Clinical Outcomes and Prognostic Factors in Complex, High-Risk Indicated Procedure (CHIP) and High-Bleeding-Risk (HBR) Patients Undergoing Percutaneous Coronary Intervention with Sirolimus-Eluting Stent Implantation: 4-Year Results
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. CHIP and HBR Subgroup Criteria
2.3. Alex Plus Stent Characteristics
2.4. Data Collection
2.5. Study Endpoints
2.6. Statistical Methods
3. Results
3.1. Patient Inclusion
3.2. Baseline Characteristics
3.3. Procedure Characteristics
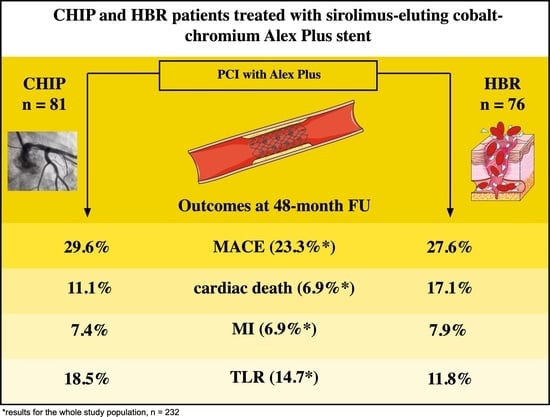
3.4. Four-Year Outcomes
3.5. Multivariable Cox Regression Analysis
4. Discussion
| Study | Comments | Death | Cardiac Death | TLR | MI |
|---|---|---|---|---|---|
| Our study: whole | 10.8% | 6.9% | 14.7% | 6.9% | |
| Our study: Non-complex | Including HBR | 8.6% | 4.6% | 12.5% | 6.6% |
| Our study: CHIP | 14.8% | 11.1% | 18.5% | 7.4% | |
| Murray [31] | - | 18.8% | - | - | - |
| Riku [32] | 5 yrs FU non-complex vs. complex PCI | 7.0 vs. 12.2% | 2.9 vs. 5.4% | 9.8 vs. 22% | 1.4 vs. 3.1% |
| Buiten [33] | 3 yrs FU SES/EES/ZES | - | 2.4%/2.5%/2.5% | 7%/9.5%/10% | 3.3%/3.9%/4.2% |
| Maeng [34] | EES/SES | 12/9.5% | 8.3/4.8% | 5.6/9.5% | 1.9/7.6% |
| Olesen [35] | 5 yrs FU ZES/SES | 16/17.9% | 6.5/7.1% | 14.8/4.8% | 8.9/7.1% |
| Wijns [36] | ZES/SES | 5.5/6% | 2.9/3.4% | 9/8.6% | 4.6/5.8 |
| Sato [37] | 5 yrs FU DM/non-DM | 7.1/8.9% | 2.4/2.7% | 9.4/8.9% | 4.7/0% |
| Stefanini [38] | BP-DES/DP-SES | 9.3/10% | 5.2/5.9% | 12/13.7% | 6/6.8% |
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alfonso, F.; Coughlan, J.J.; Giacoppo, D.; Kastrati, A.; Byrne, R.A. Management of in-stent restenosis. EuroIntervention 2022, 18, e103–e123. [Google Scholar] [CrossRef]
- Tamez, H.; Secemsky, E.A.; Valsdottir, L.R.; Moussa, I.D.; Song, Y.; Simonton, C.A.; Gibson, C.M.; Popma, J.J.; Yeh, R.W. Long-term outcomes of percutaneous coronary intervention for in-stent restenosis among Medicare beneficiaries. EuroIntervention 2021, 17, e380–e387. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Q.; Hu, J.; Zhang, R.; Gao, T.; Rong, S.; Dong, H. Global research trends in in-stent neoatherosclerosis: A CiteSpace-based visual analysis. Front. Cardiovasc. Med. 2022, 9, 1025858. [Google Scholar] [CrossRef]
- Wanha, W.; Bil, J.; Januszek, R.; Gilis-Malinowska, N.; Figatowski, T.; Milewski, M.; Pawlik, A.; Staszczak, B.; Wybraniec, M.; Tomasiewicz, B.; et al. Long-Term Outcomes Following Drug-Eluting Balloons Versus Thin-Strut Drug-Eluting Stents for Treatment of In-Stent Restenosis (DEB-Dragon-Registry). Circ. Cardiovasc. Interv. 2021, 14, e010868. [Google Scholar] [CrossRef]
- Genereux, P.; Madhavan, M.V.; Mintz, G.S.; Maehara, A.; Palmerini, T.; Lasalle, L.; Xu, K.; McAndrew, T.; Kirtane, A.; Lansky, A.J.; et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) TRIALS. J. Am. Coll. Cardiol. 2014, 63, 1845–1854. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yang, L.; Ju, H.; Zhang, F.; Wu, J.; He, B.; Chen, Y. Prevalence and prognosis of coronary stent gap detected by multi-detector CT: A follow-up study. Eur. Radiol. 2012, 22, 1896–1903. [Google Scholar] [CrossRef]
- Condello, F.; Spaccarotella, C.; Sorrentino, S.; Indolfi, C.; Stefanini, G.G.; Polimeni, A. Stent Thrombosis and Restenosis with Contemporary Drug-Eluting Stents: Predictors and Current Evidence. J. Clin. Med. 2023, 12, 1238. [Google Scholar] [CrossRef]
- Bil, J.; Gil, R.J.; Kern, A.; Pawlowski, T.; Seweryniak, P.; Sliwinski, Z. Novel sirolimus-eluting stent Prolim(R) with a biodegradable polymer in the all-comers population: One year clinical results with quantitative coronary angiography and optical coherence tomography analysis. BMC Cardiovasc. Disord. 2015, 15, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, R.J.; Bil, J.; Pawlowski, T.; Yuldashev, N.; Kolakowski, L.; Janczak, J.; Jablonski, W.; Palinski, P. The use of bioresorbable vascular scaffold Absorb BVS(R) in patients with stable coronary artery disease: One-year results with special focus on the hybrid bioresorbable vascular scaffolds and drug eluting stents treatment. Kardiol. Pol. 2016, 74, 627–633. [Google Scholar] [CrossRef] [Green Version]
- Shlofmitz, E.; Iantorno, M.; Waksman, R. Restenosis of Drug-Eluting Stents: A New Classification System Based on Disease Mechanism to Guide Treatment and State-of-the-Art Review. Circ. Cardiovasc. Interv. 2019, 12, e007023. [Google Scholar] [CrossRef] [PubMed]
- Chieffo, A.; Burzotta, F.; Pappalardo, F.; Briguori, C.; Garbo, R.; Masiero, G.; Nicolini, E.; Ribichini, F.; Trani, C.; Alvarez, B.C.; et al. Clinical expert consensus document on the use of percutaneous left ventricular assist support devices during complex high-risk indicated PCI: Italian Society of Interventional Cardiology Working Group Endorsed by Spanish and Portuguese Interventional Cardiology Societies. Int. J. Cardiol. 2019, 293, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.; Mehran, R.; Colleran, R.; Angiolillo, D.J.; Byrne, R.A.; Capodanno, D.; Cuisset, T.; Cutlip, D.; Eerdmans, P.; Eikelboom, J.; et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: A consensus document from the Academic Research Consortium for High Bleeding Risk. Eur. Heart J. 2019, 40, 2632–2653. [Google Scholar] [CrossRef] [Green Version]
- Rola, P.; Kulczycki, J.J.; Barycki, M.; Wlodarczak, S.; Furtan, L.; Kedzierska, M.; Giniewicz, K.; Doroszko, A.; Lesiak, M.; Wlodarczak, A. Comparison of Orbital Atherectomy and Rotational Atherectomy in Calcified Left Main Disease: Short-Term Outcomes. J. Clin. Med. 2023, 12, 4025. [Google Scholar] [CrossRef]
- Marchese, A.; Tito, A.; Paparella, D.; Colombo, A. A cascade of multiple complications hampering a complex high-risk percutaneous coronary intervention (CHIP-PCI): When ingenuity overcomes troubles! Clin. Case Rep. 2020, 8, 3362–3368. [Google Scholar] [CrossRef]
- Iannaccone, M.; Franchin, L.; Burzotta, F.; Botti, G.; Pazzanese, V.; Briguori, C.; Trani, C.; Piva, T.; De Marco, F.; Masiero, G.; et al. Impact of in-Hospital Left Ventricular Ejection Fraction Recovery on Long-Term Outcomes in Patients Who Underwent Impella Support for HR PCI or Cardiogenic Shock: A Sub-Analysis from the IMP-IT Registry. J. Pers. Med. 2023, 13, 826. [Google Scholar] [CrossRef]
- Marin, F.; Pighi, M.; Zucchelli, F.; Ruzzarin, A.; Russo, G.; Aurigemma, C.; Romagnoli, E.; Ferrero, V.; Piccoli, A.; Scarsini, R.; et al. Predictors and Prognostic Impact of Left Ventricular Ejection Fraction Recovery after Impella-Supported Percutaneous Coronary Interventions in Acute Myocardial Infarction. J. Pers. Med. 2022, 12, 1576. [Google Scholar] [CrossRef]
- Costa, F.; Garcia-Ruiz, V.; Licordari, R.; Fimiani, L. The High Bleeding Risk Patient with Coronary Artery Disease. Cardiol. Clin. 2020, 38, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Bass, T.A. High-Risk Percutaneous Coronary Interventions in Modern Day Clinical Practice: Current Concepts and Challenges. Circ. Cardiovasc. Interv. 2015, 8, e003405. [Google Scholar] [CrossRef]
- Byun, S.; Choo, E.H.; Oh, G.C.; Lim, S.; Choi, I.J.; Lee, K.Y.; Lee, S.N.; Hwang, B.H.; Kim, C.J.; Park, M.W.; et al. Temporal Trends of Major Bleeding and Its Prediction by the Academic Research Consortium-High Bleeding Risk Criteria in Acute Myocardial Infarction. J. Clin. Med. 2022, 11, 988. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Kim, M.H.; Lee, K.M.; Ko, Y.G.; Yoon, C.H.; Jo, M.K.; Yun, S.C. Comparison of Performance between ARC-HBR Criteria and PRECISE-DAPT Score in Patients Undergoing Percutaneous Coronary Intervention. J. Clin. Med. 2021, 10, 2566. [Google Scholar] [CrossRef] [PubMed]
- Buszman, P.P.; Michalak, M.J.; Pruski, M.; Fernandez, C.; Jelonek, M.; Janas, A.; Savard, C.; Gwiazdowska-Nowotka, B.; Zurakowski, A.; Wojakowski, W.; et al. Comparable vascular response of a new generation sirolimus eluting stents when compared to fluoropolymer everolimus eluting stents in the porcine coronary restenosis model. Cardiol. J. 2016, 23, 657–666. [Google Scholar] [CrossRef] [Green Version]
- Dobrolinska, M.; Gasior, P.; Roleder, T.; Roleder-Dylewska, M.; Smolka, G.; Ochala, A.; Kedhi, E.; Wojakowski, W. Short-term healing response after implantation of the thin-strut, fast-releasing sirolimus-eluting biodegradable polymer-coated Alex Plus stent: Optical coherence tomography study. Adv. Interv. Cardiol./Postępy W Kardiol. Interwencyjnej 2020, 16, 187–191. [Google Scholar] [CrossRef]
- Ryan, T.J.; Faxon, D.P.; Gunnar, R.M.; Kennedy, J.W.; King, S.B., 3rd; Loop, F.D.; Peterson, K.L.; Reeves, T.J.; Williams, D.O.; Winters, W.L., Jr.; et al. Guidelines for percutaneous transluminal coronary angioplasty. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty). Circulation 1988, 78, 486–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooq, V.; van Klaveren, D.; Steyerberg, E.W.; Meliga, E.; Vergouwe, Y.; Chieffo, A.; Kappetein, A.P.; Colombo, A.; Holmes, D.R., Jr.; Mack, M.; et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: Development and validation of SYNTAX score II. Lancet 2013, 381, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Woelders, E.C.I.; Luijkx, J.J.P.; Rodwell, L.; Winkler, P.J.C.; Dimitriu-Leen, A.C.; Smits, P.C.; van Royen, N.; Hof, A.; Damman, P.; van Geuns, R.J.M. Outcomes with P2Y12 inhibitor monotherapy after PCI according to bleeding risk: A Bayesian meta-analysis. Cardiovasc. Revasc. Med. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Eccleston, D.; Duong, M.N.; Chowdhury, E.; Schwarz, N.; Reid, C.; Liew, D.; Conradie, A.; Worthley, S.G. Early vs. Late Readmission following Percutaneous Coronary Intervention: Predictors and Impact on Long-Term Outcomes. J. Clin. Med. 2023, 12, 1684. [Google Scholar] [CrossRef]
- Sakurai, R.; Inajima, T.; Kaneda, H.; Nagai, R.; Hashimoto, H. Sirolimus-eluting stents reduce long-term mortality compared with bare metal stents in ST-segment elevation myocardial infarction: A meta-analysis of randomized controlled trials. Int. J. Cardiol. 2013, 167, 162–167. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Sakakura, K.; Fujita, H. Complex and high-risk intervention in indicated patients (CHIP) in contemporary clinical practice. Cardiovasc. Interv. Ther. 2023, 38, 269–274. [Google Scholar] [CrossRef]
- Achim, A.; Szigethy, T.; Olajos, D.; Molnar, L.; Papp, R.; Barczi, G.; Kakonyi, K.; Edes, I.F.; Becker, D.; Merkely, B.; et al. Switching from Proximal to Distal Radial Artery Access for Coronary Chronic Total Occlusion Recanalization. Front. Cardiovasc. Med. 2022, 9, 895457. [Google Scholar] [CrossRef]
- Achim, A.; Szucsborus, T.; Sasi, V.; Nagy, F.; Jambrik, Z.; Nemes, A.; Varga, A.; Homorodean, C.; Bertrand, O.F.; Ruzsa, Z. Safety and Feasibility of Distal Radial Balloon Aortic Valvuloplasty: The DR-BAV Study. JACC Cardiovasc. Interv. 2022, 15, 679–681. [Google Scholar] [CrossRef]
- Murray, C.S.G.; Zamora, C.; Shitole, S.G.; Christa, P.; Lee, U.J.; Bortnick, A.E.; Kizer, J.R.; Rodriguez, C.J. Race-Ethnic Differences of ST-Elevation Myocardial Infarction: Findings from a New York Health System Registry. Ethn. Dis. 2022, 32, 193–202. [Google Scholar] [CrossRef]
- Riku, S.; Suzuki, S.; Yokoi, T.; Sakaguchi, T.; Yamamoto, T.; Jinno, Y.; Tanaka, A.; Ishii, H.; Inden, Y.; Murohara, T. <Editors’ Choice> Very long-term clinical outcomes after percutaneous coronary intervention for complex vs non-complex lesions: 10-year outcomes following sirolimus-eluting stent implantation. Nagoya J. Med. Sci. 2022, 84, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Buiten, R.A.; Ploumen, E.H.; Zocca, P.; Doggen, C.J.M.; van der Heijden, L.C.; Kok, M.M.; Danse, P.W.; Schotborgh, C.E.; Scholte, M.; de Man, F.; et al. Outcomes in Patients Treated with Thin-Strut, Very Thin-Strut, or Ultrathin-Strut Drug-Eluting Stents in Small Coronary Vessels: A Prespecified Analysis of the Randomized BIO-RESORT Trial. JAMA Cardiol. 2019, 4, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Maeng, M.; Baranauskas, A.; Christiansen, E.H.; Kaltoft, A.; Holm, N.R.; Krusell, L.R.; Ravkilde, J.; Tilsted, H.H.; Thayssen, P.; Jensen, L.O. A 10-month angiographic and 4-year clinical outcome of everolimus-eluting versus sirolimus-eluting coronary stents in patients with diabetes mellitus (the DiabeDES IV randomized angiography trial). Catheter. Cardiovasc. Interv. 2015, 86, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Olesen, K.K.; Tilsted, H.H.; Jensen, L.O.; Kaltoft, A.; Krusell, L.R.; Ravkilde, J.; Christiansen, E.H.; Madsen, M.; Thayssen, P.; Sorensen, H.T.; et al. Long-term outcome of sirolimus-eluting and zotarolimus-eluting coronary stent implantation in patients with and without diabetes mellitus (a Danish organization for randomized trials on clinical outcome III substudy). Am. J. Cardiol. 2015, 115, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Wijns, W.; Steg, P.G.; Mauri, L.; Kurowski, V.; Parikh, K.; Gao, R.; Bode, C.; Greenwood, J.P.; Lipsic, E.; Alamgir, F.; et al. Endeavour zotarolimus-eluting stent reduces stent thrombosis and improves clinical outcomes compared with cypher sirolimus-eluting stent: 4-year results of the PROTECT randomized trial. Eur. Heart J. 2014, 35, 2812–2820. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Ono, T.; Morimoto, Y.; Kawai, H.; Fuke, S.; Ikeda, T.; Saito, H. Five-year clinical outcomes after implantation of sirolimus-eluting stents in patients with and without diabetes mellitus. Cardiovasc. Interv. Ther. 2012, 27, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, G.G.; Byrne, R.A.; Serruys, P.W.; de Waha, A.; Meier, B.; Massberg, S.; Juni, P.; Schomig, A.; Windecker, S.; Kastrati, A. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: A pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur. Heart J. 2012, 33, 1214–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brener, S.J.; Cunn, G.J.; Desai, P.H.; Faroqui, M.; Ha, L.D.; Handa, G.; Kutkut, I.; Raza, A.S.; Sacchi, T.J. A Novel Risk Score to Predict One-Year Mortality in Patients Undergoing Complex High-Risk Indicated Percutaneous Coronary Intervention (CHIP-PCI). J. Invasive Cardiol. 2021, 33, E253–E258. [Google Scholar]
- Mattke, S.; Hanson, M.; Bentele, M.; Kandzari, D.E. Cost and Mortality Implications of Lower Event Rates after Implantation of an Ultrathin-Strut Coronary Stent Compared with a Thin-Strut Stent Over Four Years. Cardiovasc. Revasc. Med. 2020, 21, 835–842. [Google Scholar] [CrossRef]
- de Waha, A.; Stefanini, G.G.; King, L.A.; Byrne, R.A.; Serruys, P.W.; Kufner, S.; Meier, B.; Juni, P.; Kastrati, A.; Windecker, S. Long-term outcomes of biodegradable polymer versus durable polymer drug-eluting stents in patients with diabetes a pooled analysis of individual patient data from 3 randomized trials. Int. J. Cardiol. 2013, 168, 5162–5166. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Sakakura, K.; Jinnouchi, H.; Taniguchi, Y.; Tsukui, T.; Watanabe, Y.; Yamamoto, K.; Seguchi, M.; Wada, H.; Fujita, H. Comparison of Long-Term Clinical Outcomes of Elective Percutaneous Coronary Intervention Between Complex and High-risk Intervention in Indicated Patients (CHIP) versus Non-CHIP. Am. J. Cardiol. 2023, 194, 1–8. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Sakakura, K.; Jinnouchi, H.; Taniguchi, Y.; Tsukui, T.; Watanabe, Y.; Yamamoto, K.; Seguchi, M.; Wada, H.; Fujita, H. Comparison of Outcomes of Elective Percutaneous Coronary Intervention between Complex and High-Risk Intervention in Indicated Patients (CHIP) versus Non-CHIP. J. Atheroscler. Thromb. 2022. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Guldener, U.; Kessler, T.; von Scheidt, M.; Hawe, J.S.; Gerhard, B.; Maier, D.; Lachmann, M.; Laugwitz, K.L.; Cassese, S.; Schomig, A.W.; et al. Machine Learning Identifies New Predictors on Restenosis Risk after Coronary Artery Stenting in 10,004 Patients with Surveillance Angiography. J. Clin. Med. 2023, 12, 2941. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Whole Population n = 232 (%) | CHIP n = 81 (%) | HBR n = 76 (%) |
|---|---|---|---|
| Females | 55 (23.7) | 22 (27) | 25 (33) |
| Age [years] | 68 ± 11 | 70 ± 11 | 77 ± 8 |
| Acute coronary syndrome type at presentation | |||
| UA | 31 (13.4) | 13 (16.0) | 11 (14.5) |
| NSTEMI | 26 (11.2) | 16 (19.8) | 11 (14.5) |
| STEMI | 32 (13.8) | 13 (16.0) | 10 (13.2) |
| Cardiogenic shock | 6 (2.6) | 3 (3.7) | 3 (3.9) |
| Arterial hypertension | 213 (91.8) | 76 (93.8) | 71 (93.4) |
| Diabetes type 2 | 97 (41.8) | 45 (55.6) | 44 (57.9) |
| Dyslipidemia | 177 (76.3) | 70 (86.4) | 61 (80.3) |
| Prior myocardial infarction | 113 (48.7) | 42 (51.9) | 43 (56.6) |
| Prior PCI | 130 (56.0) | 48 (59.3) | 49 (64.5) |
| Prior CABG | 22 (9.5) | 12 (14.8) | 11 (14.5) |
| Chronic kidney disease | 42 (18.1) | 16 (19.8) | 27 (35.5) |
| Prior stroke | 17 (7.3) | 6 (7.4) | 10 (13.2) |
| Peripheral artery disease | 25 (10.8) | 12 (14.8) | 9 (11.8) |
| Chronic obstructive pulmonary disease | 13 (5.6) | 6 (7.4) | 6 (7.9) |
| Echocardiographic parameters | |||
| LVEDd [mm] | 50.4 ± 9.0 | 52.0 ± 7.9 | 51.5 ± 8.5 |
| IVSd [mm] | 11.4 ± 2.1 | 11.4 ± 2.4 | 11.6 ± 1.7 |
| PWDd [mm] | 10.5 ± 1.6 | 10.6 ± 1.9 | 10.5 ± 1.6 |
| LA [mm] | 40.4 ± 5.9 | 41.0 ± 6.0 | 43.2 ± 5.8 |
| TAPSE [mm] | 22.0 ± 4.3 | 22.2 ± 4.5 | 21.3 ± 4.8 |
| LVEF [%] | 49.5 ± 10.5 | 48.9 ± 10.4 | 47.0 ± 12.2 |
| Severe mitral regurgitation | 6 (3.1) | 2 (2.7) | 4 (6.1) |
| Severe aortic regurgitation | 1 (0.5) | 0 | 0 |
| Severe aortic stenosis | 4 (2.1) | 3 (4.1) | 3 (4.5) |
| Parameter | Whole Population n = 232 | CHIP n = 81 | HBR n = 76 |
|---|---|---|---|
| White blood cells [109/L] | 8.5 ± 2.7 | 8.6 ± 2.3 | 8.0 ± 2.2 |
| Hemoglobin [g/dL] | 13.4 ± 1.7 | 13.1 ± 1.7 | 12.1 ± 1.9 |
| Red blood cells [1012/L] | 4.4 ± 0.5 | 4.3 ± 0.5 | 4.1 ± 0.6 |
| Platelets [109/L] | 222.9 ± 65 | 215.8 ± 63 | 222.5 ± 64.7 |
| Glucose [md/dL] | 136.4 ± 64.9 | 154.8 ± 75.3 | 148.3 ± 77.4 |
| HbA1c [%] | 6.3 (6.0–7.3) | 6.6 (6.1–7.3) | 6.4 (6.0–7.1) |
| Total cholesterol [md/dL] | 163.9 ± 50.9 | 161.5 ± 60.6 | 143.4 ± 41.4 |
| HDL [md/dL] | 45.7 ± 14.6 | 44.0 ± 43.5 | 44.7 ± 13.1 |
| LDL [md/dL] | 89.8 ± 40.5 | 83.0 ± 42.6 | 76.0 ± 35.8 |
| Triglycerides [md/dL] | 142 ± 33.9 | 170.7 ± 89.9 | 113.1 ± 62.3 |
| Creatine [md/dL] | 1.1 ± 0.7 | 1.2 ± 0.9 | 1.4 ± 0.8 |
| eGFR | 70.5 ± 23.2 | 67.4 ± 23.4 | 56.6 ± 20.4 |
| TnI at admission [ng/mL] | 108 (15.8–235) | 211.5 (28.2–754.5) | 62.5 (19.0–1446) |
| Max. TnI [ng/mL] | 1110 (49.8–11,573) | 1263 (88.2–13,371) | 372 (44.0–8802) |
| CK | 134.5 (84–326) | 169 (75–319) | 118 (72–334) |
| CK max | 173 (90–473) | 183 (80–390) | 156 (74–363) |
| CK-MB | 18 (13.5–30) | 20 (13–34.5) | 18 (13–29.8) |
| CK-MB max | 22.5 (15–48.5) | 26.5 (14.2–68) | 23.5 (14.2–40) |
| Parameter | Whole Population n = 232 (%) | CHIP n = 81 (%) | HBR n = 76 (%) |
|---|---|---|---|
| Lesion location | |||
| LM | 9 (3.9) | 8 (9.9) | 5 (6.6) |
| LAD | 72 (31) | 21 (25.9) | 31 (40.8) |
| LCx | 61 (26.3) | 16 (19.8) | 14 (8.4) |
| RCA | 90 (38.8) | 36 (44.4) | 29 (38.2) |
| VG | 6 (2.6) | 6 (7.4) | 2 (2.6) |
| Lesion type | |||
| A | 38 (16.4) | 12 (14.8) | 14 (18.4) |
| B1 | 66 (28.4) | 17 (21) | 24 (31.6) |
| B2 | 37 (15.9) | 9 (11.1) | 8 (10.5) |
| C | 91 (39.2) | 43 (53.1) | 30 (39.5) |
| Heavy calcification | 18 (7.8) | 9 (11.1) | 6 (7.9) |
| Coronary bifurcation | 23 (9.9) | 12 (14.8) | 6 (7.9) |
| SYNTAX | 13.9 ± 8.7 | 16.0 ± 8.4 | 14.8 ± 9.2 |
| SYNTAX II PCI | 32.9 ± 11.0 | 35.6 ± 10.1 | 40.3 ± 10.5 |
| SYNTAX II CABG | 29.1 ± 10.8 | 29.9 ± 10.5 | 28.4 ± 10.5 |
| EuroScore II | 1.6 (0.9–3.3) | 2.5 (1.3–4.3) | 3.6 (1.8–6.6) |
| Lesion predilatation | 143 (61.6) | 47 (58.0) | 48 (63.2) |
| Stent diameter [mm] | 3.2 ± 0.5 | 3.3 ± 0.5 | 3.1 ± 0.5 |
| Stent length [mm] | 21.2 ± 10.9 | 26.7 ± 14.3 | 20.3 ± 8.8 |
| Stent pressure [atm] | 15.3 ± 2.7 | 15.5 ± 2.6 | 15.4 ± 2.5 |
| 2nd stent | 90 (39) | 71 (87.7) | 34 (44.7) |
| Stent postdilatation | 88 (37.9) | 34 (42.0) | 54 (35.8) |
| Access site | |||
| Transradial | 193 (83.2) | 64 (79) | 56 (73.7) |
| Transfemoral | 43 (18.5) | 19 (23.5) | 20 (26.3) |
| Guiding catheter | |||
| 6F | 222 (95.7) | 76 (93.8) | 71 (93.4) |
| 7F | 11 (4.7) | 5 (6.2) | 5 (6.6) |
| Dissection | 16 (6.9) | 11 (13.6) | 3 (3.9) |
| MI type 4a | 5 (2.2) | 1 (1.2) | 1 (1.3) |
| Parameter | Whole Population n = 232 (%) | CHIP n = 81 (%) | HBR n = 76 (%) |
|---|---|---|---|
| Acetylsalicylic acid | 232 (100) | 81 (100) | 76 (100) |
| P2Y12 | |||
| Clopidogrel | 214 (92.2) | 74 (91.4) | 72 (94.7) |
| Prasugrel | 1 (0.4) | 1 (1.2) | 0 |
| Ticagrelor | 17 (7.3) | 6 (7.4) | 4 (5.3) |
| Beta-blocker | 223 (96.1) | 80 (98.8) | 73 (96.1) |
| Ca-blocker | 53 (22.8) | 21 (25.9) | 12 (15.8) |
| Angiotensin-converting enzyme inhibitor | 190 (81.9) | 66 (81.5) | 60 (78.9) |
| Angiotensin receptor blocker | 36 (15.5) | 14 (17.3) | 13 (17.1) |
| Diuretic | 125 (53.9) | 51 (63) | 61 (80.3) |
| Mineralocorticoid receptor antagonist | 48 (20.7) | 16 (19.8) | 20 (26.3) |
| Nitrates | 13 (5.6) | 8 (9.9) | 7 (9.2) |
| Vitamin K antagonist | 17 (7.3) | 4 (4.9) | 16 (21.1) |
| Novel oral anticoagulant | 11 (4.7) | 5 (6.1) | 10 (13.1) |
| Statin | 230 (99.1) | 81 (100) | 76 (100) |
| Hypoglycemic medications | 62 (26.7) | 29 (35.8) | 23 (30.3) |
| Insulin | 33 (14.2) | 20 (24.7) | 18 (23.7) |
| Year | Death | Cardiac Death | TLR | MI | MACE |
|---|---|---|---|---|---|
| Whole population (n = 232) | |||||
| 1st year | 17 (7.3) | 11 (4.7) | 18 (7.8) | 9 (3.9) | 27 (11.6) |
| 2nd year | 19 (8.2) | 13 (5.6) | 28 (12.1) | 11 (4.7) | 39 (16.8) |
| 3rd year | 21 (9.1) | 15 (6.5) | 31 (13.4) | 11 (4.7) | 44 (18.9) |
| 4th year | 25 (10.8) | 16 (6.9) | 34 (14.7) | 16 (6.9) | 54 (23.3) |
| CHIP (n = 81) | |||||
| 1st year | 9 (11.1) | 6 (7.4) | 10 (12.3) | 4 (4.9) | 14 (17.3) |
| 2nd year | 10 (12.3) | 7 (8.6) | 13 (16.1) | 4 (4.9) | 18 (22.2) |
| 3rd year | 11 (13.6) | 8 (9.9) | 14 (17.3) | 4 (4.9) | 20 (24.7) |
| 4th year | 12 (14.8) | 9 (11.1) | 15 (18.5) | 6 (7.4) | 24 (29.6) |
| HBR (n = 76) | |||||
| 1st year | 11 (14.5) | 9 (11.8) | 6 (7.9) | 5 (6.6) | 13 (17.1) |
| 2nd year | 13 (17.1) | 10 (13.2) | 8 (10.5) | 5 (6.6) | 16 (21.1) |
| 3rd year | 15 (19.7) | 12 (15.8) | 9 (11.8) | 5 (6.6) | 19 (25.0) |
| 4th year | 17 (22.4) | 13 (17.1) | 9 (11.8) | 6 (7.9) | 21 (27.6) |
| CHIP + HBR (n = 35) | |||||
| 1st year | 8 (22.9) | 6 (17.1) | 4 (11.4) | 3 (8.6) | 8 (22.9) |
| 2nd year | 10 (28.6) | 7 (20.0) | 4 (11.4) | 3 (8.6) | 9 (25.7) |
| 3rd year | 12 (34.3) | 8 (22.9) | 4 (11.4) | 3 (8.6) | 9 (25.7) |
| 4th year | 14 (40.0) | 9 (25.7) | 4 (11.4) | 4 (11.4) | 11 (31.4) |
| Characteristic | Multivariable Analysis for MACEs | ||
|---|---|---|---|
| HR | 95% CI | p | |
| Whole population (n = 232) | |||
| Lesion in left main | 3.88 | 1.42, 10.6 | 0.008 |
| Calcification | 2.70 | 1.17, 6.23 | 0.020 |
| Second stent | 2.06 | 1.11, 3.84 | 0.023 |
| EuroScore II | |||
| <3 | — | — | |
| 3–5 | 1.92 | 0.83, 4.42 | 0.125 |
| >5 | 2.87 | 1.32, 6.23 | 0.008 |
| Prior PCI | 2.09 | 1.03, 4.21 | 0.040 |
| CHIP (n = 81) | |||
| Prior CABG | 3.02 | 1.02, 8.92 | 0.045 |
| Chronic kidney disease | 5.07 | 1.44, 17.9 | 0.011 |
| Beta-blocker | 0.00 | 0.00, 0.06 | <0.001 |
| Diuretics | 3.02 | 0.80, 11.4 | 0.103 |
| HBR (n = 76) | |||
| EuroScore II | |||
| <3 | — | — | |
| 3–5 | 4.12 | 1.16, 14.6 | 0.028 |
| >5 | 2.19 | 0.67, 7.19 | 0.196 |
| Cardiogenic shock | 13.0 | 1.99, 85.4 | 0.007 |
| Smoking | 2.62 | 0.94, 7.26 | 0.065 |
| Hypoglycemic drugs | 5.30 | 1.90, 14.8 | 0.001 |
| Characteristic | Multivariable Analysis for TLR | ||
|---|---|---|---|
| HR | 95% CI | p | |
| Whole population (n = 232) | |||
| Lesion in left main | 14.9 | 3.95, 56.2 | <0.001 |
| Calcification | 3.07 | 1.12, 8.37 | 0.029 |
| Second stent | 4.09 | 1.72, 9.75 | 0.001 |
| CHIP (n = 81) | |||
| Prior CABG | 3.94 | 1.15, 13.5 | 0.029 |
| HBR (n = 76) | |||
| Male sex | 0.15 | 0.03, 0.71 | 0.017 |
| Postdilatation | 5.62 | 1.06, 29.9 | 0.043 |
| Smoking | 5.70 | 1.02, 31.7 | 0.047 |
| Alpha-adrenolytic | 5.22 | 1.04, 26.3 | 0.045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyczyński, M.; Kern, A.; Buller, P.; Wańha, W.; Gil, R.J.; Bil, J. Clinical Outcomes and Prognostic Factors in Complex, High-Risk Indicated Procedure (CHIP) and High-Bleeding-Risk (HBR) Patients Undergoing Percutaneous Coronary Intervention with Sirolimus-Eluting Stent Implantation: 4-Year Results. J. Clin. Med. 2023, 12, 5313. https://doi.org/10.3390/jcm12165313
Tyczyński M, Kern A, Buller P, Wańha W, Gil RJ, Bil J. Clinical Outcomes and Prognostic Factors in Complex, High-Risk Indicated Procedure (CHIP) and High-Bleeding-Risk (HBR) Patients Undergoing Percutaneous Coronary Intervention with Sirolimus-Eluting Stent Implantation: 4-Year Results. Journal of Clinical Medicine. 2023; 12(16):5313. https://doi.org/10.3390/jcm12165313
Chicago/Turabian StyleTyczyński, Maciej, Adam Kern, Patryk Buller, Wojciech Wańha, Robert J. Gil, and Jacek Bil. 2023. "Clinical Outcomes and Prognostic Factors in Complex, High-Risk Indicated Procedure (CHIP) and High-Bleeding-Risk (HBR) Patients Undergoing Percutaneous Coronary Intervention with Sirolimus-Eluting Stent Implantation: 4-Year Results" Journal of Clinical Medicine 12, no. 16: 5313. https://doi.org/10.3390/jcm12165313
APA StyleTyczyński, M., Kern, A., Buller, P., Wańha, W., Gil, R. J., & Bil, J. (2023). Clinical Outcomes and Prognostic Factors in Complex, High-Risk Indicated Procedure (CHIP) and High-Bleeding-Risk (HBR) Patients Undergoing Percutaneous Coronary Intervention with Sirolimus-Eluting Stent Implantation: 4-Year Results. Journal of Clinical Medicine, 12(16), 5313. https://doi.org/10.3390/jcm12165313