Positive Association between Macular Pigment Optical Density and Glomerular Filtration Rate: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Measurement of MPOD
2.4. Measurement of Estimated Glomerular Filtration Rate (eGFR)
2.5. Other Measurements
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
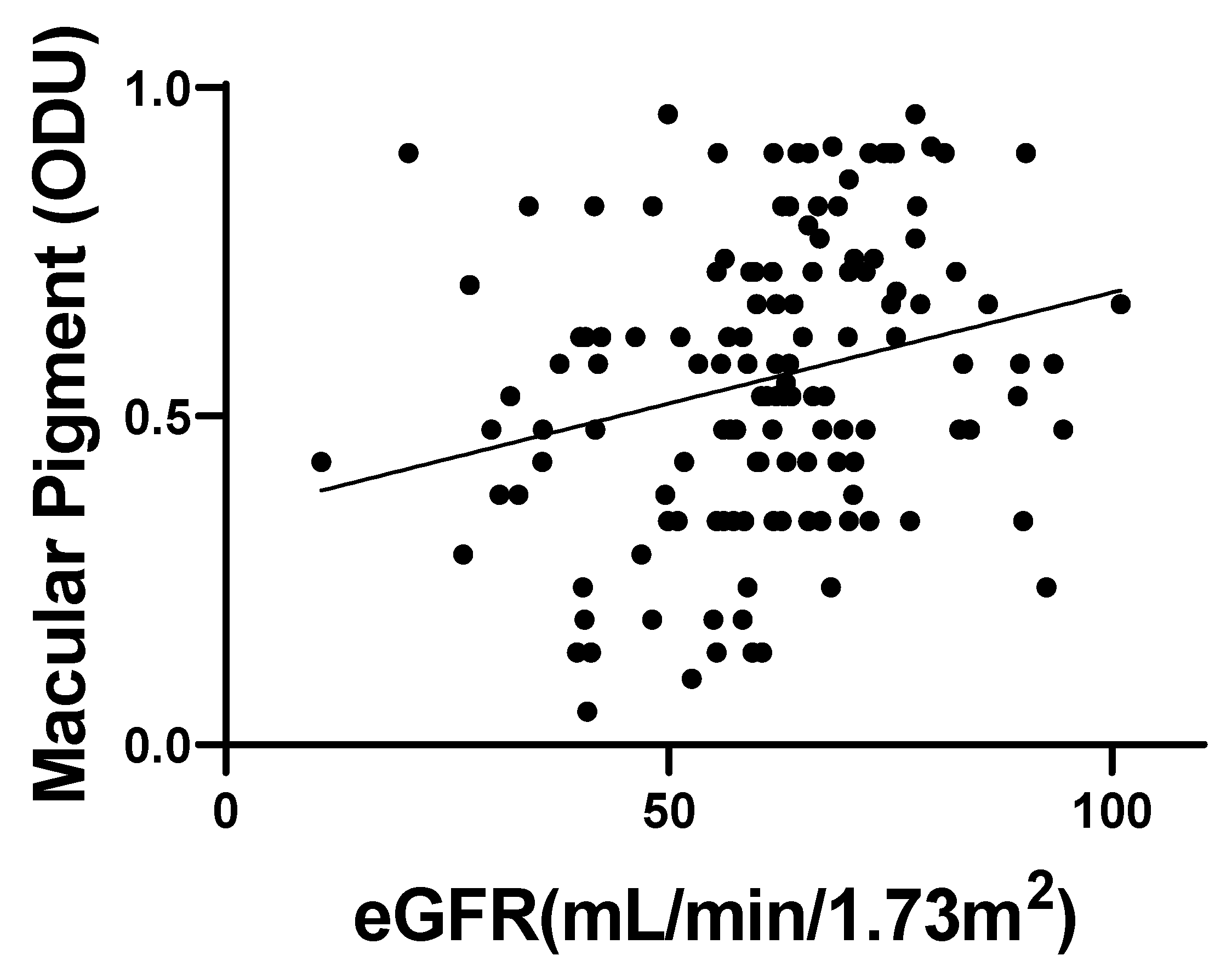
3.2. MPOD and eGFR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bone, R.A.; Landrum, J.T.; Friedes, L.M.; Gomez, C.M.; Kilburn, M.D.; Menendez, E.; Vidal, I.; Wang, W. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp. Eye Res. 1997, 64, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Malinow, M.R.; Feeney-Burns, L.; Peterson, L.H.; Klein, M.L.; Neuringer, M. Diet-related macular anomalies in monkeys. Investig. Ophthalmol. Vis. Sci. 1980, 19, 857–863. [Google Scholar]
- Edge, R.; McGarvey, D.J.; Truscott, T.G. The carotenoids as anti-oxidants—A review. J. Photochem. Photobiol. B 1997, 41, 189–200. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, C.C.; Abreu, F.; Adamis, A.P.; Basu, K.; Eichenbaum, D.A.; Haskova, Z.; Lin, H.; Loewenstein, A.; Mohan, S.; Pearce, I.A.; et al. YOSEMITE and RHINE Investigators. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): Two randomised, double-masked, phase 3 trials. Lancet 2022, 399, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Jiang, S.; Gericke, A. Age-Related Macular Degeneration: Role of Oxidative Stress and Blood Vessels. Int. J. Mol. Sci. 2021, 22, 1296. [Google Scholar] [CrossRef]
- Beatty, S.; Koh, H.-H. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef]
- Yu, D.Y.; Cringle, S.J. Retinal degeneration and local oxygen metabolism. Exp. Eye Res. 2005, 80, 745–751. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Mayne, S.T.; Gomez, C.M.; Tibor, S.E.; Twaroska, E.E. Macular pigment in donor eyes with and without AMD: A case-control study. Investig. Ophthalmol. Vis. Sci. 2001, 42, 235–240. [Google Scholar]
- Obana, A.; Hiramitsu, T.; Gohto, Y.; Ohira, A.; Mizuno, S.; Hirano, T.; Bernstein, P.S.; Fujii, H.; Iseki, K.; Tanito, M.; et al. Macular carotenoid levels of normal subjects and age-related maculopathy patients in a Japanese population. Ophthalmology 2008, 115, 147–157. [Google Scholar] [CrossRef]
- Howells, O.; Eperjesi, F.; Bartlett, H. Measuring macular pigment optical density in vivo: A review of techniques. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 315–347. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T. Heterochromatic flicker photometry. Arch. Biochem. Biophys. 2004, 430, 137–142. [Google Scholar] [CrossRef]
- Mares, J.A.; LaRowe, T.L.; Snodderly, D.M.; Moeller, S.M.; Gruber, M.J.; Klein, M.L.; Wooten, B.R.; Johnson, E.J.; Chappell, R.J.; CAREDS Macular Pigment Study Group and Investigators. Predictors of Optical Density of Lutein and Zeaxanthin in retinas of older women in the Carotenoids in Age-Related Eye Disease Study, an ancillary study of the Women’s Health Initiative. Am. J. Clin. Nutr. 2006, 84, 1107–1122. [Google Scholar] [CrossRef]
- Obana, A.; Gohto, Y.; Asaoka, R.; Gellermann, W.; Bernstein, P.S. Lutein and zeaxanthin distribution in the healthy macula and its association with various demographic factors examined in pseudophakic eyes. Antioxidants 2021, 10, 1857. [Google Scholar] [CrossRef] [PubMed]
- Berendschot, T.T.; van Norren, D. On the age dependency of the macular pigment optical density. Exp. Eye Res. 2005, 81, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Hammond, B.R.; Curran-Celentano, J.; Judd, S.; Fuld, K.; Krinsky, N.I.; Wooten, B.R.; Snodderly, D.M. Sex differences in macular pigment optical density: Relation to plasma carotenoid concentrations and dietary patterns. Vis. Res. 1996, 36, 2001–2012. [Google Scholar] [CrossRef] [PubMed]
- Hammond, B.R.; Fuld, K.; Snodderly, D.M. Iris color and macular pigment optical density. Exp. Eye Res. 1996, 62, 293–297. [Google Scholar] [CrossRef]
- Hammond, B.R.; Wooten, B.R.; Snodderly, D.M. Cigarette smoking and retinal carotenoids: Implications for age-related macular degeneration. Vis. Res. 1996, 36, 3003–3009. [Google Scholar] [CrossRef]
- Gupta, A.; Raman, R.; Biswas, S.; Rajan, R.; Kulothungan, V.; Sharma, T. Association between various types of obesity and macular pigment optical density. Eye 2012, 26, 259–266. [Google Scholar] [CrossRef]
- Nusinovici, S.; Sabanayagam, C.; Teo, B.W.; Tan, G.S.W.; Wong, T.Y. Vision impairment in CKD patients: Epidemiology, mechanisms, differential diagnoses, and prevention. Am. J. Kidney Dis. 2019, 73, 846–857. [Google Scholar] [CrossRef]
- Keir, L.S.; Firth, R.; Aponik, L.; Feitelberg, D.; Sakimoto, S.; Aguilar, E.; Welsh, G.I.; Richards, A.; Usui, Y.; Satchell, S.C.; et al. VEGF regulates local inhibitory complement proteins in the eye and kidney. J. Clin. Investig. 2017, 127, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Nishi, T.; Saeki, K.; Obayashi, K.; Miyata, K.; Tone, N.; Tsujinaka, H.; Yamashita, M.; Masuda, N.; Mizusawa, Y.; Okamoto, M.; et al. The effect of blue-blocking intraocular lenses on circadian biological rhythm: Protocol for a randomised controlled trial (CLOCK-IOL colour study). BMJ Open 2015, 5, e007930. [Google Scholar] [CrossRef] [PubMed]
- Chylack, L.T.; Wolfe, J.K.; Singer, D.M.; Leske, M.C.; Bullimore, M.A.; Bailey, I.L.; Friend, J.; McCarthy, D.; Wu, S.Y. The lens opacities classification system III. The longitudinal study of cataract study group. Arch. Ophthalmol. 1993, 111, 831–836. [Google Scholar] [CrossRef]
- Van der Veen, R.L.; Berendschot, T.T.; Hendrikse, F.; Carden, D.; Makridaki, M.; Murray, I.J. A new desktop instrument for measuring macular pigment optical density based on a novel technique for setting flicker thresholds. Ophthalmic Physiol. Opt. 2009, 29, 127–137. [Google Scholar] [CrossRef]
- Obana, A.; Gohto, Y.; Moriyama, T.; Seto, T.; Sasano, H.; Okazaki, S. Reliability of a commercially available heterochromatic flicker photometer, the MPS2, for measuring the macular pigment optical density of a Japanese population. Jpn. J. Ophthalmol. 2018, 62, 473–480. [Google Scholar] [CrossRef]
- Obayashi, K.; Saeki, K.; Kurumatani, N. Nighttime BP in elderly individuals with prediabetes/diabetes with and without CKD: The HEIJO-KYO study. Clin. J. Am. Soc. Nephrol. 2016, 11, 867–874. [Google Scholar] [CrossRef]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Rodicio, J.L.; Campo, C.; Ruilope, L.M. Microalbuminuria in essential hypertension. Kidney Int. Suppl. 1998, 68, S51–S54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choi, J.; Moon, J.W.; Shin, H.J. Chronic kidney disease, early age-related macular degeneration, and peripheral retinal drusen. Ophthal. Epidemiol. 2011, 18, 259–263. [Google Scholar] [CrossRef]
- Klein, R.; Knudtson, M.D.; Lee, K.E.; Klein, B.E. Serum Cystatin C level, kidney disease markers, and incidence of age-related macular degeneration: The Beaver Dam Eye Study. Arch. Ophthalmol. 2009, 127, 193–199. [Google Scholar] [CrossRef]
- Liew, G.; Mitchell, P.; Wong, T.Y.; Iyengar, S.K.; Wang, J.J. CKD increases the risk of age-related macular degeneration. J. Am. Soc. Nephrol. 2008, 19, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Saaddine, J.B.; Klein, R.; Rothenberg, R.; Chou, C.F.; Il’yasova, D. Early age-related macular degeneration with cardiovascular and renal comorbidities: An analysis of the national health and nutrition examination survey, 2005–2008. Ophthal Epidemiol. 2017, 24, 413–419. [Google Scholar] [CrossRef]
- Cheung, C.M.; Li, X.; Cheng, C.Y.; Zheng, Y.; Mitchell, P.; Wang, J.J.; Wong, T.Y. Prevalence, racial variations, and risk factors of age-related macular degeneration in Singaporean Chinese, Indians, and Malays. Ophthalmology 2014, 121, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadeh, M.; Obana, A.; Gohto, Y.; Seto, T.; Gellermann, W. Autofluorescence imaging of macular pigment: Influence and correction of ocular media opacities. J. Biomed. Opt. 2014, 19, 096010. [Google Scholar] [CrossRef] [PubMed]
- Akuffo, K.O.; Nolan, J.; Stack, J.; Power, R.; Kirwan, C.; Moran, R.; Corcoran, L.; Owens, N.; Beatty, S. The Impact of Cataract, and Its Surgical Removal, on Measures of Macular Pigment Using the Heidelberg Spectralis HRA+OCT MultiColor Device. Investig. Opthalmol. Vis. Sci. 2016, 57, 2552–2563. [Google Scholar] [CrossRef] [PubMed]
- Obana, A.; Gohto, Y.; Sasano, H.; Gellermann, W.; Sharifzadeh, M.; Seto, T.; Bernstein, P.S. Grade of Cataract and Its Influence on Measurement of Macular Pigment Optical Density Using Autofluorescence Imaging. Investig. Opthalmol. Vis. Sci. 2018, 59, 3011–3019. [Google Scholar] [CrossRef]
- Obana, A.; Ote, K.; Hashimoto, F.; Asaoka, R.; Gohto, Y.; Okazaki, S.; Yamada, H. Correction for the Influence of Cataract on Macular Pigment Measurement by Autofluorescence Technique Using Deep Learning. Transl. Vis. Sci. Technol. 2021, 10, 18. [Google Scholar] [CrossRef]
- Van Norren, D.; Vos, J.J. Light damage to the retina: An historical approach. Eye 2016, 30, 169–172. [Google Scholar] [CrossRef]
- Narimatsu, T.; Negishi, K.; Miyake, S.; Hirasawa, M.; Osada, H.; Kurihara, T.; Tsubota, K.; Ozawa, Y. Blue light-induced inflammatory marker expression in the retinal pigment epithelium-choroid of mice and the protective effect of a yellow intraocular lens material in vivo. Exp. Eye Res. 2015, 132, 48–51. [Google Scholar] [CrossRef]
- Oberg, B.P.; McMenamin, E.; Lucas, F.L.; McMonagle, E.; Morrow, J.; Ikizler, T.A.; Himmelfarb, J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004, 65, 1009–1016. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group; SanGiovanni, J.P.; Chew, E.Y.; Clemons, T.E.; Ferris, F.L.; Gensler, G.; Lindblad, A.S.; Milton, R.C.; Seddon, J.M.; Sperduto, R.D. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22: AREDS Report No. 22. Arch. Ophthalmol. 2007, 125, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study 2 (AREDS2) Research Group; Chew, E.Y.; SanGiovanni, J.P.; Ferris, F.L.; Wong, W.T.; Agron, E.; Clemons, T.E.; Sperduto, R.; Danis, R.; Chandra, S.R.; et al. Lutein/zeaxanthin for the treatment of age-related cataract: AREDS2 randomized trial report no. 4. JAMA Ophthalmol. 2013, 131, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cai, X.; Zhang, N.; Li, Y.; Mao, Y.; Ge, S.; Yao, Y.; Gao, H. Relation Between Dietary Carotenoid Intake, Serum Concentration, and Mortality Risk of CKD Patients Among US Adults: National Health and Nutrition Examination Survey 2001–2014. Front. Med. 2022, 9, 871767. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Obana, A.; Gohto, Y.; Okazaki, S.; Gellermann, W.; Ohira, A. Macular pigment density changes in Japanese individuals supplemented with lutein or zeaxanthin: Quantification via resonance Raman spectrophotometry and autofluorescence imaging. Jpn. J. Ophthalmol. 2012, 56, 488–496. [Google Scholar] [CrossRef]

| Characteristics | Pigment High | Pigment Low | p |
|---|---|---|---|
| Number (%) | 70 (51.1) | 67 (49.0) | |
| Age, mean (SD), years | 74.9 (6.5) | 75.2 (6.7) | 0.82 |
| Sex (men), number (%) | 43 (61.4) | 39 (58.2) | 0.73 |
| Abdominal circumference, mean (SD), cm | 86.3 (9.5) | 84.3 (9.6) | 0.21 |
| Diabetes, number (%) | 10 (14.3) | 13 (19.4) | 0.50 |
| Hypertension, number (%) | 27 (38.6) | 33 (49.2) | 0.23 |
| Current smoking, number (%) | 27 (38.6) | 25 (37.3) | 1.00 |
| IOL (Clear), number (%) | 40 (57.1) | 29 (43.2) | 0.13 |
| BCVA before surgery, mean (SD), logMAR units | 0.48 (0.38) | 0.48 (0.42) | 0.97 |
| BCVA after surgery, mean (SD), logMAR units | 0.012 (0.11) | 0.028 (0.13) | 0.44 |
| eGFR (SD), mL/min/1.73 m2 | 64.2 (15.3) | 58.1 (16.3) | 0.02 |
| (CKD-EPI definition) | 64.8 (12.8) | 59.9 (15.1) | 0.04 |
| β * | 95% CI | p | |
|---|---|---|---|
| Crude model | 0.0034 | 0.0011–0.0056 | <0.01 |
| Adjusted model 1 | 0.0034 | 0.0011–0.0057 | <0.01 |
| Adjusted model 2 | 0.0035 | 0.0011–0.0059 | <0.01 |
| Adjusted model 3 | 0.0033 | 0.00090–0.0058 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsujinaka, H.; Saeki, K.; Obayashi, K.; Nishi, T.; Ueda, T.; Ogata, N. Positive Association between Macular Pigment Optical Density and Glomerular Filtration Rate: A Cross-Sectional Study. J. Clin. Med. 2023, 12, 5312. https://doi.org/10.3390/jcm12165312
Tsujinaka H, Saeki K, Obayashi K, Nishi T, Ueda T, Ogata N. Positive Association between Macular Pigment Optical Density and Glomerular Filtration Rate: A Cross-Sectional Study. Journal of Clinical Medicine. 2023; 12(16):5312. https://doi.org/10.3390/jcm12165312
Chicago/Turabian StyleTsujinaka, Hiroki, Keigo Saeki, Kenji Obayashi, Tomo Nishi, Tetsuo Ueda, and Nahoko Ogata. 2023. "Positive Association between Macular Pigment Optical Density and Glomerular Filtration Rate: A Cross-Sectional Study" Journal of Clinical Medicine 12, no. 16: 5312. https://doi.org/10.3390/jcm12165312
APA StyleTsujinaka, H., Saeki, K., Obayashi, K., Nishi, T., Ueda, T., & Ogata, N. (2023). Positive Association between Macular Pigment Optical Density and Glomerular Filtration Rate: A Cross-Sectional Study. Journal of Clinical Medicine, 12(16), 5312. https://doi.org/10.3390/jcm12165312






