Femoral or Axillary Cannulation for Extracorporeal Circulation during Minimally Invasive Heart Valve Surgery (FAMI): Protocol for a Multi-Center Prospective Randomized Trial
Abstract
:1. Introduction
1.1. Background
1.2. Objectives
1.3. Trial Design
1.4. Methods
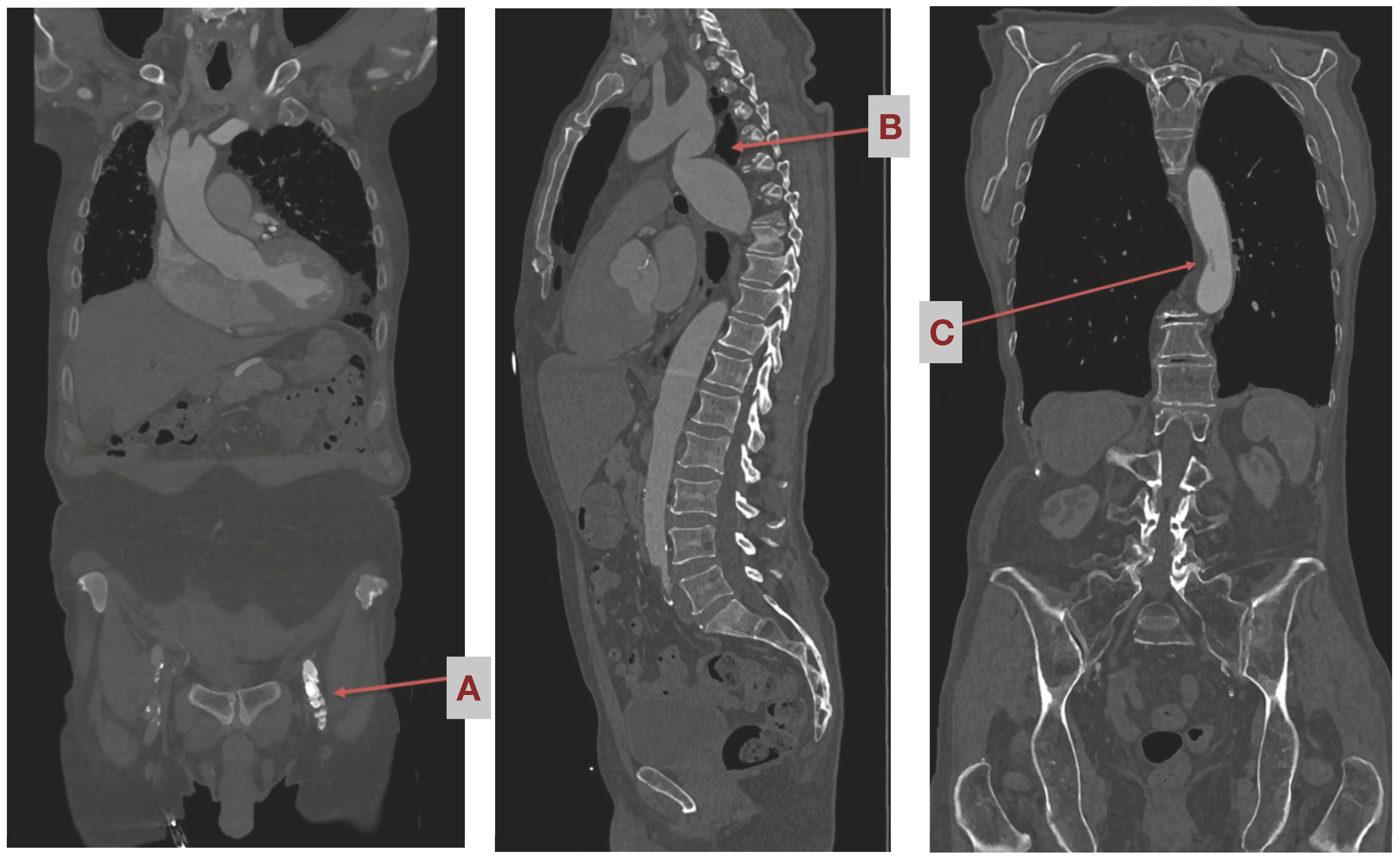
Study Settings Pre-Operatively
1.5. Eligibility Criteria
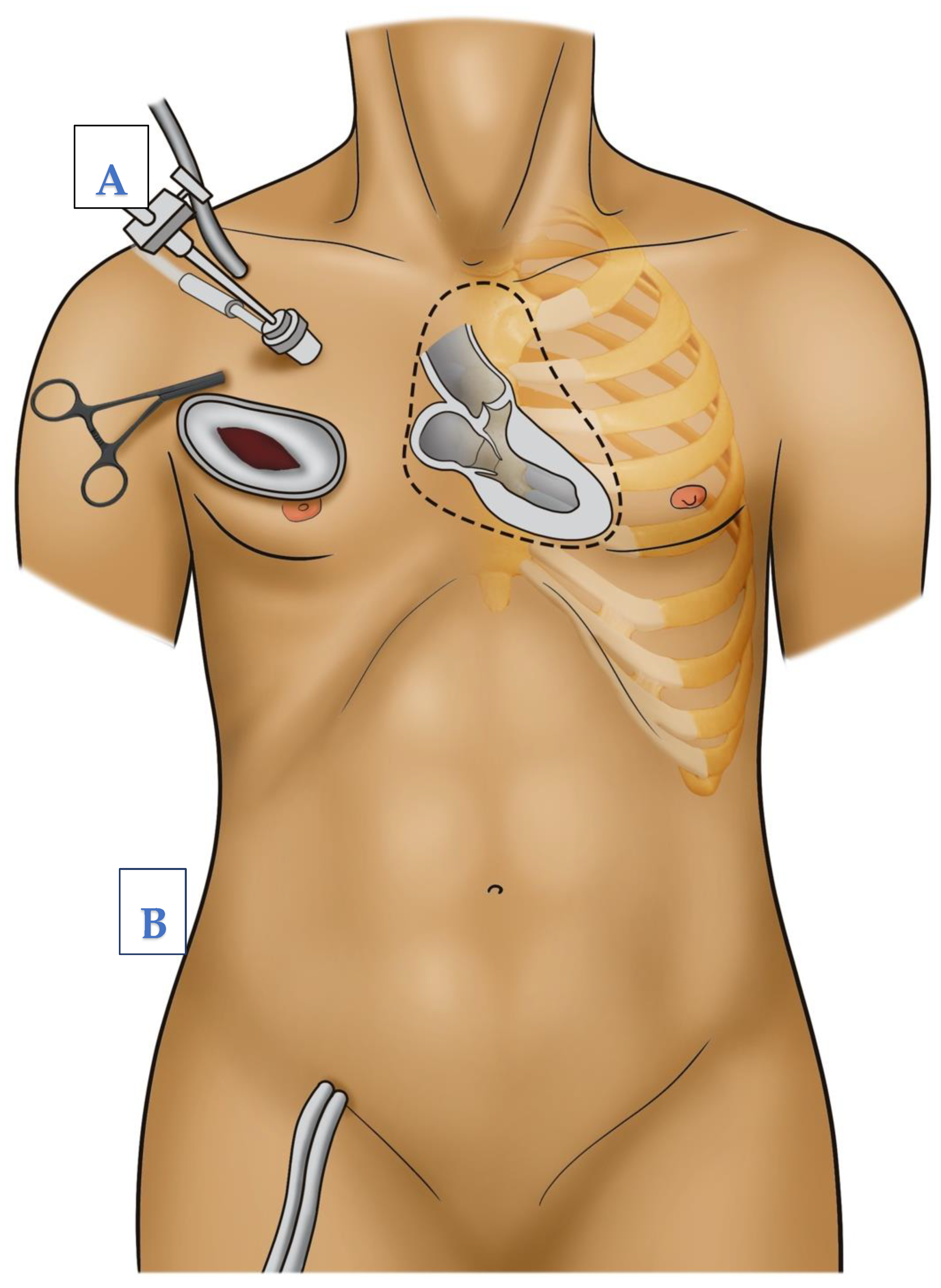
1.6. Surgical Procedure Step-by-Step
- In cases of aortic valve surgery, after the induction of sufficient cardioplegia, the aorta is incised transversally. Then, the aortic valve procedure, repair, or replacement is performed as needed. Afterwards, control of the coronary ostia and control of the leaflets, then double row closure of the aorta, are performed
- In cases of mitral valve surgery, a retraction suture is placed at the level of the right atrium to expose the interatrial grove. The left atrium is then opened at the level of the interatrial grove and exposed using a retractor, which is stabilized by another stab incision, parasternal at the level of the same intercostal space used for thoracotomy (see Figure 3). Then, the mitral valve procedure, repair, or replacement is performed as needed. Afterwards, the waterprobe of the left atrium is closed using continuous double layered sutures.
2. Outcomes
2.1. Primary Endpoint
2.2. Secondary Endpoints
- Rate of transient ischemic attacks;
- Modified Rankin Scale (0–6 points) at discharge;
- Rate of postoperative delirium (mean CAM-ICU score);
- Neurological status assessed by mean NIH Stroke Scale at discharge;
- Rate of seizures generalized and focal;
- Hours on ventilator post-procedure;
- Long-term survival rate (1 year);
- Rate of access-site or access-related bleeding;
- Rate of wound healing disorders in the cannulation area;
- Rate of iatrogenic aortic dissection;
- Thromboembolic events of limbs;
- Rate of pseudoaneurysm or other vascular complications at access site.
3. Study Visits
3.1. Provisions for Post-Trial Care
3.2. Sample Size and Statistical Analysis
4. Data Management
5. Plans to Promote Participant Retention and Complete Follow-Up
6. Feasibility of Recruitment/Trial Sites
7. Discussion
8. Trial Status
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MIS | minimal invasive surgery |
| CT | computer tomography |
| NIH Stroke Scale | National Institutes of Health Stroke Scale |
| CAM ICU Score | confusion assessment method for intensive care unit |
| FAMI | Femoral or axillary cannulation for extracorporeal circulation during minimally invasive heart valve surgery |
References
- McClure, R.S.; Cohn, L.H.; Wiegerinck, E.; Couper, G.S.; Aranki, S.F.; Bolman, R.M., III; Dividson, M.J.; Chen, F.Y. Early and late outcomes in minimally invasive mitral valve repair: An eleven-year experience in 707 patients. J. Thorac. Cardiovasc. Surg. 2009, 137, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Modi, P.; Hassan, A.; Chitwood, W.R. Minimally invasive mitral valve surgery: A systematic review and meta-analysis. Eur. J. Cardiothorac. Surg. 2008, 34, 943–952. [Google Scholar] [PubMed]
- Murzi, M.; Cerillo, A.G.; Miceli, A.; Bevilacqua, S.; Kallushi, E.; Farneti, P.; Solinas, M.; Glauber, M. Antegrade and retrograde arterial perfusion strategy in minimally invasive mitral-valve surgery: A propensity score analysis on 1280 patients. Eur. J. Cardiothorac. Surg. 2013, 43, e167–e172. [Google Scholar] [CrossRef]
- Murzi, M.; Cerillo, A.G.; Gasbarri, T.; Margaryan, R.; Kallushi, E.; Farneti, P.; Solinas, M. Antegrade and retrograde perfusion in minimally invasive mitral valve surgery with transthoracic aortic clamping: A single-institution experience with 1632 patients over 12 years. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 363–368. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.R.; Henry, M.; Rahouma, M.; Khan, F.M.; Wingo, M.; Hameed, I.; Di Franco, A.; Guy, T.S.; Girardi, L.N.; Gaudino, M. Systematic preoperative CT scan is associated with reduced risk of stroke in minimally invasive mitral valve surgery: A meta-analysis. Int. J. Cardiol. 2019, 278, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Grossi, E.A.; Loulmet, D.F.; Schwartz, C.F.; Ursomanno, P.; Zias, E.A.; Dellis, S.L.; Galloway, A.C. Evolution of operative techniques and perfusion strategies for minimally invasive mitral valve repair. J. Thorac. Cardiovasc. Surg. 2012, 143 (Suppl. S4), S68–S70. [Google Scholar] [CrossRef] [PubMed]


| Course of Study | ||||
|---|---|---|---|---|
| Inclusion | Reference | Visits | ||
| Time | -t1 (Pre-OP) | t0 (Intra-OP) | t1–3: Post-OP | t7 or Date of Discharge: Follow Up |
| Screening | X | X | ||
| Informed consent | X | |||
| Clinical examination | X | X | X | X |
| CT-angiography | X | X * | X * | |
| Modified Rankin Scale | X | X | X | |
| NIH Stroke scale | X | X | X | |
| CAM-ICU | X | X | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruse, J.; Silaschi, M.; Velten, M.; Wittmann, M.; Alaj, E.; Ahmad, A.E.-S.; Zimmer, S.; Borger, M.A.; Bakhtiary, F. Femoral or Axillary Cannulation for Extracorporeal Circulation during Minimally Invasive Heart Valve Surgery (FAMI): Protocol for a Multi-Center Prospective Randomized Trial. J. Clin. Med. 2023, 12, 5344. https://doi.org/10.3390/jcm12165344
Kruse J, Silaschi M, Velten M, Wittmann M, Alaj E, Ahmad AE-S, Zimmer S, Borger MA, Bakhtiary F. Femoral or Axillary Cannulation for Extracorporeal Circulation during Minimally Invasive Heart Valve Surgery (FAMI): Protocol for a Multi-Center Prospective Randomized Trial. Journal of Clinical Medicine. 2023; 12(16):5344. https://doi.org/10.3390/jcm12165344
Chicago/Turabian StyleKruse, Jacqueline, Miriam Silaschi, Markus Velten, Maria Wittmann, Eissa Alaj, Ali El-Sayed Ahmad, Sebastian Zimmer, Michael A. Borger, and Farhad Bakhtiary. 2023. "Femoral or Axillary Cannulation for Extracorporeal Circulation during Minimally Invasive Heart Valve Surgery (FAMI): Protocol for a Multi-Center Prospective Randomized Trial" Journal of Clinical Medicine 12, no. 16: 5344. https://doi.org/10.3390/jcm12165344
APA StyleKruse, J., Silaschi, M., Velten, M., Wittmann, M., Alaj, E., Ahmad, A. E.-S., Zimmer, S., Borger, M. A., & Bakhtiary, F. (2023). Femoral or Axillary Cannulation for Extracorporeal Circulation during Minimally Invasive Heart Valve Surgery (FAMI): Protocol for a Multi-Center Prospective Randomized Trial. Journal of Clinical Medicine, 12(16), 5344. https://doi.org/10.3390/jcm12165344









