Safety and Feasibility Study of the Medical Care Pit Walking Support System for Rehabilitation of Acute Stroke Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Study Schedule
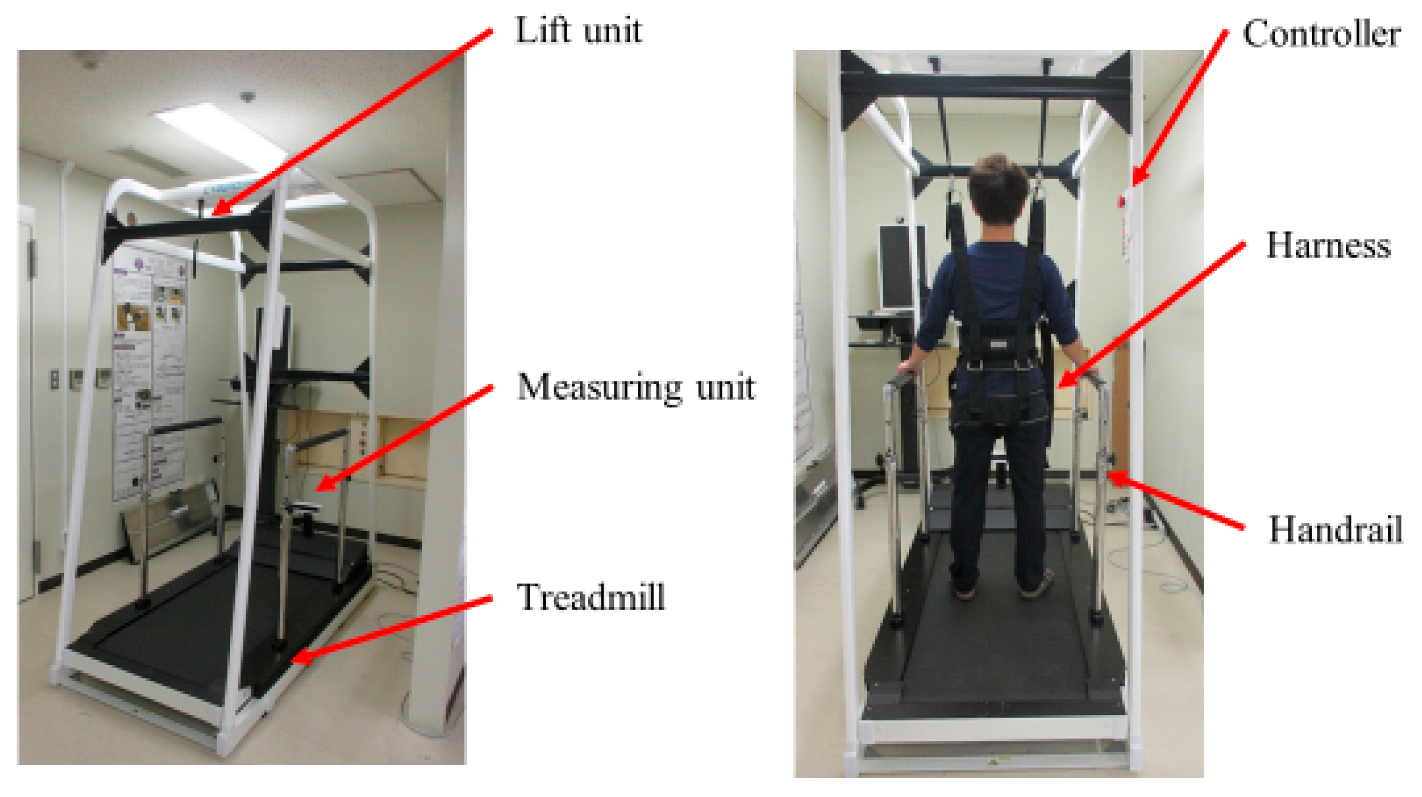
2.4. Intervention (Medical Care Pit Walking Support System)
2.5. Outcome Measures
- (Q1)
- What is your satisfaction with this device throughout the whole?
- (Q2)
- What is your satisfaction with the gait assessment using this device?
- (Q3)
- What is your satisfaction with the gait training using this device?
- (Q4)
- What is your anxiety about the gait training using this device?
- (Q5)
- What is your anxiety about falling when you use this device?
2.6. Criteria for Discontinuation (Discontinuation of Protocol Treatment)
- (1)
- The patient requested to withdraw from the study or withdrew consent.
- (2)
- An event that met the exclusion criteria occurred during the study period.
- (3)
- When a disease or other condition developed that made it difficult to continue the research.
- (4)
- When a patient’s medical doctor judged that discontinuation was appropriate, based on the evaluation of efficacy or assurance of safety.
- (5)
- The discontinuation of the entire study as a whole.
2.7. Ethical Statement
2.8. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Completion Rate and Adverse Events
3.3. Improvements in Functional Parameters
3.4. Qualitative Evaluation of MCP Use and Features
- Handrails and harnesses (trunk belts) can be used to safely implement walking exercises in the acute period.
- Gait measurement (left and right stride length) can be carried out conveniently.
- Objective values can be easily calculated, and regular evaluations can be carried out in the acute period.
- Easy feedback to patients on the results of gait measurement.
4. Discussion
- It is not easy to provide real-time feedback to the physical therapist and patients using objective gait parameters while visual feedback of gait analysis (e.g., step-length symmetry and toe clearance during walking) is delayed by analysis time.
- In general, numerous medical staff and sufficient laboratory space are required to obtain gait parameters in walking-dependent patients with acute stroke. It is expected that equipment which measures the posture of a stroke with a camera in a simple way will be introduced into clinical practice and simplify this step [21,22].
- Physical therapists provide assistance to stroke patients who require it, but the physical therapist’s own fatigue may interrupt the walking exercise. Since developing appropriate motor learning requires intensive repetition in sufficient amounts [2,23], physical therapists with many patients may not be able to meet this demand. There is also a need to focus on reducing the physical burden for long-term care providers, including family members, as well as for patients themselves.
4.1. Limitations of MCP
4.2. Limitations of Study
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Zhang, H. Efficacy of very early mobilization in patients with acute stroke: A systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 11776–11784. [Google Scholar] [CrossRef]
- Duncan, P.W.; Sullivan, K.J.; Behrman, A.L.; Azen, S.P.; Wu, S.S.; Nadeau, S.E.; Dobkin, B.H.; Rose, D.K.; Tilson, J.K.; Cen, S.; et al. Body-weight-supported treadmill rehabilitation after stroke. N. Engl. J. Med. 2011, 364, 2026–2036. [Google Scholar] [CrossRef]
- Sennfält, S.; Norrving, B.; Petersson, J.; Ullberg, T. Long-Term Survival and Function after Stroke: A longitudinal observational study from the Swedish Stroke Register. Stroke 2019, 50, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef] [PubMed]
- Sczesny-Kaiser, M.; Trost, R.; Aach, M.; Schildhauer, T.A.; Schwenkreis, P.; Tegenthoff, M. A Randomized and Controlled Crossover Study Investigating the Improvement of Walking and Posture Functions in Chronic Stroke Patients Using HAL Exoskeleton—The HALESTRO Study (HAL-Exoskeleton STROke Study). Front. Neurosci. 2019, 13, 259. [Google Scholar] [CrossRef] [PubMed]
- Anastasiev, A.; Kadone, H.; Marushima, A.; Watanabe, H.; Zaboronok, A.; Watanabe, S.; Matsumura, A.; Suzuki, K.; Matsumaru, Y.; Ishikawa, E. Supervised Myoelectrical Hand Gesture Recognition in Post-Acute Stroke Patients with Upper Limb Paresis on Affected and Non-Affected Sides. Sensors 2022, 22, 8733. [Google Scholar] [CrossRef]
- Bui, J.; Luauté, J.; Farnè, A. Enhancing Upper Limb Rehabilitation of Stroke Patients with Virtual Reality: A Mini Review. Front. Virtual Real. 2021, 2, 595771. [Google Scholar] [CrossRef]
- Li, G.; Cheng, L.; Gao, Z.; Xia, X.; Jiang, J. Development of an Untethered Adaptive Thumb Exoskeleton for Delicate Rehabilitation Assistance. IEEE Trans. Robot. 2022, 38, 3514–3529. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R.; Jaasko, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [CrossRef]
- Holden, M.K.; Gill, K.M.; Magliozzi, M.R. Gait assessment for neurologically impaired patients. Standards for outcome assessment. Phys. Ther. 1986, 66, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Ogasawara, K.; Kuroda, S.; Itabashi, R.; Toyoda, K.; Itoh, Y.; Iguchi, Y.; Shiokawa, Y.; Takagi, Y.; Ohtsuki, T.; et al. Japan Stroke Society Guideline 2021 for the Treatment of Stroke. Int. J. Stroke 2022, 17, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The fugl-meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabilit. Neural Repair 2002, 16, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Dobkin, B.H. Clinical practice. Rehabilitation after stroke. N. Engl. J. Med. 2005, 352, 1677–1684. [Google Scholar] [CrossRef]
- Visintin, M.; Barbeau, H.; Korner-Bitensky, N.; Mayo, N.E. A new approach to retrain gait in stroke patients through body weight support and treadmill stimulation. Stroke 1998, 29, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Moseley, A.M.; Stark, A.; Cameron, I.D.; Pollock, A. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst. Rev. 2003, Cd002840. [Google Scholar] [CrossRef]
- Hesse, S.; Schmidt, H.; Werner, C.; Bardeleben, A. Upper and lower extremity robotic devices for rehabilitation and for studying motor control. Curr. Opin. Neurol. 2003, 16, 705–710. [Google Scholar] [CrossRef]
- Jezernik, S.; Colombo, G.; Keller, T.; Frueh, H.; Morari, M. Robotic orthosis lokomat: A rehabilitation and research tool. Neuromodulation 2003, 6, 108–115. [Google Scholar] [CrossRef]
- Mehrholz, J.; Thomas, S.; Kugler, J.; Pohl, M.; Elsner, B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst. Rev. 2020, 10, Cd006185. [Google Scholar] [CrossRef]
- Yeung, L.F.; Yang, Z.; Cheng, K.C.; Du, D.; Tong, R.K. Effects of camera viewing angles on tracking kinematic gait patterns using Azure Kinect, Kinect v2 and Orbbec Astra Pro v2. Gait Posture 2021, 87, 19–26. [Google Scholar] [CrossRef]
- Gissot, A.S.; Barbieri, G.; Iacobelis, M.; Paindavoine, M.; Pérennou, D. Measuring trunk orientation with a CMOS camera: Feasibility and accuracy. Gait Posture 2007, 26, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Bütefisch, C.; Hummelsheim, H.; Denzler, P.; Mauritz, K.H. Repetitive training of isolated movements improves the outcome of motor rehabilitation of the centrally paretic hand. J. Neurol. Sci. 1995, 130, 59–68. [Google Scholar] [CrossRef]
- Hsieh, J.; Putman, K.; DeJong, G.; Smout, R.; Horn, S. *Poster 76: Physical and Occupational Therapy in Inpatient Stroke Rehabilitation: The Contribution of Therapists and Their Extenders. Arch. Phys. Med. Rehabil. 2010, 91, e28. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Putman, K.; Nichols, D.; McGinty, M.E.; DeJong, G.; Smout, R.J.; Horn, S. Physical and occupational therapy in inpatient stroke rehabilitation: The contribution of therapy extenders. Am. J. Phys. Med. Rehabil. 2010, 89, 887–898. [Google Scholar] [CrossRef]
- Dobkin, B.H.; Plummer-D’Amato, P.; Elashoff, R.; Lee, J. International randomized clinical trial, stroke inpatient rehabilitation with reinforcement of walking speed (SIRROWS), improves outcomes. Neurorehabilit. Neural Repair 2010, 24, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Moseley, A.; Wales, A.; Herbert, R.; Schurr, K.; Moore, S. Observation and analysis of hemiplegic gait: Stance phase. Aust. J. Physiother. 1993, 39, 259–267. [Google Scholar] [CrossRef]
- Sato, K.; Inoue, T.; Maeda, K.; Shimizu, A.; Murotani, K.; Ueshima, J.; Ishida, Y.; Ogawa, T.; Suenaga, M. Early Wearing of Knee-Ankle-Foot Orthosis Improves Functional Prognosis in Patients after Stroke. J. Stroke Cerebrovasc. Dis. 2022, 31, 106261. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.; Hiratsuka, K.; Haruna, H.; Kojima, N.; Himuro, N. Efficacy of Knee-Ankle-Foot Orthosis on Functional Mobility and Activities of Daily Living in Patients with Stroke: A Systematic Review of Case Reports. J. Rehabil. Med. 2022, 54, jrm00290. [Google Scholar] [CrossRef]
- Choo, Y.J.; Chang, M.C. Effectiveness of an ankle-foot orthosis on walking in patients with stroke: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 15879. [Google Scholar] [CrossRef]
- Morris, M.E.; Matyas, T.A.; Bach, T.M.; Goldie, P.A. Electrogoniometric feedback: Its effect on genu recurvatum in stroke. Arch. Phys. Med. Rehabil. 1992, 73, 1147–1154. [Google Scholar] [PubMed]
- Takahashi, Y.; Okada, K.; Noda, T.; Teramae, T.; Nakamura, T.; Haruyama, K.; Okuyama, K.; Tsujimoto, K.; Mizuno, K.; Morimoto, J.; et al. Robotized Knee-Ankle-Foot Orthosis-Assisted Gait Training on Genu Recurvatum during Gait in Patients with Chronic Stroke: A Feasibility Study and Case Report. J. Clin. Med. 2023, 12, 415. [Google Scholar] [CrossRef] [PubMed]
- Sankai, Y.; Sakurai, T. Exoskeletal cyborg-type robot. Sci. Robot. 2018, 3, eaat3912. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Marushima, A.; Kadone, H.; Shimizu, Y.; Kubota, S.; Hino, T.; Sato, M.; Ito, Y.; Hayakawa, M.; Tsurushima, H.; et al. Efficacy and Safety Study of Wearable Cyborg HAL (Hybrid Assistive Limb) in Hemiplegic Patients With Acute Stroke (EARLY GAIT Study): Protocols for a Randomized Controlled Trial. Front. Neurosci. 2021, 15, 666562. [Google Scholar] [CrossRef]
- Leong, S.C.; Tang, Y.M.; Toh, F.M.; Fong, K.N.K. Examining the effectiveness of virtual, augmented, and mixed reality (VAMR) therapy for upper limb recovery and activities of daily living in stroke patients: A systematic review and meta-analysis. J. Neuroeng. Rehabil. 2022, 19, 93. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Saitoh, E.; Tanabe, S.; Tanikawa, H.; Sasaki, S.; Kato, D.; Kagaya, H.; Itoh, N.; Konosu, H. The features of Gait Exercise Assist Robot: Precise assist control and enriched feedback. NeuroRehabilitation 2017, 41, 77–84. [Google Scholar] [CrossRef]
- Tomida, K.; Sonoda, S.; Hirano, S.; Suzuki, A.; Tanino, G.; Kawakami, K.; Saitoh, E.; Kagaya, H. Randomized Controlled Trial of Gait Training Using Gait Exercise Assist Robot (GEAR) in Stroke Patients with Hemiplegia. J. Stroke Cerebrovasc. Dis. 2019, 28, 2421–2428. [Google Scholar] [CrossRef]

| Age (Years) | 60.7 ± 16.3 | |
|---|---|---|
| Sex | Men | 3 |
| Women | 5 | |
| Height (cm) | 160.1 ± 5.6 | |
| Weight (kg) | 65.7 ± 14.3 | |
| Type of Stroke | Ischemic | 3 |
| Hemorrhagic | 5 | |
| Side of Paresis | Right | 5 |
| Left | 3 | |
| Time Since Stroke (d) | 14.1 ± 6.5 | |
| Study Period (d) | 10.5 ± 1.6 |
| Evaluation Index | Pre | Post | p | |
|---|---|---|---|---|
| Motor Paralysis | FMA-LE | 21.1 ± 7.2 | 22.6 ± 6.5 | 0.106 |
| Independent Walking | FAC | 2.2 ± 0.8 | 3.1 ± 1.3 | 0.020 |
| Gait Measurement | Right Step Length (m) | 0.24 ± 0.07 | 0.36 ± 0.07 | 0.025 |
| Left Step Length (m) | 0.21 ± 0.10 | 0.35 ± 0.08 | 0.017 | |
| Step Lengh Symmetry (Paretic/Non-Paretic) | 1.70 ± 1.51 | 1.04 ± 0.11 | 0.263 | |
| Cadence (Steps/Min) | 86.6 ± 48.0 | 89.2 ± 40.3 | 0.674 | |
| Treadmill Speed (km/h) | 1.1 ± 0.1 | 1.6 ± 0.5 | 0.018 | |
| Gait Training Burden | Burden on Patient (Modified Borg Scale) | 2.7 ± 1.6 | 2.0 ± 1.6 | 0.014 |
| Burden on Medical Staff (Modified Borg Scale) | 1.5 ± 0.9 | 1.0 ± 0.7 | 0.167 |
| Evaluation Index | 1st Session with MCP | 4th Session with MCP | P |
|---|---|---|---|
| From Physical Therapist | |||
| Q1: Satisfaction with MCP (Whole) | 2.6 ± 1.3 | 1.8 ± 0.8 | 0.216 |
| Q2: Satisfaction with MCP (Gait Assessment) | 2.2 ± 1.0 | 1.6 ± 0.5 | 0.096 |
| Q3: Satisfaction with MCP (Gait Training) | 2.1 ± 0.8 | 1.7 ± 0.4 | 0.180 |
| Q4: Anxiety with MCP (Gait Training) | 3.8 ± 2.3 | 1.0 ± 1.6 | 0.027 |
| Q5: Anxiety with MCP (Falling) | 3.1 ± 2.2 | 0.8 ± 1.3 | 0.045 |
| From Patient | |||
| Q1: Satisfaction with MCP (Whole) | 1.8 ± 0.6 | 2.0 ± 0.7 | 0.317 |
| Q2: Satisfaction with MCP (Gait Assessment) | 1.8 ± 0.6 | 1.8 ± 0.6 | 1.000 |
| Q3: Satisfaction with MCP (Gait Training) | 1.7 ± 0.7 | 1.8 ± 0.6 | 0.317 |
| Q4: Anxiety with MCP (Gait Training) | 2.0 ± 2.4 | 0.7 ± 1.0 | 0.068 |
| Q5: Anxiety with MCP (Falling) | 1.3 ± 2.4 | 0.7 ± 1.0 | 0.257 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, H.; Mathis, B.J.; Ueno, T.; Taketomi, M.; Kubota, S.; Marushima, A.; Kawamoto, H.; Sankai, Y.; Matsumura, A.; Hada, Y. Safety and Feasibility Study of the Medical Care Pit Walking Support System for Rehabilitation of Acute Stroke Patients. J. Clin. Med. 2023, 12, 5389. https://doi.org/10.3390/jcm12165389
Watanabe H, Mathis BJ, Ueno T, Taketomi M, Kubota S, Marushima A, Kawamoto H, Sankai Y, Matsumura A, Hada Y. Safety and Feasibility Study of the Medical Care Pit Walking Support System for Rehabilitation of Acute Stroke Patients. Journal of Clinical Medicine. 2023; 12(16):5389. https://doi.org/10.3390/jcm12165389
Chicago/Turabian StyleWatanabe, Hiroki, Bryan J. Mathis, Tomoyuki Ueno, Masakazu Taketomi, Shigeki Kubota, Aiki Marushima, Hiroaki Kawamoto, Yoshiyuki Sankai, Akira Matsumura, and Yasushi Hada. 2023. "Safety and Feasibility Study of the Medical Care Pit Walking Support System for Rehabilitation of Acute Stroke Patients" Journal of Clinical Medicine 12, no. 16: 5389. https://doi.org/10.3390/jcm12165389
APA StyleWatanabe, H., Mathis, B. J., Ueno, T., Taketomi, M., Kubota, S., Marushima, A., Kawamoto, H., Sankai, Y., Matsumura, A., & Hada, Y. (2023). Safety and Feasibility Study of the Medical Care Pit Walking Support System for Rehabilitation of Acute Stroke Patients. Journal of Clinical Medicine, 12(16), 5389. https://doi.org/10.3390/jcm12165389








