Textbook Outcome after Gastrectomy for Gastric Cancer Is Associated with Improved Overall and Disease-Free Survival
Abstract
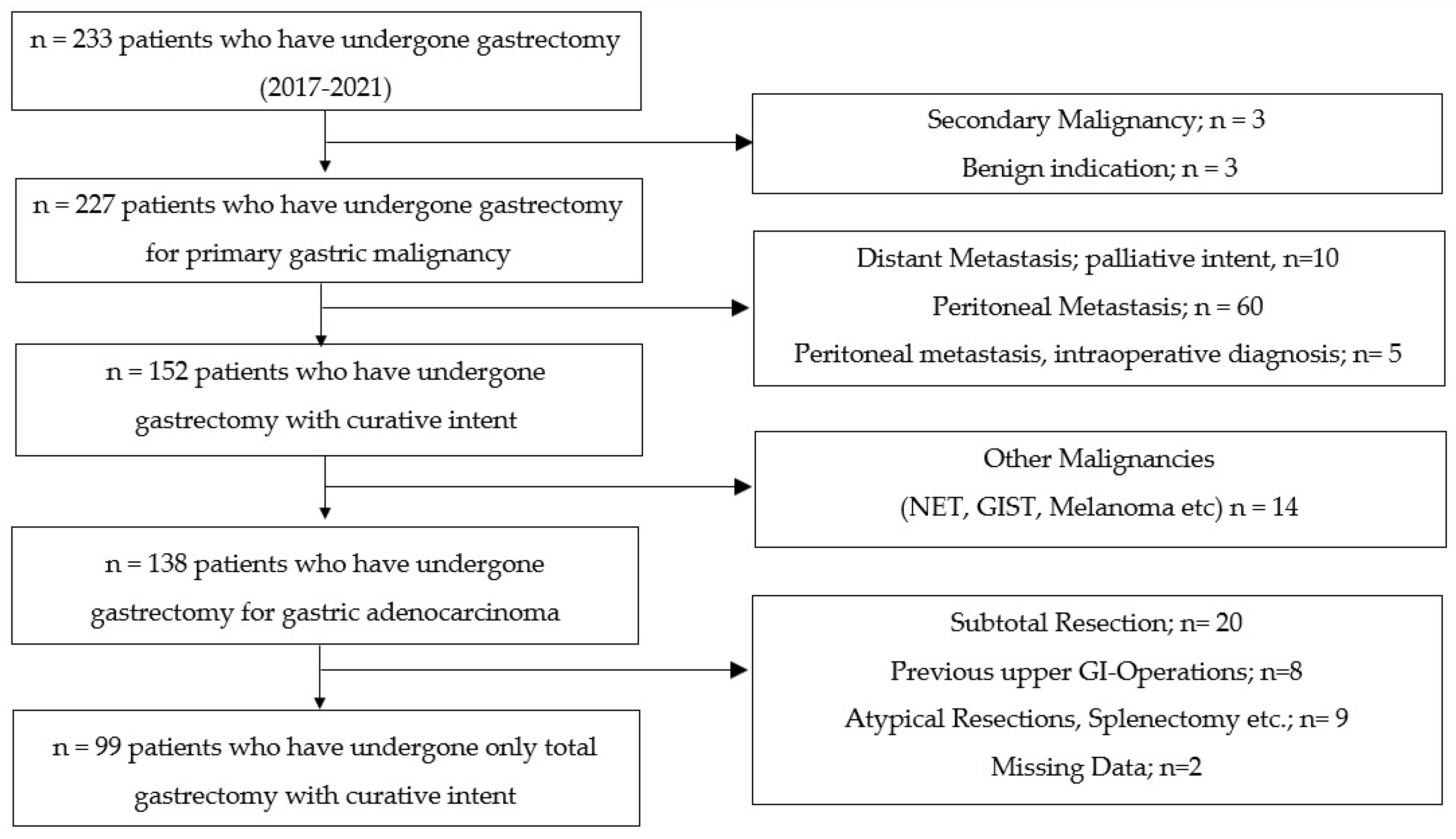
:1. Introduction
2. Patients and Methods
2.1. Data Collection
2.2. Preoperative Evaluation and Multimodal Therapy
2.3. Surgical Procedure
2.4. Perioperative Management
2.5. Histological Evaluation
2.6. Definition of Textbook Outcome
2.7. Statistical Analysis
3. Results
3.1. Reasons for Failure to Achieve Textbook Outcome
3.2. Baseline Characteristics
3.3. Tumor Location and Histopathological Characteristics
3.4. Multimodal Therapy and Perioperative Characteristics
3.5. Factors Affecting Textbook Outcome
3.6. Textbook Outcome and Survival Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.; Vignat, J.; Laversanne, M.; et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–2040: A population-based modelling study. eClinicalMedicine 2022, 47, 101404. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Cai, M.; Shuai, X.; Gao, J.; Wang, G.; Tao, K. Multimodal treatments for resectable gastric cancer: A systematic review and network meta-analysis. Eur. J. Surg. Oncol. EJSO 2019, 45, 1796–1805. [Google Scholar] [CrossRef] [PubMed]
- Zheng-Yan, L.; Yong-Liang, Z.; Feng, Q.; Yan, S.; Pei-Wu, Y. Morbidity and short-term surgical outcomes of robotic versus laparo-scopic distal gastrectomy for gastric cancer: A large cohort study. Surg. Endosc. 2021, 35, 3572–3583. [Google Scholar] [CrossRef]
- Lu, J.; Wu, D.; Wang, H.-G.; Zheng, C.-H.; Li, P.; Xie, J.-W.; Wang, J.-B.; Chen, Q.-Y.; Cao, L.-L.; Lin, M.; et al. 114O Assessment of robotic versus laparoscopic distal gastrectomy for gastric cancer: A randomized controlled trial. Ann. Oncol. 2021, 31, S1287. [Google Scholar] [CrossRef]
- Wang, S.; Xu, L.; Wang, Q.; Li, J.; Bai, B.; Li, Z.; Wu, X.; Yu, P.; Li, X.; Yin, J. Postoperative complications and prognosis after radical gastrectomy for gastric cancer: A systematic review and meta-analysis of observational studies. World J. Surg. Oncol. 2019, 17, 52. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.X.; Sanford, D.E.; Squires, M.H.; Moses, L.E.; Yan, Y.; Poultsides, G.A.; Votanopoulos, K.I.; Weber, S.M.; Bloomston, M.; Pawlik, T.M.; et al. Interaction of Postoperative Morbidity and Receipt of Adjuvant Therapy on Long-Term Survival After Resection for Gastric Adenocarcinoma: Results from the U.S. Gastric Cancer Collaborative. Ann. Surg. Oncol. 2016, 23, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Costantino, C.L.; Mullen, J.T. Morbidity and Mortality of Total Gastrectomy: A Comprehensive Analysis of 90-Day Outcomes. J. Gastrointest. Surg. 2019, 23, 1340–1348. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, P.; Shi, Y.; Tang, B.; Hao, Y.; Zhao, Y.; Qian, F. Evaluation of Clavien–Dindo classification in patients undergoing total gastrectomy for gastric cancer. Med. Oncol. 2015, 32, 120. [Google Scholar] [CrossRef]
- Raziee, H.R.; Cardoso, R.; Seevaratnam, R.; Mahar, A.; Helyer, L.; Law, C.; Coburn, N. Systematic review of the predictors of positive margins in gastric cancer surgery and the effect on survival. Gastric Cancer 2012, 15, 116–124. [Google Scholar] [CrossRef]
- Khanjani, N.; Mirzaei, S.; Nasrolahi, H.; Hamedi, S.H.; Mosalaei, A.; Omidvari, S.; Ahmadloo, N.; Ansari, M.; Sobhani, F.; Mohammadianpanah, M. Insufficient lymph node assessment in gastric adenocarcinoma. J. Egypt. Natl. Cancer Inst. 2019, 31, 2. [Google Scholar] [CrossRef]
- Wang, B.; Nolan, R.; Marshall, H. COVID-19 Immunisation, Willingness to Be Vaccinated and Vaccination Strategies to Improve Vaccine Uptake in Australia. Vaccines 2022, 9, 1467. [Google Scholar] [CrossRef]
- Marano, L.; Verre, L.; Carbone, L.; Poto, G.E.; Fusario, D.; Venezia, D.F.; Calomino, N.; Kaźmierczak-Siedlecka, K.; Polom, K.; Marrelli, D.; et al. Current Trends in Volume and Surgical Outcomes in Gastric Cancer. J. Clin. Med. 2023, 12, 2708. [Google Scholar] [CrossRef]
- Busweiler, L.A.D.; Schouwenburg, M.G.; Henegouwen, M.I.v.B.; Kolfschoten, N.E.; de Jong, P.C.; Rozema, T.; Wijnhoven, B.P.L.; van Hillegersberg, R.; Wouters, M.W.J.M.; van Sandick, J.W.; et al. Textbook outcome as a composite measure in oesophagogastric cancer surgery. Br. J. Surg. 2017, 104, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Kalff, M.C.; van Berge Henegouwen, M.I.; Gisbertz, S.S. Textbook outcome for esophageal cancer surgery: An international con-sensus-based update of a quality measure. Dis. Esophagus 2021, 34, doab011. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.; on behalf of the PRESTO Group; Gupta, V.; Amirazodi, E.; Allen-Ayodabo, C.; Jivraj, N.; Jeong, Y.; Davis, L.E.; Mahar, A.L.; De Mestral, C.; et al. Gastrectomy case volume and textbook outcome: An analysis of the Population Registry of Esophageal and Stomach Tumours of Ontario (PRESTO). Gastric Cancer 2020, 23, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Soh, C.H.; Hassan, S.W.U.; Sacre, J.; Maier, A.B. Morbidity Measures Predicting Mortality in Inpatients: A Systematic Review. J. Am. Med. Dir. Assoc. 2020, 21, 462–468.e7. [Google Scholar] [CrossRef] [PubMed]
- Moehler, M.; Baltin, C.T.H.; Ebert, M.; Fischbach, W.; Gockel, I.; Grenacher, L.; Hölscher, A.H.; Lordick, F.; Malfertheiner, P.; Messmann, H.; et al. International comparison of the German evidence-based S3-guidelines on the diagnosis and multimodal treatment of early and locally advanced gastric cancer, including adenocarcinoma of the lower esophagus. Gastric Cancer 2015, 18, 550–563. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.; Song, J.H.; Choi, S.; Cho, M.; Son, T.; Kim, H.-I.; Hyung, W.J. Intracorporeal esophagojejunostomy using a linear stapler in laparoscopic total gastrectomy: Comparison with circular stapling technique. BMC Surg. 2020, 20, 100. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarwicz, M.K.; Wittekind, C.; Amin, M.B. TNM Classification of Maligant Tumours, 8th ed.; Wiley Blackwell: Oxford, UK, 2017. [Google Scholar]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo Classification of Surgical Complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Sędłak, K.; Rawicz-Pruszyński, K.; Mlak, R.; Gęca, K.; Skórzewska, M.; Pelc, Z.; Małecka-Massalska, T.; Polkowski, W.P. Union is strength: Textbook outcome with perioperative chemotherapy compliance decreases the risk of death in advanced gastric cancer patients. Eur. J. Surg. Oncol. EJSO 2022, 48, 356–361. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017, 20, 1–19. [Google Scholar] [CrossRef]
- Chen, Q.; Ning, Z.; Liu, Z.; Zhou, Y.; He, Q.; Tian, Y.; Hao, H.; Lin, W.; Jiang, L.; Zhao, G.; et al. Textbook Outcome as a measure of surgical quality assessment and prognosis in gastric neuroendocrine carcinoma: A large multicenter sample analysis. Chin. J. Cancer Res. 2021, 33, 433–446. [Google Scholar] [CrossRef]
- Bolger, J.C.; Al Azzawi, M.; Whooley, J.; Bolger, E.M.; Trench, L.; Allen, J.; Kelly, M.E.; Brosnan, C.; Arumugasamy, M.; Robb, W.B. Surgery by a minimally invasive approach is associated with improved textbook outcomes in oesophageal and gastric cancer. Eur. J. Surg. Oncol. EJSO 2021, 47, 2332–2339. [Google Scholar] [CrossRef]
- Garbarino, G.M.; Laracca, G.G.; Lucarini, A.; Piccolino, G.; Mercantini, P.; Costa, A.; Tonini, G.; Canali, G.; Muttillo, E.M.; Costa, G. Laparoscopic versus Open Surgery for Gastric Cancer in Western Countries: A Systematic Review and Meta-Analysis of Short- and Long-Term Outcomes. J. Clin. Med. 2022, 11, 3590. [Google Scholar] [CrossRef] [PubMed]
- Blank, S.; Schmidt, T.; Heger, P.; Strowitzki, M.J.; Sisic, L.; Heger, U.; Nienhueser, H.; Haag, G.M.; Bruckner, T.; Mihaljevic, A.L.; et al. Surgical strategies in true adenocarcinoma of the esophagogastric junction (AEG II): Thoracoabdominal or abdominal approach? Gastric Cancer 2018, 21, 303–314. [Google Scholar] [CrossRef]
- Fransen, L.F.C.; Berkelmans, G.H.K.; Asti, E.; Henegouwen, M.I.v.B.; Berlth, F.; Bonavina, L.; Brown, A.; Bruns, C.; van Daele, E.; Gisbertz, S.S.; et al. The Effect of Postoperative Complications After Minimally Invasive Esophagectomy on Long-term Survival. Ann. Surg. 2020, 274, e1129–e1137. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Manceñido, F.; Jimenez-Fonseca, P.; Carmona-Bayonas, A.; Arrazubi, V.; Hernandez, R.; Cano, J.M.; Custodio, A.; Pijaume, C.P.; Aguado, G.; Lago, N.M.; et al. Is advanced esophageal adenocarcinoma a distinct entity from intestinal subtype gastric cancer? Data from the AGAMENON-SEOM Registry. Gastric Cancer 2021, 24, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Cui, Z.L.; Ghias, M.; Li, X.; Goodloe, R.; Wang, C.; Liepa, A.M.; Hess, L.M. Treatment heterogeneity and overall survival in patients with advanced/metastatic gastric or gastroesophageal junction adenocarcinoma in the United States. J. Gastrointest. Oncol. 2022, 13, 949–957. [Google Scholar] [CrossRef] [PubMed]

| Textbook Outcome Metric | Not Achieved n = 47 |
|---|---|
| Incomplete Resection | 3 (3.1%) |
| Nodes Sampled ≤ 25 | 9 (9.1%) |
| Postoperative severe complications (CD ≥ 3b) | 19 (19.2%) |
| Pulmonary | 11(11.11%) |
| Infectious | 8 (8.1%) |
| Pneumonia | 7 (7.1%) |
| Anastomotic Leak (AL) | 6 (6.1%) |
| Cardiac | 5 (5.1%) |
| Renal | 5 (5.1%) |
| Thromboembolic | 4 (4.0%) |
| Hepatic | 1 (1.0%) |
| Reinterventions | 17 (17.2%) |
| Esophagogastroduodenoscopy (EGD) | 10 (10.1%) |
| Drainage | 14 (14.1%) |
| Other | 5 (5.1%) |
| Unplanned ICU/IMC | 14 (14.1%) |
| Prolonged Hospital stay > 21 Days | 15 (15.1%) |
| Readmission within 30-Days | 7 (7.1%) |
| SSI | 3 (3.03%) |
| GI Bleeding | 2 (1.8%) |
| Pain | 2 (1.8%) |
| Other | 1 (1.0%) |
| Mortality within 30-Days | 2 (1.8%) |
| Characteristic | Total | Textbook Outcome n = 52 | Non-Textbook Outcome n = 47 | p-Value |
|---|---|---|---|---|
| Sex | 0.99 | |||
| Male Female | 61 38 | 32 (61.5%) 20 (38.5%) | 29 (61.7%) 18 (38.3%) | |
| Age (Years) | 61.27 (29–90) 2 | 67.04 (33–86) 2 | 0.03 | |
| BMI (kg/m2) | 25.06 (+4.18) 1 | 26.34 (5.32) 1 | 0.195 | |
| Pre-OP Hemoglobin (g/dL) | 11.93 (1.40) 1 | 12.04 (1.65) 1 | 0.74 | |
| Pre-OP Anemia (male < 13.5, female < 12 g/dL) | 67 | 38 (74.5%) | 29 (63.0%) | 0.22 |
| ASA Score | 0.19 | |||
| 1 2 3 4 | 1 40 52 1 | 1 (2.0%) 25 (50.0%) 23 (46.0%) 1 (2.0%) | 0 (0%) 15 (34.1%) 29 (65.9%) 0 (0%) | |
| CCI tertiles | 0.08 | |||
| ≤4 ≥5 | 45 54 | 28 (53.8%) 24 (46.2%) | 17 (36.2%) 30 (63.8%) | |
| Cardiovascular Disease | 59 | 28 (53.8%) | 31 (66.0%) | 0.22 |
| Pulmonary Disease | 31 | 15 (28.8%) | 16 (34.0%) | 0.58 |
| Renal Disease | 4 | 2 (3.8%) | 2 (4.3%) | 0.92 |
| Hepatic Disease | 1 | 0 (0%) | 1 (2.1%) | 0.29 |
| Diabetes Mellitus | 16 | 9 (17.3%) | 7 (14.9%) | 0.75 |
| Neurological Disease | 14 | 5 (9.6%) | 9 (19.1%) | 0.17 |
| Prior Abdominal Operation * | 33 | 19 (36.5%) | 14 (29.8%) | 0.48 |
| Characteristic | Total | TO n = 52 | Non-TO n = 47 | p-Value |
|---|---|---|---|---|
| Tumor Location | 0.22 | |||
| Gastroesophageal Junction (GEJ) | 39 | 17 (32.1%) | 22 (46.8%) | |
| Gastric | 60 | 35 (67.3%) | 25 (53.2%) | |
| Lauren subtype | 0.81 | |||
| Diffuse Intestinal Mixed | 30 26 9 | 17 (47.2%) 13 (36.1%) 6 (16.7%) | 13 (48.1%) 11 (40.7%) 3 (11.1%) | |
| Adenocarcinoma | 0.35 | |||
| G2–G3 Adenocarcinoma * Signet ring cell carcinoma (SRCC) Partial SRCC | 57 23 19 | 27 (51.9%) 15 (28.8%) 10 (19.2%) | 30 (63.8%) 8 (17.0%) 9 (19.1%) | |
| Pre-T Stage | ||||
| T1 T2 T3 T4 | 10 16 43 20 | 3 (6.3%) 7 (14.6%) 24 (50.0%) 14 (29.2%) | 7 (17.1%) 9 (22.0%) 19 (46.3%) 6 (14.6%) | |
| Pre-N Stage | 0.16 | |||
| N0 N1 N2 N3 | 31 45 11 2 | 14 (29.2%) 24 (50.0%) 8 (16.7%) 2 (4.2%) | 17 (41.5%) 21 (51.2%) 3 (7.3%) 0 (0%) | |
| Post-T Stage | 0.24 | |||
| T0 T1 T2 T3 T4 | 10 24 19 39 6 | 6 (11.5%) 11 (21.2%) 12 (23.1%) 21 (40.4%) 2 (3.8%) | 4 (8.7%) 13 (28.3%) 7 (15.2%) 18 (39.1%) 4 (8.7%) | |
| Post-N Stage | 0.66 | |||
| N0 N1 N2 N3 | 62 17 7 12 | 32 (61.5%) 9 (17.3%) 3 (5.8%) 8 (15.4%) | 30 (65.2%) 8 (17.4%) 4 (8.7%) 4 (8.7%) | |
| Grade (G) | 0.74 | |||
| G2 G3 | 16 54 | 5 (15.2%) 28 (84.8%) | 11 (29.7%) 26 (70.3%) | |
| Venous Invasion (V) | 0.15 | |||
| V0 V1 | 94 4 | 51 (98.1%) 1 (1.9%) | 43 (93.5%) 3 (6.5%) | |
| Lymphatic vessel Invasion (L) | 0.25 | |||
| L0 L1 | 73 24 | 39 (75.0%) 13 (25.0%) | 34 (75.6%) 11 (24.4%) | |
| Perineural invasion (PN) | 0.95 | |||
| PN0 PN1 | 44 18 | 22 (68.8%) 10 (31.3%) | 22 (73.3%) 8 (26.7%) |
| Characteristic | Total | Textbook Outcome n = 52 | Non-Textbook Outcome n = 47 | p-Value |
|---|---|---|---|---|
| NCT | 0.02 | |||
| None received | 13 | 2 (3.8%) | 11 (23.4%) | |
| FLOT | 67 | 41 (78.8%) | 26 (55.3%) | |
| FLO | 16 | 7 (13.5%) | 9 (19.1%) | |
| Other | 3 | 2 (3.8%) | 1 (2.1%) | |
| Discontinued NCT | 0.04 | |||
| <4 Cycles | 19 | 6 (11.5%) | 13 (27.7%) | |
| ≥4 Cycles | 80 | 46 (88.5%) | 34 (72.3%) | |
| Adjuvant Chemotherapy | 0.08 | |||
| None | 17 | 4 (14.8%) | 13 (44.8%) | |
| FLOT | 24 | 15 (55.6%) | 9 (31.0%) | |
| FLO | 8 | 5 (18.5%) | 3 (10.3%) | |
| Other | 7 | 3 (11.1%) | 4 (13.8%) | |
| Discontinued Adjuvant Chemotherapy | 0.05 | |||
| <4 Cycles | 28 | 11 (40.7%) | 17 (68.0%) | |
| ≥4 Cycles | 24 | 16 (59.3%) | 8 (32.0%) | |
| Type of resection | 0.15 | |||
| Total | 62 | 36 (69.2%) | 26 (55.3%) | |
| Trans-hiatal Extended Gastrectomy | 37 | 16 (30.8%) | 21 (44.7%) | |
| Anastomosis | 0.27 | |||
| 25mm Circular | 86 | 40(85.1%) | 46(88.5%) | |
| 29mm Circular | 5 | 4(8.5%) | 1(1.9%) | |
| Linear | 8 | 3(6.5%) | 5(9.6%) | |
| Surgical Approach | 0.02 | |||
| Open Laparoscopic | 27 72 | 8 (15.4%) 44 (84.6%) | 19 (40.4%) 28 (59.6%) | |
| Operation Duration (min) | 287.62 1 | 287.87 1 | 0.99 | |
| Days of Stay | 12.67 1 | 21.28 1 | <0.01 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| p-Value | OR (CI 95%) | p-Value | OR (CI 95%) | |
| Age | 0.03 | 6.997 (1.133–43.212) | 0.27 | 3.626 (0.362–36.240) |
| Hyperlipidemia | 0.02 | 2.966 (1.171–7.504) | 0.35 | 1.724 (0.547–5.430) |
| Tumor Location (AEG) | 0.22 | 0.601 (0.265–1.360) | ||
| ASA | 0.07 | 0.477 (0.212–1.076) | ||
| CCI | 0.15 | 0.530 (0.220–1.279) | ||
| Trans-hiatal Extended Gastrectomy | 0.15 | 0.550 (0.241–1.253) | ||
| Open Surgery | <0.01 | 3.894 (1.528–10.699) | 0.01 | 3.715 (1.334–10.351) |
| Discontinued NCT | <0.01 | 7.638 (1.595–36.584) | 0.02 | 4.278 (1.176–15.553) |
| Overall Survival | Disease Free Survival | |||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Univariate Analysis | Multivariate Analysis | ||||
| p-Value | OR (CI 95%) | p-Value | OR (CI 95%) | p-Value | OR (CI 95%) | |
| TO | 0.02 | 0.135 (0.027–0.681) | 0.04 | 0.448 (0.211–0.954) | 0.069 | 0.496 (0.232–1.057) |
| Sex (Male) | 0.69 | 1.326 (0.331–5.309) | 0.21 | 1.634 (0.741–3.600) | ||
| Age | 0.76 | 0.992 (1.044–1.007) | 0.09 | 1.025 (0.996–1.057) | ||
| BMI > 30 | 0.35 | 0.409 (0.051–3.275) | 0.34 | 0.639 (0.243–1.680) | ||
| UICC > 2a | 0.37 | 2.407 (0.289–20.005) | 0.37 | 1.536 (0.621–3.805) | ||
| CCI > 4 | 0.22 | 3.634 (0.454–29.084) | 0.03 | 2.844 (1.079–7.493) | 0.035 | 2.605 (0.983–6.905) |
| Completion of Neoadjuvant Treatment | 0.21 | 0.409 (0.102–1.640) | 0.41 | 0.672 (0.273–1.653) | ||
| Open Surgery | 0.95 | 1.045 (0.213–5.137) | 0.90 | 1.059 (0.425–2.635) | ||
| ASA > 2 | 0.45 | 1.682 (0.419–6.754) | 0.70 | 1.153 (0.551–2.412) | ||
| Tumor Location (AEG) | 0.11 | 2.935 (0.781–11.032) | 0.32 | 1.474 (0.694–3.130) | ||
| Trans-hiatal-Extended Gastrectomy | 0.12 | 2.973 (0.791–11.174) | 0.31 | 1.483 (0.699–3.149) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çetinkaya-Hosgör, C.; Seika, P.; Raakow, J.; Kröll, D.; Dobrindt, E.M.; Maurer, M.M.; Martin, F.; Ossami Saidy, R.R.; Thuss-Patience, P.; Pratschke, J.; et al. Textbook Outcome after Gastrectomy for Gastric Cancer Is Associated with Improved Overall and Disease-Free Survival. J. Clin. Med. 2023, 12, 5419. https://doi.org/10.3390/jcm12165419
Çetinkaya-Hosgör C, Seika P, Raakow J, Kröll D, Dobrindt EM, Maurer MM, Martin F, Ossami Saidy RR, Thuss-Patience P, Pratschke J, et al. Textbook Outcome after Gastrectomy for Gastric Cancer Is Associated with Improved Overall and Disease-Free Survival. Journal of Clinical Medicine. 2023; 12(16):5419. https://doi.org/10.3390/jcm12165419
Chicago/Turabian StyleÇetinkaya-Hosgör, Candan, Philippa Seika, Jonas Raakow, Dino Kröll, Eva Maria Dobrindt, Max Magnus Maurer, Friederike Martin, Ramin Raul Ossami Saidy, Peter Thuss-Patience, Johann Pratschke, and et al. 2023. "Textbook Outcome after Gastrectomy for Gastric Cancer Is Associated with Improved Overall and Disease-Free Survival" Journal of Clinical Medicine 12, no. 16: 5419. https://doi.org/10.3390/jcm12165419








