, E. Transcatheter aortic valve replacement for structural degeneration of previously implanted transcatheter valves (TAVR-in-TAVR): A systematic review. Eur. J. Cardio-Thoracic Surg. 2022, 61, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Okuno, T.; Demirel, C.; Tomii, D.; Heg, D.; Häner, J.; Siontis, G.C.; Lanz, J.; Räber, L.; Strotecky, S.; Fürholz, M.; et al. Long-term risk of unplanned percutaneous coronary intervention after transcatheter aortic valve replacement. Eurointervention 2022, 18, 797–803. [Google Scholar] [CrossRef]
- Faroux, L.; Lhermusier, T.; Vincent, F.; Nombela-Franco, L.; Tchétché, D.; Barbanti, M.; Abdel-Wahab, M.; Windecker, S.; Auffret, V.; Campanha-Borges, D.C.; et al. ST-Segment Elevation Myocardial Infarction Following Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2021, 77, 2187–2199. [Google Scholar] [CrossRef] [PubMed]
- De Backer, O.; Landes, U.; Fuchs, A.; Yoon, S.-H.; Mathiassen, O.N.; Sedaghat, A.; Kim, W.-K.; Pilgrim, T.; Buzzatti, N.; Ruile, P.; et al. Coronary Access after TAVR-in-TAVR as Evaluated by Multidetector Computed Tomography. JACC Cardiovasc. Interv. 2020, 13, 2528–2538. [Google Scholar] [CrossRef]
- Williams, M.R.; Jilaihawi, H.; Makkar, R.; O’Neill, W.W.; Guyton, R.; Malaisrie, S.C.; Brown, D.L.; Blanke, P.; Leipsic, J.A.; Pibarot, P.; et al. The PARTNER 3 Bicuspid Registry for Transcatheter Aortic Valve Replacement in Low-Surgical-Risk Patients. JACC Cardiovasc. Interv. 2022, 15, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.K.; Ramlawi, B.; Deeb, G.M.; Zahr, F.; Song, H.K.; Kleiman, N.S.; Chetcuti, S.J.; Michelena, H.I.; Mangi, A.A.; Skiles, J.A.; et al. Transcatheter Aortic Valve Replacement in Low-risk Patients with Bicuspid Aortic Valve Stenosis. JAMA Cardiol. 2021, 6, 50–57. [Google Scholar] [CrossRef]
- Kanjanahattakij, N.; Horn, B.; Vutthikraivit, W.; Biso, S.M.; Ziccardi, M.R.; Lu, M.L.R.; Rattanawong, P. Comparing outcomes after transcatheter aortic valve replacement in patients with stenotic bicuspid and tricuspid aortic valve: A systematic review and meta-analysis. Clin. Cardiol. 2018, 41, 896–902. [Google Scholar] [CrossRef] [Green Version]
- Roberts, W.C.; Ko, J.M. Frequency by Decades of Unicuspid, Bicuspid, and Tricuspid Aortic Valves in Adults Having Isolated Aortic Valve Replacement for Aortic Stenosis, with or without Associated Aortic Regurgitation. Circulation 2005, 111, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.K.; Delgado, V.; Bax, J.J. Bicuspid Aortic Valve. Circ. Cardiovasc. Imaging 2017, 10, e005987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scharfschwerdt, M.; Meyer-Saraei, R.; Schmidtke, C.; Sievers, H.-H. Hemodynamics of the Edwards Sapien XT transcatheter heart valve in noncircular aortic annuli. J. Thorac. Cardiovasc. Surg. 2014, 148, 126–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchetche, D.; de Biase, C.; van Gils, L.; Parma, R.; Ochala, A.; Lefevre, T.; Hovasse, T.; De Backer, O.; Sondergaard, L.; Bleiziffer, S.; et al. Bicuspid Aortic Valve Anatomy and Relationship with Devices: The BAVARD Multicenter Registry. Circ. Cardiovasc. Interv. 2019, 12, e007107. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Webb, J.G.; Leon, M.B.; Makkar, R. Transcatheter aortic valve replacement in bicuspid aortic valve stenosis. Prog. Cardiovasc. Dis. 2020, 63, 482–487. [Google Scholar] [CrossRef]
- Xiong, T.-Y.; Ben Ali, W.; Feng, Y.; Hayashida, K.; Jilaihawi, H.; Latib, A.; Lee, M.K.-Y.; Leon, M.B.; Makkar, R.R.; Modine, T.; et al. Transcatheter aortic valve implantation in patients with bicuspid valve morphology: A roadmap towards standardization. Nat. Rev. Cardiol. 2022, 20, 52–67. [Google Scholar] [CrossRef]
- Pasic, M.; Unbehaun, A.; Buz, S.; Drews, T.; Hetzer, R. Annular Rupture During Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2015, 8 Pt A, 1–9. [Google Scholar] [CrossRef] [Green Version]
- John, D.; Buellesfeld, L.; Yuecel, S.; Mueller, R.; Latsios, G.; Beucher, H.; Gerckens, U.; Grube, E. Correlation of Device Landing Zone Calcification and Acute Procedural Success in Patients Undergoing Transcatheter Aortic Valve Implantations with the Self-Expanding CoreValve Prosthesis. JACC Cardiovasc. Interv. 2010, 3, 233–243. [Google Scholar] [CrossRef] [Green Version]
- Langer, N.B.; Hamid, N.B.; Nazif, T.M.; Khalique, O.K.; Vahl, T.P.; White, J.; Terre, J.; Hastings, R.; Leung, D.; Hahn, R.T.; et al. Injuries to the Aorta, Aortic Annulus, and Left Ventricle During Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2017, 10, e004735. [Google Scholar] [CrossRef] [Green Version]
- Barbanti, M.; Yang, T.-H.; Rodès Cabau, J.; Tamburino, C.; Wood, D.A.; Jilaihawi, H.; Blanke, P.; Makkar, R.R.; Latib, A.; Colombo, A.; et al. Anatomical and Procedural Features Associated with Aortic Root Rupture During Balloon-Expandable Transcatheter Aortic Valve Replacement. Circulation 2013, 128, 244–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollari, F.; Dell’Aquila, A.M.; Söhn, C.; Marianowicz, J.; Wiehofsky, P.; Schwab, J.; Pauschinger, M.; Hitzl, W.; Fischlein, T.; Pfeiffer, S. Risk factors for paravalvular leak after transcatheter aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2019, 157, 1406–1415.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pibarot, P.; Hahn, R.T.; Weissman, N.J.; Arsenault, M.; Beaudoin, J.; Bernier, M.; Dahou, A.; Khalique, O.K.; Asch, F.M.; Toubal, O.; et al. Association of Paravalvular Regurgitation with 1-Year Outcomes after Transcatheter Aortic Valve Replacement with the SAPIEN 3 Valve. JAMA Cardiol. 2017, 2, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Thyregod, H.G.H.; Ihlemann, N.; Jørgensen, T.H.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Five-Year Clinical and Echocardiographic Outcomes From the NOTION Randomized Clinical Trial in Patients at Lower Surgical Risk. Circulation 2019, 139, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Seeger, J.; Gonska, B.; Otto, M.; Rottbauer, W.; Wöhrle, J. Cerebral Embolic Protection During Transcatheter Aortic Valve Replacement Significantly Reduces Death and Stroke Compared with Unprotected Procedures. JACC Cardiovasc. Interv. 2017, 10, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, S.R.; Kodali, S.; Makkar, R.; Mehran, R.; Lazar, R.M.; Zivadinov, R.; Dwyer, M.G.; Jilaihawi, H.; Virmani, R.; Anwaruddin, S.; et al. Protection Against Cerebral Embolism During Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2017, 69, 367–377. [Google Scholar] [CrossRef]
- Kapadia, S.R.; Makkar, R.; Leon, M.; Abdel-Wahab, M.; Waggoner, T.; Massberg, S.; Rottbauer, W.; Horr, S.; Sondergaard, L.; Karha, J.; et al. Cerebral Embolic Protection during Transcatheter Aortic-Valve Replacement. N. Engl. J. Med. 2022, 387, 1253–1263. [Google Scholar] [CrossRef]
- Nombela-Franco, L.; Webb, J.G.; De Jaegere, P.P.; Toggweiler, S.; Nuis, R.-J.; Dager, A.E.; Amat-Santos, I.J.; Cheung, A.; Ye, J.; Binder, R.K.; et al. Timing, Predictive Factors, and Prognostic Value of Cerebrovascular Events in a Large Cohort of Patients Undergoing Transcatheter Aortic Valve Implantation. Circulation 2012, 126, 3041–3053. [Google Scholar] [CrossRef] [PubMed]
- Nombela-Franco, L.; Rodés-Cabau, J.; DeLarochellière, R.; Larose, E.; Doyle, D.; Villeneuve, J.; Bergeron, S.; Bernier, M.; Amat-Santos, I.J.; Mok, M.; et al. Predictive Factors, Efficacy, and Safety of Balloon Post-Dilation after Transcatheter Aortic Valve Implantation with a Balloon-Expandable Valve. JACC Cardiovasc. Interv. 2012, 5, 499–512. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, H.B.; Nombela-Franco, L.; Urena, M.; Mok, M.; Pasian, S.; Doyle, D.; DeLarochellière, R.; Côté, M.; Laflamme, L.; DeLarochellière, H.; et al. Coronary Obstruction Following Transcatheter Aortic Valve Implantation. JACC Cardiovasc. Interv. 2013, 6, 452–461. [Google Scholar] [CrossRef] [Green Version]
- Giordana, F.; Bruno, F.; Conrotto, F.; Saglietto, A.; D’Ascenzo, F.; Marra, W.G.; Dvir, D.; Webb, J.; D’Onofrio, A.; Camboni, D.; et al. Incidence, predictors and outcomes of valve-in-valve TAVI: A systematic review and meta-analysis. Int. J. Cardiol. 2020, 316, 64–69. [Google Scholar] [CrossRef]
- Palmerini, T.; Chakravarty, T.; Saia, F.; Bruno, A.G.; Bacchi-Reggiani, M.-L.; Marrozzini, C.; Patel, C.; Patel, V.; Testa, L.; Bedogni, F.; et al. Coronary Protection to Prevent Coronary Obstruction During TAVR. JACC Cardiovasc. Interv. 2020, 13, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.M.; Greenbaum, A.B.; Babaliaros, V.C.; Rogers, T.; Eng, M.H.; Paone, G.; Leshnower, B.G.; Reisman, M.; Satler, L.; Waksman, R.; et al. The BASILICA Trial. JACC Cardiovasc. Interv. 2019, 12, 1240–1252. [Google Scholar] [CrossRef] [PubMed]
- Abramowitz, Y.; Chakravarty, T.; Jilaihawi, H.; Kashif, M.; Kazuno, Y.; Takahashi, N.; Maeno, Y.; Nakamura, M.; Cheng, W.; Makkar, R.R. Clinical impact of coronary protection during transcatheter aortic valve implantation: First reported series of patients. Eurointervention 2015, 11, 572–581. [Google Scholar] [CrossRef]
- Jabbour, R.J.; Tanaka, A.; Finkelstein, A.; Mack, M.; Tamburino, C.; Van Mieghem, N.; de Backer, O.; Testa, L.; Gatto, P.; Purita, P.; et al. Delayed Coronary Obstruction after Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2018, 71, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.A.; Schaefer, U.; Guetta, V.; Hildick-Smith, D.; Möllmann, H.; Dumonteil, N.; Modine, T.; Bosmans, J.; Petronio, A.S.; Moat, N.; et al. Transcatheter Aortic Valve Implantation for Pure Severe Native Aortic Valve Regurgitation. J. Am. Coll. Cardiol. 2013, 61, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, F.J.; Deutsch, M.-A.; Seiffert, M.; Yoon, S.-H.; Codner, P.; Wickramarachchi, U.; Latib, A.; Petronio, A.S.; Rodés-Cabau, J.; Taramasso, M.; et al. Safety and Efficacy of Transcatheter Aortic Valve Replacement in the Treatment of Pure Aortic Regurgitation in Native Valves and Failing Surgical Bioprostheses. JACC Cardiovasc. Interv. 2017, 10, 1048–1056. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Schmidt, T.; Bleiziffer, S.; Schofer, N.; Fiorina, C.; Munoz-Garcia, A.J.; Yzeiraj, E.; Amat-Santos, I.J.; Tchetche, D.; Jung, C.; et al. Transcatheter Aortic Valve Replacement in Pure Native Aortic Valve Regurgitation. J. Am. Coll. Cardiol. 2017, 70, 2752–2763. [Google Scholar] [CrossRef]
- Mauri, V.; Kim, W.-K.; Abumayyaleh, M.; Walther, T.; Moellmann, H.; Schaefer, U.; Conradi, L.; Hengstenberg, C.; Hilker, M.; Wahlers, T.; et al. Short-Term Outcome and Hemodynamic Performance of Next-Generation Self-Expanding Versus Balloon-Expandable Transcatheter Aortic Valves in Patients with Small Aortic Annulus. Circ. Cardiovasc. Interv. 2017, 10, e005013. [Google Scholar] [CrossRef]
- Herrmann, H.C.; Abdel-Wahab, M.; Attizzani, G.F.; Batchelor, W.; Bleiziffer, S.; Verdoliva, S.; Chang, Y.; Gada, H.; Gillam, L.; Guerrero, M.; et al. Rationale and design of the SMall Annuli Randomized To Evolut or SAPIEN Trial (SMART Trial). Am. Heart J. 2022, 243, 92–102. [Google Scholar] [CrossRef]
- Bahlmann, E.; Cramariuc, D.; Minners, J.; Lønnebakken, M.T.; Ray, S.; Gohlke-Baerwolf, C.; Nienaber, C.A.; Jander, N.; Seifert, R.; Chambers, J.B.; et al. Small aortic root in aortic valve stenosis: Clinical characteristics and prognostic implications. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Pibarot, P. Prosthesis-patient mismatch: Definition, clinical impact, and prevention. Heart 2006, 92, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Fallon, J.M.; DeSimone, J.P.; Brennan, J.M.; O’Brien, S.; Thibault, D.P.; DiScipio, A.W.; Pibarot, P.; Jacobs, J.P.; Malenka, D.J. The Incidence and Consequence of Prosthesis-Patient Mismatch after Surgical Aortic Valve Replacement. Ann. Thorac. Surg. 2018, 106, 14–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas-Ferraz, A.B.; Tirado-Conte, G.; Dagenais, F.; Ruel, M.; Al-Atassi, T.; Dumont, E.; Mohammadi, S.; Bernier, M.; Pibarot, P.; Rodés-Cabau, J. Aortic Stenosis and Small Aortic Annulus. Circulation 2019, 139, 2685–2702. [Google Scholar] [CrossRef]
- Puri, R.; Byrne, J.; Muller, R.; Baumbach, H.; Eltchaninoff, H.; Redwood, S.; Cheema, A.; Dubois, C.; Ihlberg, L.; Wijeysundera, H.C.; et al. Transcatheter aortic valve implantation in patients with small aortic annuli using a 20 mm balloon-expanding valve. Heart 2017, 103, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Rodés-Cabau, J.; Pibarot, P.; Suri, R.M.; Kodali, S.; Thourani, V.H.; Szeto, W.Y.; Svensson, L.G.; Dumonteil, N.; Xu, K.; Hahn, R.T.; et al. Impact of Aortic Annulus Size on Valve Hemodynamics and Clinical Outcomes after Transcatheter and Surgical Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2014, 7, 701–711. [Google Scholar] [CrossRef] [Green Version]
- Coutinho, G.F.; Correia, P.M.; Paupério, G.; de Oliveira, F.; Antunes, M.J. Aortic root enlargement does not increase the surgical risk and short-term patient outcome? Eur. J. Cardio-Thoracic Surg. 2011, 40, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Witberg, G.; Regev, E.; Chen, S.; Assali, A.; Barbash, I.M.; Planer, D.; Vaknin-Assa, H.; Guetta, V.; Vukasinovic, V.; Orvin, K.; et al. The Prognostic Effects of Coronary Disease Severity and Completeness of Revascularization on Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2017, 10, 1428–1435. [Google Scholar] [CrossRef]
- Sankaramangalam, K.; Banerjee, K.; Kandregula, K.; Mohananey, D.; Parashar, A.; Jones, B.M.; Jobanputra, Y.; Mick, S.; Krishnaswamy, A.; Svensson, L.G.; et al. Impact of Coronary Artery Disease on 30-Day and 1-Year Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement: A Meta-Analysis. J. Am. Heart Assoc. 2017, 6, e006092. [Google Scholar] [CrossRef] [Green Version]
- Faroux, L.; Guimaraes, L.; Wintzer-Wehekind, J.; Junquera, L.; Ferreira-Neto, A.N.; del Val, D.; Muntané-Carol, G.; Mohammadi, S.; Paradis, J.-M.; Rodés-Cabau, J. Coronary Artery Disease and Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2019, 74, 362–372. [Google Scholar] [CrossRef]
- Patrick, W.L.; Chen, Z.; Han, J.J.; Smood, B.; Rao, A.; Khurshan, F.; Yarlagadda, S.; Iyengar, A.; Kelly, J.J.; Grimm, J.C.; et al. Patients with Atrial Fibrillation Benefit from SAVR with Surgical Ablation Compared to TAVR Alone. Cardiol. Ther. 2022, 11, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Toggweiler, S.; Boone, R.H.; Rodés-Cabau, J.; Humphries, K.H.; Lee, M.; Nombela-Franco, L.; Bagur, R.; Willson, A.B.; Binder, R.K.; Gurvitch, R.; et al. Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2012, 59, 2068–2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nombela-Franco, L.; Ribeiro, H.B.; Urena, M.; Allende, R.; Amat-Santos, I.; DeLarochellière, R.; Dumont, E.; Doyle, D.; DeLarochellière, H.; Laflamme, J.; et al. Significant mitral regurgitation left untreated at the time of aortic valve replacement: A comprehensive review of a frequent entity in the transcatheter aortic valve replacement era. J. Am. Coll. Cardiol. 2014, 63, 2643–2658. [Google Scholar] [CrossRef] [Green Version]
- Löw, K.; Steffen, J.; Theiss, H.; Orban, M.; Rizas, K.D.; Haum, M.; Doldi, P.M.; Stolz, L.; Gmeiner, J.; Hagl, C.; et al. CTA-determined tricuspid annular dilatation is associated with persistence of tricuspid regurgitation after transcatheter aortic valve replacement. Clin. Res. Cardiol. 2023, 112, 645–655. [Google Scholar] [CrossRef]
- Bäz, L.; Möbius-Winkler, S.; Diab, M.; Kräplin, T.; Westphal, J.G.; Ibrahim, K.; Schulze, P.C.; Franz, M. Prognostic relevance of mitral and tricuspid regurgitation after transcatheter aortic valve implantation: Impact of follow-up time point for decision-making. Front. Cardiovasc. Med. 2023, 10, 990373. [Google Scholar] [CrossRef] [PubMed]
- Rotman, O.M.; Bianchi, M.; Ghosh, R.P.; Kovarovic, B.; Bluestein, D. Principles of TAVR valve design, modelling, and testing. Expert Rev. Med. Devices 2018, 15, 771–791. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juarez-Casso, F.M.; Crestanello, J.A. The Evolving Role of Surgical Aortic Valve Replacement in the Era of Transcatheter Valvular Procedures. J. Clin. Med. 2023, 12, 5299. https://doi.org/10.3390/jcm12165299
Juarez-Casso FM, Crestanello JA. The Evolving Role of Surgical Aortic Valve Replacement in the Era of Transcatheter Valvular Procedures. Journal of Clinical Medicine. 2023; 12(16):5299. https://doi.org/10.3390/jcm12165299
Chicago/Turabian StyleJuarez-Casso, Fernando M., and Juan A. Crestanello. 2023. "The Evolving Role of Surgical Aortic Valve Replacement in the Era of Transcatheter Valvular Procedures" Journal of Clinical Medicine 12, no. 16: 5299. https://doi.org/10.3390/jcm12165299



