The Effectiveness and Safety of Autologous Platelet Concentrates as Hemostatic Agents after Tooth Extraction in Patients on Anticoagulant Therapy: A Systematic Review of Randomized, Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search and Review Selection
2.2. Data Extraction
2.3. Risk of Bias Assessment
- (A) Low risk of bias (plausible bias unlikely to seriously alter the results) if all the criteria were met.
- (B) Unclear risk of bias (plausible bias that raises some doubt about the results) if one or more key domains had an unclear risk of bias.
- (C) High risk of bias (plausible bias that seriously weakens confidence in the results) if one or more criteria were not met.
2.4. Assessing the Certainty of the Evidence
3. Results
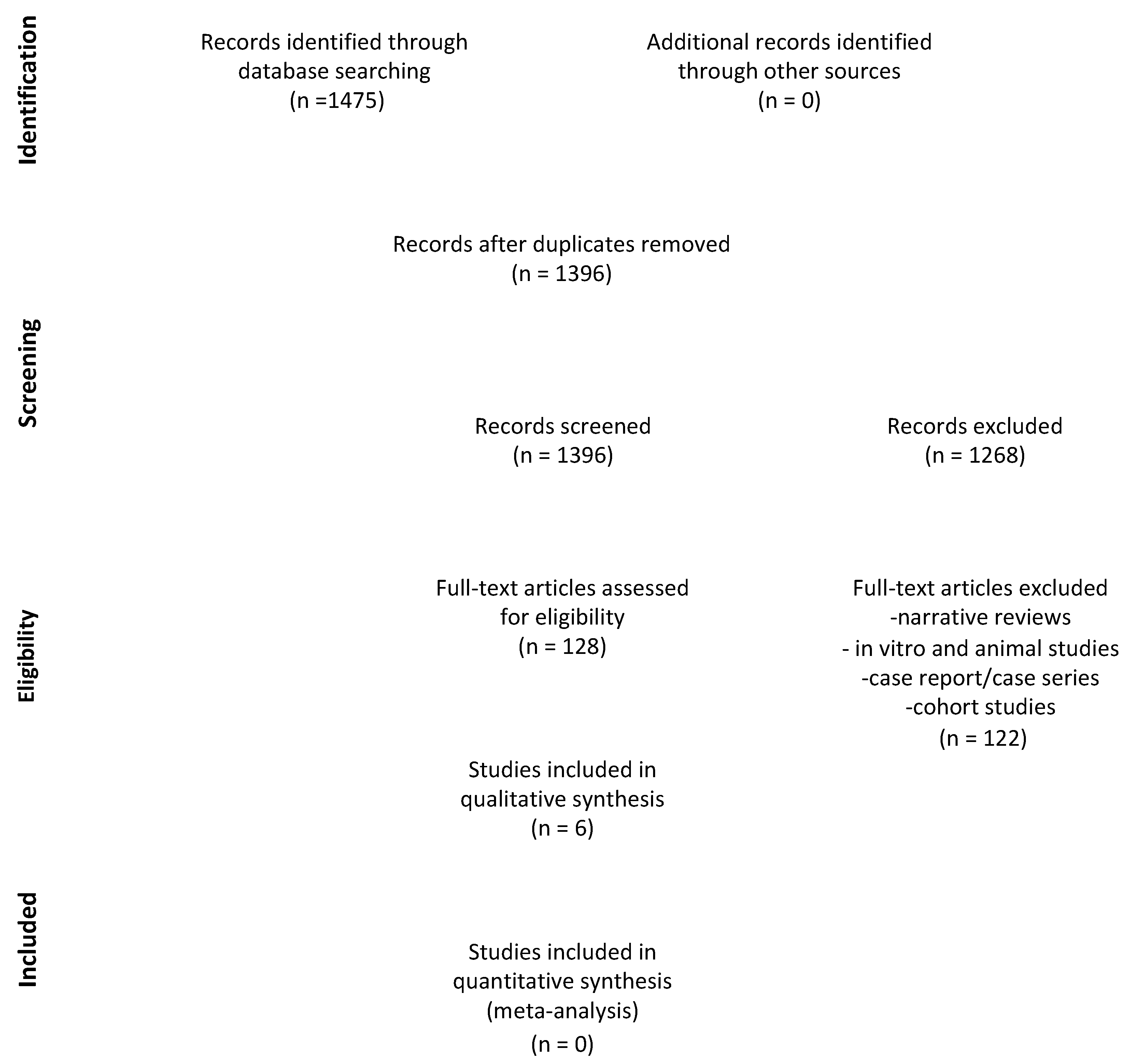
3.1. Search Results
3.2. Characteristics of the Studies Included
3.3. Summary of Clinical Findings
3.3.1. Time to Hemostasis
3.3.2. Post-Operative Bleeding
3.3.3. Pain Score and Wound Healing
3.4. Risk of Bias of the Included Studies
3.5. Certainty of the Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katzung, B.G. Basic & Klinical Pharmacology, 15th ed.; McGraw Hill/Medical: London, UK, 2017; pp. 826–833. [Google Scholar]
- Curto, A.; Albaladejo, A. Implications of apixaban for dental treatments. J. Clin. Exp. Dent. 2016, 8, e611–e614. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Mohan, B.; Babbar, V.; Dang, N. Practices and Perceptions of Doctors for Patients on Anti-platelets during Dental Surgery: A National Survey. J. Maxillofac. Oral Surg. 2014, 13, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.W.; Tan, S.H.X.; Teo, G.N.; Tan, T.S.; Ng, W.H. Should we stop dual anti-platelet therapy for dental extractions? An umbrella review for this dental dilemma. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e708–e716. [Google Scholar] [CrossRef]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.-C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS The Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC Endorsed by the European Stroke Organisation (ESO). Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K Antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef]
- Li, L.; Zhang, W.; Yang, Y.; Zhao, L.; Zhou, X.; Zhang, J. Dental management of patient with dual antiplatelet therapy: A meta-analysis. Clin. Oral Investig. 2019, 23, 1615–1623. [Google Scholar] [CrossRef]
- Miclotte, I.; Vanhaverbeke, M.; Agbaje, J.O.; Legrand, P.; Vanassche, T.; Verhamme, P.; Politis, C. Pragmatic approach to manage new oral anticoagulants in patients undergoing dental extractions: A prospective case-control study. Clin. Oral Investig. 2017, 21, 2183–2188. [Google Scholar] [CrossRef] [PubMed]
- Sammartino, G.; Ehrenfest, D.M.D.; Carile, F.; Tia, M.; Bucci, P. Prevention of Hemorrhagic Complications After Dental Extractions Into Open Heart Surgery Patients Under Anticoagulant Therapy: The Use of Leukocyte- and Platelet-Rich Fibrin. J. Oral Implant. 2011, 37, 681–690. [Google Scholar] [CrossRef]
- Caliskan, M.; Tukel, H.C.; Benlidavi, M.; Deniz, A. Is it necessary to alter anticoagulation therapy for tooth extraction in patients taking direct oral anticoagulants? Med. Oral Patol. Oral Cir. Bucal 2017, 22, e767. [Google Scholar] [CrossRef]
- Salam, S.; Yusuf, H.; Milosevic, A. Bleeding after dental extractions in patients taking warfarin. Br. J. Oral Maxillofac. Surg. 2007, 45, 463–466. [Google Scholar] [CrossRef]
- Khalil, H.; Abdullah, W.A. Dental extraction in patients on warfarin treatment. Clin. Cosmet. Investig. Dent. 2014, 6, 65–69. [Google Scholar] [CrossRef]
- Morimoto, Y.; Niwa, H.; Minematsu, K. Hemostatic Management of Tooth Extractions in Patients on Oral Antithrombotic Therapy. J. Oral Maxillofac. Surg. 2008, 66, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.W.G.; Rodrigues, R.R.; de Sousa, L.H.T.; Carvalho, F.S.R.; Chaves, F.N.; Fernandes, C.P.; Pereira, K.M.A.; Soares, E.C.S. Local hemostatic measures in anticoagulated patients undergoing oral surgery: A systematized literature review. Acta Cir. Bras. 2013, 28, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Kwak, E.-J.; Nam, S.; Park, K.-M.; Kim, S.-Y.; Huh, J.; Park, W. Bleeding related to dental treatment in patients taking novel oral anticoagulants (NOACs): A retrospective study. Clin. Oral Investig. 2019, 23, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.; Goss, A.; Lloyd, J.; Tocchetti, R. Tranexamic acid mouthwash versus autologous fibrin glue in patients takingwarfarin undergoing dental extractions: A randomized prospective clinical study. J. Oral Maxillofac. Surg. 2003, 61, 1432–1435. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Sinjari, B.; Murmura, G.; Mijiritsky, E.; Iaculli, F.; Mortellaro, C.; Tetè, S. Hemostasis control in dental extractions in patients receiving oral anticoagulant therapy: An approach with calcium sulfate. J. Craniofac. Surg. 2014, 25, 843–846. [Google Scholar] [CrossRef]
- Soares, E.C.S.; Costa, F.W.G.; Bezerra, T.P.; Nogueira, C.B.P.; Silva, P.G.D.B.; Batista, S.H.B.; Sousa, F.B.; Fonteles, C.S.R. Postoperative hemostatic efficacy of gauze soaked in tranexamic acid, fibrin sponge, and dry gauze compression following dental extractions in anticoagulated patients with cardiovascular disease: A prospective, randomized study. J. Oral Maxillofac. Surg. 2015, 19, 209–216. [Google Scholar] [CrossRef]
- Bodner, L.; Weinstein, J.M.; Baumgarten, A.K. Efficacy of fibrin sealant in patients on various levels of oral anticoagulant undergoing oral surgery. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1998, 86, 421–424. [Google Scholar] [CrossRef]
- Al-Belasy, F.A.; Amer, M.Z. Hemostatic effect of n-butyl-2-cyanoacrylate (histoacryl) glue in warfarin-treated patients undergoing oral surgery. J. Oral Maxillofac. Surg. 2003, 61, 1405–1409. [Google Scholar] [CrossRef]
- Marjanovic, M. Use of thrombin powder after tooth extraction in patients receiving anticoagulant therapy. Vojnosanit. Pregl. 2002, 59, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Souto, J.; Oliver, A.; Zuazu-Jausoro, I.; Vives, A.; Fontcuberta, J. Oral surgery in anticoagulated patients without reducing the dose of oral anticoagulant: A prospective randomized study. J. Oral Maxillofac. Surg. 1996, 54, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Sammartino, G.; Marenzi, G.; Miro, A.; Ungaro, F.; Nappi, A.; Sammartino, J.C.; Quaglia, F.; Mortellaro, C. Local Delivery of the Hemostatic Agent Tranexamic Acid in Chronically Anticoagulated Patients. J. Craniofacial Surg. 2012, 23, e648–e652. [Google Scholar] [CrossRef] [PubMed]
- Blinder, D.; Manor, Y.; Martinowitz, U.; Taicher, S.; Hashomer, T. Dental extractions in patients maintained on continued oral anticoagulant: Comparison of local hemostatic modalities. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1999, 88, 137–140. [Google Scholar]
- Bajkin, B.V.; Selaković, S.D.; Mirković, S.M.; Šarčev, I.N.; Tadić, A.J.; Milekić, B.R. Comparison of efficacy of local hemostatic modalities in anticoagulated patients undergoing tooth extractions. Vojn. Pregl. 2014, 71, 1097–1101. [Google Scholar] [CrossRef]
- Ockerman, A.; Miclotte, I.; Vanhaverbeke, M.; Verhamme, P.; Poortmans, L.-L.; Vanassche, T.; Politis, C.; Jacobs, R. Local haemostatic measures after tooth removal in patients on antithrombotic therapy: A systematic review. Clin. Oral Investig. 2019, 23, 1695–1708. [Google Scholar] [CrossRef] [PubMed]
- Lekovic, V.; Camargo, P.M.; Weinlaender, M.; Vasilic, N.; Kenney, E.B. Comparison of platelet rich plasma, bovine porous bone mineral, and guided tissue regeneration versus platelet rich plasma and bovine porous bone mineral in the treatment of intrabony defects: A reentry study. J. Periodontal Res. 2002, 73, 198–205. [Google Scholar] [CrossRef]
- Del Corso, M.; Vervelle, A.; Simonpieri, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.; Dohan Ehrenfest, D.M. Current Knowledge and Perspectives for the Use of Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF) in Oral and Maxillofacial Surgery Part 1: Periodontal and Dentoalveolar Surgery. Curr. Pharm. Biotechnol. 2012, 13, 1207–1230. [Google Scholar] [CrossRef]
- Anitua, E. A New Approach to Bone Regeneration, Plasma Rich in Growth Factors; Puesta al Dia Publicaciones S.L.: Madrid, Spain, 2001. [Google Scholar]
- Dohan Ehrenfest, D.M.; de Peppo, G.M.; Doglioli, P.; Sammartino, G. Slow release of growth factors and thrombospondin-1 in Choukroun’s platelet-rich fibrin (PRF): A gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors 2009, 27, 63–69. [Google Scholar] [CrossRef]
- Gasparro, R.; Qorri, E.; Valletta, A.; Masucci, M.; Sammartino, P.; Amato, A.; Marenzi, G. Non-Transfusional Hemocomponents: From Biology to the Clinic—A Literature Review. Bioengineering 2018, 5, 27. [Google Scholar] [CrossRef]
- D’Esposito, V.; Passaretti, F.; Perruolo, G.; Ambrosio, M.R.; Valentino, R.; Oriente, F.; Raciti, G.A.; Nigro, C.; Miele, C.; Sammartino, G.; et al. Platelet-Rich Plasma Increases Growth and Motility of Adipose Tissue-Derived Mesenchymal Stem Cells and Controls Adipocyte Secretory Function. J. Cell. Biochem. 2015, 116, 2408–2418. [Google Scholar] [CrossRef] [PubMed]
- Cabaro, S.; D’esposito, V.; Gasparro, R.; Borriello, F.; Granata, F.; Mosca, G.; Passaretti, F.; Sammartino, J.C.; Beguinot, F.; Sammartino, G.; et al. White cell and platelet content affects the release of bioactive factors in different blood-derived scaffolds. Platelets 2018, 29, 463–467. [Google Scholar] [CrossRef]
- Marenzi, G.; Riccitiello, F.; Tia, M.; di Lauro, A.; Sammartino, G. Influence of Leukocyte- and Platelet-Rich Fibrin (L-PRF) in the Healing of Simple Postextraction Sockets: A Split-Mouth Study. BioMed Res. Int. 2015, 2015, 369273. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.-L.; et al. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin. Oral Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Miron, R.J.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Choukroun, J.; Ghanaati, S.; Ehrenfest, D.M.D.; Piattelli, A.; Sammartino, G.; et al. Optimized Platelet-Rich Fibrin with the Low-Speed Concept: Growth Factor Release, Biocompatibility, and Cellular Response. J. Periodontol. 2017, 88, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Farina, R.; Trombelli, L. Wound Healing of Extraction Sockets. In Oral Wound Healing: Cell Biology and Clinical Management; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 195–228. [Google Scholar]
- Gasparro, R.; Sammartino, G.; Mariniello, M.; di Lauro, A.E.; Spagnuolo, G.; Marenzi, G.; Elbourne, D.; Egger, M.; Altman, D.G. Treatment of periodontal pockets at the distal aspect of mandibular second molar after surgical removal of impacted third molar and application of L-PRF: A split-mouth randomized clinical trial. Quintessence Int. 2020, 51, 204–211. [Google Scholar]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Brancaccio, Y.; Antonelli, A.; Barone, S.; Bennardo, F.; Fortunato, L.; Giudice, A. Evaluation of local hemostatic efficacy after dental extractions in patients taking antiplatelet drugs: A randomized clinical trial. Clin. Oral Investig. 2021, 25, 1159–1167. [Google Scholar] [CrossRef]
- Eldibany, R. Platelet rich fibrin versus Hemcon dental dressing following dental extraction in patients under anticoagulant therapy. Tanta Dent. J. 2014, 11, 75–84. [Google Scholar] [CrossRef]
- Giudice, A.; Esposito, M.; Bennardo, F.; Brancaccio, Y.; Buti, J.; Fortunato, L. Dental extractions for patients on oral antiplatelet: A within-person randomised controlled trial comparing haemostatic plugs, advanced-platelet-rich fibrin (A-PRF+) plugs, leukocyte- and platelet-rich fibrin (L-PRF) plugs and suturing alone. Int. J. Oral Implantol. 2019, 12, 77–87. [Google Scholar]
- Giuffrè, G.; Caputo, G.; Misso, S.; Peluso, F. Platelet-rich plasma treatment and hemostasis in patients with hemorrhagic risk. Minerva Stomatol. 2006, 55, 599–609. [Google Scholar] [PubMed]
- Sarkar, S.; Prashanth, N.; Shobha, E.; Rangan, V.; Nikhila, G. Efficacy of Platelet Rich Fibrin versus chitosan as a hemostatic agent following dental extraction in patients on antiplatelet therapy. J. Oral Biol. Craniofacial Res. 2019, 9, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Rajendra, K.; Vempalli, S.; Kadiyala, M.; Karipineni, S.; Gunturu, S.; Patil, D.B. Effect of platelet-rich fibrin versus chitosan-based Axiostat hemostatic agent following dental extraction in cardiac patients on antiplatelet therapy: A comparative study. Natl. J. Maxillofac. Surg. 2021, 12, 361–366. [Google Scholar] [CrossRef]
- LaPelusa, A.; Dave, H.D. Physiology, Hemostasis; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Sammartino, G.; Gasparro, R.; Spagnuolo, G.; Miniello, A.; Blasi, A.; Marenzi, G. Influence of the Antithrombotic Therapy in the Healing of Simple Post-Extraction Sockets: A Randomized Clinical Trial. J. Clin. Med. 2022, 11, 3654. [Google Scholar] [CrossRef]
- Weisel, J.W. Structure of fibrin: Impact on clot stability. J. Thromb. Haemost. 2007, 5 (Suppl. S1), 116–124. [Google Scholar] [CrossRef]
- Patel, S.; Singh, R.; Preuss, C.V.; Patel, N. StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Jupalli, A.; Iqbal, A.M. Enoxaparin; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Pollack, R.P.; Bouwsma, O.J. Applications of oxidized regenerated cellulose in periodontal therapy: Part 1. Compendium 1992, 13, 888. [Google Scholar]
- Vezeau, P.J. Dental extraction wound management: Medicating postextraction sockets. J. Oral Maxillofac. Surg. 2000, 58, 531–537. [Google Scholar] [CrossRef]
- Garcia-Blanco, M.; Puia, S.A.; Hilber, E.M. Randomized clinical trial comparing three local hemostatic agents for dental extractions in patients under chronic anticoagulant therapy–A comparative study. Ann. Maxillofac. Surg. 2020, 10, 292–296. [Google Scholar] [CrossRef]
- Martinez, M.; Tsakiris, D.A. Management of Antithrombotic Agents in Oral Surgery Maria Martinez and Dimitrios A. Tsakiris. Dent. J. 2015, 3, 93–101. [Google Scholar] [CrossRef]
- Borie, E.; Rosas, E.; Kuramochi, G.; Etcheberry, S.; Olate, S.; Weber, B. Oral Applications of Cyanoacrylate Adhesives: A Literature Review. BioMed Res. Int. 2019, 2019, 8217602. [Google Scholar] [CrossRef]
- Ali, T.; Keenan, J.; Mason, J.; Hseih, J.-T.; Batstone, M. Prospective study examining the use of thrombin–gelatin matrix (Floseal) to prevent post dental extraction haemorrhage in patients with inherited bleeding disorders. Int. J. Oral Maxillofac. Surg. 2022, 51, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Rattan, V. Efficacy of Feracrylum as Topical Hemostatic Agent in Therapeutically Anticoagulated Patients Undergoing Dental Extraction: A Comparative Study. J. Maxillofac. Oral Surg. 2019, 18, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Zaib, A.; Shaheryar, M.; Shakil, M.; Sarfraz, A.; Sarfraz, Z.; Cherrez-Ojeda, I. Local Tranexamic Acid for Preventing Hemorrhage in Anticoagulated Patients Undergoing Dental and Minor Oral Procedures: A Systematic Review and Meta-Analysis. Healthcare 2022, 10, 2523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hui, D.; Du, C.; Sun, H.; Peng, W.; Pu, X.; Li, Z.; Sun, J.; Zhou, C. Preparation and application of chitosan biomaterials in dentistry. Int. J. Biol. Macromol. 2021, 167, 1198–1210. [Google Scholar] [CrossRef]
- Passaretti, F.; Tia, M.; D’esposito, V.; De Pascale, M.; Del Corso, M.; Sepulveres, R.; Liguoro, D.; Valentino, R.; Beguinot, F.; Formisano, P.; et al. Growth-promoting action and growth factor release by different platelet derivatives. Platelets 2014, 25, 252–256. [Google Scholar] [CrossRef]
- Perry, D.J.; Noakes, T.J.C.; Helliwell, P.S. Guidelines for the management of patients on oral anticoagulants requiring dental surgery. Br. Dent. J. 2007, 203, 389–393. [Google Scholar] [CrossRef]
- Dinkova, A.S.; Atanasov, D.T.; Vladimirova-Kitova, L.G. Discontinuation of Oral Antiplatelet Agents before Dental Extraction—Necessity or Myth? Folia Med. 2017, 59, 336–343. [Google Scholar] [CrossRef]
- Lewandowski, B.; Myszka, A.; Migut, M.; Czenczek-Lewandowska, E.; Brodowski, R. Analysing the effectiveness of topical bleeding care following tooth extraction in patients receiving dual antiplatelet therapy-retrospective observational study. BMC Oral Health 2021, 21, 31. [Google Scholar] [CrossRef]
- Iwata, E.; Tachibana, A.; Kusumoto, J.; Hasegawa, T.; Kadoya, R.; Enomoto, Y.; Takata, N.; Akashi, M. Risk factors associated with post-extraction bleeding in patients on warfarin or direct-acting oral anticoagulants: A retrospective cohort study. Oral Maxillofac. Surg. 2022, 26, 641–648. [Google Scholar] [CrossRef]
- Cryer, P.E.; Haymond, M.W.; Santiago, J.V.; Shah, S.D. Norepinephrine and Epinephrine Release and Adrenergic Mediation of Smoking-Associated Hemodynamic and Metabolic Events. N. Engl. J. Med. 1976, 295, 573–577. [Google Scholar] [CrossRef] [PubMed]


| Pubmed | (“tooth extraction”[MeSH Terms] OR “dental extraction*”[All Fields] OR “dental avulsion”[All Fields] OR “hemorrhagic risk” [Title]) AND (“platelet-rich plasma”[Title/Abstract] OR “platelet-rich fibrin”[Title/Abstract] OR “PRF”[Title/Abstract] OR “PRP”[Title/Abstract] OR “antiplatelet therapy”[Title/Abstract] OR “anticoagulant therapy”[Title/Abstract] OR “antiplatelet drugs”[Title/Abstract] OR “platelet rich in growth factors”[Title/Abstract] OR “anticoagulant drugs”[Title/Abstract] OR “autologous platelet concentrates”[Title/Abstract] OR “hemocomponents”[Title/Abstract] OR “concentrated growth factors”[Title/Abstract] OR “CGF”[Title/Abstract] OR “hemostasis”[Title/Abstract]) |
| Scopus | (TITLE-ABS-KEY (tooth extraction*) OR TITLE-ABS-KEY (dental extraction*)) AND (TITLE-ABS-KEY (“ platelet-rich plasma “) OR TITLE-ABS-KEY (“platelet-rich fibrin”) OR TITLE-ABS-KEY (“PRP”) OR TITLE-ABS-KEY (“PRF”) OR TITLE-ABS-KEY (“antiplatelet therapy”) OR TITLE-ABS (“anticoagulant therapy”) OR TITLE-ABS-KEY (“ autologous platelet concentrates “) OR TITLE-ABS-KEY (“anticoagulant drugs”) OR TITLE-ABS-KEY (“antiplatelet drugs”) OR TITLE-ABS-KEY (“ concentrated growth factors”) OR TITLE-ABS-KEY (“ CGF “) OR TITLE-ABS-KEY (“ hemocomponents “) OR TITLE-ABS-KEY (hemostasis)) |
| CENTRAL | (hemostasis in tooth extraction) |
| Author, Year of Publication | Country | Number of Patients | Age (Mean) Range) | Gender (M, F) | Patients’ Characteristics | Intervention | APC Specifications | Control | Follow-Up | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Brancaccio, 2020 [42] | Magna Graecia University of Catanzaro, Italy | 102 patients divided into 4 groups: Control group (suturing alone) and 3 different hemostatic agents—HEM group, L-PRF group and A-PRF group (test groups). | 64.1 ± 17.4 | 74; 28 | Patients undergoing long-term treatment with oral antiplatelets: acetylsalicylic acid 100 mg (72 patients), Clopidogrel 75 mg (15 patients), acetylsalicylic acid 100 mg + clopidogrel 75 mg (6 patients), Ticagrelor 90 mg (5 patients) and ticagrelor 90 mg + acetylsalicylic acid 100 mg (4 patients). | All patients were subjected to 4 dental extractions of non-adjacent teeth at the same time and to the application of different hemostatic agents (A-PRF+, L-PRF, HEM). | For A-PRF+, two test tubes were used, corresponding to 18 mL of venous blood, which were centrifuged at 1300 rpm for 8 min in the DUO PRF centrifuge. For L-PRF, two test tubes were used, corresponding to 12 mL of blood, which were centrifuged at 2700 rpm for 18 min using the IntraSpin centrifuge. | Natural healing using suturing alone | 30 min (frequency of post-operative bleeding), 2 weeks (healing of the surgical wound after the extraction) | Post-operative bleeding (Souto classification), any possible complications and wound healing |
| Eldibany R.M. et al., 2014 [43] | Alexandria University, Egipt | 20 patients divided into 2 groups: group A (test group = 10 patients) where PRF was inserted into the extraction socket and group B (control group: 10 patients) where the extraction socket was packed by HDD. | 46.65; 36–62 | 11; 9 | All patients were cardiac patients and had undergone heart valve replacement and were currently taking warfarin. | All patients required extraction of a single mandibular posterior tooth. The extractions were performed for all patients without altering the dose of the anticoagulant and in the test group the extraction sockets were packed with PRF. | For the PRF preparation, 20 mL of blood were collected without any anticoagulant from the brachial vein 12 min before the extraction. The blood was transferred and equally divided into two 10 mL sterile glass tubes and was immediately centrifuged using a table centrifuge at 3000 rpm for 12 min. | HemCon dental dressing (HDD) 10 mm × 12 mm, a hemostatic agent. | 1–7 days | Duration of bleeding and pain (VAS) |
| Giudice A. et al., 2019 [44] | Magna Graecia University of Catanzaro, Italy | 40 patients equally divided into 4 groups: suturing alone (control group = 10 patients) and suturing + hemostatic plug HEM (10 patients) suturing + A-PRF+ (10 patients), suturing + L-PRF into the socket (10 patients) as test groups. | 60.9; 18–88 | 28;12 | Patients undergoing oral antiplatelet therapy (type of drug not specified). | For all patients requiring the extraction of at least four non-adjacent teeth (incisors, canines, premolars, and molars). After the extractions, hemostatic plugs (HEM) were put in the sockets in the. A-PRF+ and L-PRF (test groups) without reducing the dose of oral antiplatelets. | For the A-PRF+, two tubes, corresponding to 18 mL of blood, were sampled and centrifuged immediately at 1300 rpm for 8 min using the PRF DUO centrifuge. For the L-PRF, two tubes corresponding to 12 mL of blood were sampled and centrifuged immediately for 18 min at 2700 rpm using the IntraSpin centrifuge. | Natural healing using suturing alone | 30 min for the frequency of post-operative bleeding and 1 and 2 weeks post-extraction for the wound healing | Complications, time to complete each procedure, post-operative bleeding, costs of the materials, patient preference and wound healing index recorded 1 and 2 weeks post-extraction by blinded assessors |
| Giuffrè G. et al., 2006 [45] | St. Sebastian Hospital, Caserta, Italy | 208 patients divided into 4 groups consisting of 52 patients each (A,B,C,D). | 64.5; 51–68 | 84; 124 | All patients were undergoing anticoagulant therapy (warfarin). The patients belonging to the first 3 groups (A, B, C), had undergone extractions without discontinuing the therapy. In group A (test group) PRP + suturing were used; in group B PRP, hemostatic sponges and suturing were used; in group C hemostatic sponges, suturing and compression by means of gauzes soaked in tranexamic acid were used; group D consisted of patients where dicumarol therapy had been suspended and replaced by heparincalcium. | All patients underwent oral surgical operations: simple tooth extractions, root extraction, and cystectomies. In group A (test group), the socket was filled with PRP only and sutured. | For the PRP preparation the blood (70 mL ± 30) was centrifuged at 180° for 10 min. so that plasma, which is full of platelets, was separated from the thick red corpuscle layer. The PRP obtained was then further centrifuged at 1800° for 10 min in order to obtain hyperconcentrated platelets. | 1: PRP + hemostatic sponges; 2: hemostatic sponges and local tranexamic acid; 3: therapy modification | 3, 12, 24, 48 h | Post-operative bleeding |
| Rajendra K. et al., 2021 [47] | University of Ambala, Haryana, India. | 300 patients divided into two categories (n = 150, respectively) as Group I (PRF dressing—test group) and Group II (Axiostat dressing). | 52.5; 35–70 | not specified | All patients had a medical history of cardiac disease and were on anti-platelet drug therapy (type of drug not specified). | Extraction of single tooth filled with PRF (test group). | PRF membrane was placed after preparing it in clean and sterile glass test tubes using centrifugation at 3000 rpm for 10 min. | Axiostat (chitosan) | 7 days post-operative | Duration of bleeding and pain |
| Sarkar S, 2019 [46] | University of Bangalore, Karnataka, India | 60 patients allocated equally in two groups: group A (test group), where PRF gel was packed into the extraction socket, while in group B (control group), chitosan hydrogel was packed. | 58.77; 35–82 | 29; 31 | Patients undergoing oral antiplatelet therapy (type of drug not specified). | For all patients requiring the extraction of a single tooth, after the extractions PRF was put in the sockets (test group). | PRF gel was prepared in patients of Group A f(test group) with 5 mL of blood that was drawn from the brachial vein of each patient and was centrifuged at 3000 rpm for 10 min. | Chitosan | 1, 3, 7 days | Time to hemostasis, pain score, alveolar osteitis, secondary hemorrhage and healing |
| Author, Year of Publication | Results | Conclusion |
|---|---|---|
| Brancaccio, 2020 [42] | Bleeding sites were in numbers of 20, 12, 2, and 5 for the suture-only group, for the HEM group, for the A-PRF+ group, and for the L-PRF group, respectively. Instead, the sites not completely healed 2 weeks after the extraction were in numbers of 31, 39, 22, and 15 for the suture-only group, for the HEM group, for the A-PRF+ group, and for the L-PRF group, respectively. | L-PRF and A-PRF represent a valid alternative to the traditional hemostatic treatment, reducing post-surgical bleeding and promoting wound healing. |
| Eldibany R.M. et al., 2014 [43] | Regarding the time to hemostasis in the test group the mean time was 47.6 ± 1.3 s while in the control group the mean time was 51.3 ± 2.1 s. Regarding the pain in the test group there was no notable post-surgical pain (VAS average 2, ranging between 1 and 3) on the 2nd post-extraction day and 0 on the following days. Instead, in the control group, four cases reported severe pain (VAS = 8) in the first 48 h following the extraction, while two cases showed post-operative pain of VAS 5 on the first two post-extraction days. | PRF has good anti-hemorrhagic properties and increases tissue healing and wound closure, thus allowing for a quick recovery without significant painful events. |
| Giudice A. et al., 2019 [44] | Two weeks after the extraction, no patient dropped out and no complication was reported. The average time to complete suturing after tooth extractions was: 1.0 ± 0.00 min at the control sites, 1.5 ± 0.41 at the HEM sites, 2.8 ± 0.61 at the A-PRF+ sites, and 2.8 ± 0.56 at the L-PRF sites. Post-operative bleeding 30 min after the extraction was present at 8, 5, 1 and 2 sites for the control, HEM, A-PRF+ and L-PRF sites, respectively. One week after the extraction the mean wound healing index was 1.05 ± 0.60 for the control, 1.18 ± 0.59 for the HEM, 1.00 ± 0.68, A-for the PRF+ and 0.95 ± 0.50 for the L-PRF sites. Two weeks after the extraction the mean wound healing index was 0.33 ± 0.53 for the control, 0.43 ± 0.50 for the HEM, 0.25 ± 0.49 for the A-PRF+ and 0.15 ± 0.36 for the L-PRF sites. One week after the extraction, nine patients preferred control sites, eight HEM, ten A-PRF+ four L-PRF and nine had no preference. Costs without counting sutures and blood centrifuges were 0.00, 14.49, 2.44 and 2.44 euros for the control, HEM, A-PRF+ and L-PRF sites, respectively. | A-PRF+ was associated with less post-operative bleeding when compared to suturing alone. |
| Giuffrè G. et al., 2006 [45] | The number of hemorrhages in the test group (group A) was 0 at 3 and 12 h, 2 at 24 h and 0 at 48 h. Patients belonging to the groups A and B showed a very good hemostasis compared to the patients of group D. As for group C (19 males), 6 patients (i.e., 11.5%) showed a good hemostasis, both immediately and in the long term, so that they could be discharged on day 2 after surgery. | The results strongly encourage using PRP regularly when carrying out surgical treatments on patients who are undergoing a coumarin therapy. |
| Rajendra K. et al., 2021 [47] | The average pain score was 1.86 ± 0.06 in Group I (test group) and 1.05 ± 0.87 in Group II (control group). Thus, a lower post-operative pain was seen with Axiostat dressing. Hemostasis was achieved in Group II participants in 1.25 ± 0.06 min and in Group I patients 1.89 ± 0.54 min. | Chitosan is a superior wound dressing material in achieving hemostasis in cardiac patients on antiplatelet medication after tooth extraction. |
| Sarkar S, 2019 [46] | All the extraction sockets with Platelet-rich fibrin achieved hemostasis in 2.64 min and sockets with Chitosan hydrogel achieved hemostasis in 1.182 min (p < 0.001). Post-operative pain in Group A sites (3.2, 1.4, 0.37 on 1st, 3rd & 7th day respectively) was significantly lower than the control sites (3.4, 1.67, 0.53 on 1st, 3rd & 7th day respectively). A total of 4 patients, 2 in each group, reported alveolar osteitis on the 7th day after extraction. There was a statistically significant (p < 0.001) better healing at the Group A extraction sites. 80% of the patients of Group A showed a healthy healing (score of 5) of the extraction sockets based on the custom-made evaluation chart by 7th post-operative day. | Chitosan hydrogel dressing thus proved to be a superior hemostatic agent compared to PRF gel, significantly shortening the clotting time following dental extraction in patients under antiplatelet therapy. However, PRF gel has superior wound healing properties compared to Chitosan with less postoperative pain following minor oral surgical procedures under local anesthesia. |
| Patients or population: 1 Patients undergoing anticoagulant therapy. Settings: 1 Dental Clinic of the University “Magna Graecia” of Catanzaro, San Sebastiano Hospital in Caserta; University of Ambala, Haryana; University of Bangalore, Karnataka; Alexandria University in Egypt. Intervention: 1 Use of autologous platelet concentrates (APCs) to gain hemostasis. Comparison: 1 Other hemostatic agents or physiological healing. | |||
| Outcomes 2 | Impact 3 | Number of participants(Studies) 4 | Certainty of the evidence (GRADE) * 5 |
| Time to hemostasis | APCs had a shorter time to achieve hemostasis (mean 1.18 min) than the control group (mean 2.64 min). | 60 (1 study) | ⊕⊕⊖⊖ Low |
| Post-operative bleeding | APCs provided for a significantly reduction of the bleeding risk compared to the control group. | 102 (1 study) | ⊕⊕⊕⊖ Moderate |
| Pain score | APCs seem to be a great method to reduce post-operative infection and pain. | 442 (3 studies) | ⊕⊕⊕⊖ Moderate |
| Wound healing | APCs provided for a better and faster wound healing. | 142 (2 studies) | ⊕⊕⊕⊖ Moderate |
| * GRADE Working Group grades of evidence: High = This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different † is low. Moderate = This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different † is moderate. Low = This research provides some indication of the likely effect. However, the likelihood that it will be substantially different † is high. Very low = This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different † is very high. † Substantially different = a large enough difference that it might affect a decision. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campana, M.D.; Aliberti, A.; Acerra, A.; Sammartino, P.; Dolce, P.; Sammartino, G.; Gasparro, R. The Effectiveness and Safety of Autologous Platelet Concentrates as Hemostatic Agents after Tooth Extraction in Patients on Anticoagulant Therapy: A Systematic Review of Randomized, Controlled Trials. J. Clin. Med. 2023, 12, 5342. https://doi.org/10.3390/jcm12165342
Campana MD, Aliberti A, Acerra A, Sammartino P, Dolce P, Sammartino G, Gasparro R. The Effectiveness and Safety of Autologous Platelet Concentrates as Hemostatic Agents after Tooth Extraction in Patients on Anticoagulant Therapy: A Systematic Review of Randomized, Controlled Trials. Journal of Clinical Medicine. 2023; 12(16):5342. https://doi.org/10.3390/jcm12165342
Chicago/Turabian StyleCampana, Maria Domenica, Angelo Aliberti, Alfonso Acerra, Pasquale Sammartino, Pasquale Dolce, Gilberto Sammartino, and Roberta Gasparro. 2023. "The Effectiveness and Safety of Autologous Platelet Concentrates as Hemostatic Agents after Tooth Extraction in Patients on Anticoagulant Therapy: A Systematic Review of Randomized, Controlled Trials" Journal of Clinical Medicine 12, no. 16: 5342. https://doi.org/10.3390/jcm12165342
APA StyleCampana, M. D., Aliberti, A., Acerra, A., Sammartino, P., Dolce, P., Sammartino, G., & Gasparro, R. (2023). The Effectiveness and Safety of Autologous Platelet Concentrates as Hemostatic Agents after Tooth Extraction in Patients on Anticoagulant Therapy: A Systematic Review of Randomized, Controlled Trials. Journal of Clinical Medicine, 12(16), 5342. https://doi.org/10.3390/jcm12165342









