Ultrasound-Guided Ethanol Ablation for Thyroglossal Duct Cyst: A Review of Technical Issues and Potential as a New Standard Treatment
Abstract
:1. Introduction
2. Overall Practice of EA for TGDC
2.1. Pre-Procedural Evaluation
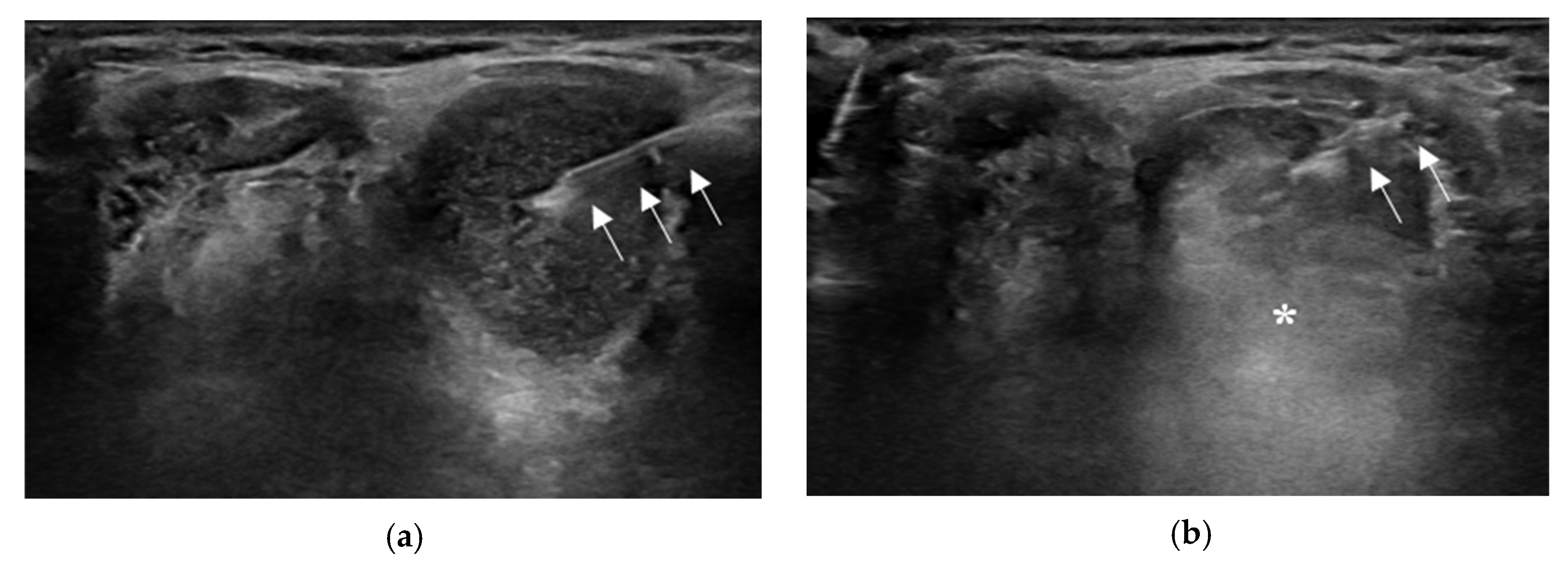
2.2. Aspiration of Internal Contents and Ethanol Injection
2.3. Technical Issues or Variations in EA for TGDC
2.3.1. Local Anesthesia
2.3.2. Needle Size Used for EA
2.3.3. Amount of Ethanol Injected
2.3.4. Retention or Aspiration of Injected Ethanol
2.3.5. EA for TGDC with Viscous Internal Contents
2.3.6. Number of Treatment Sessions
3. EA Results for TGDC
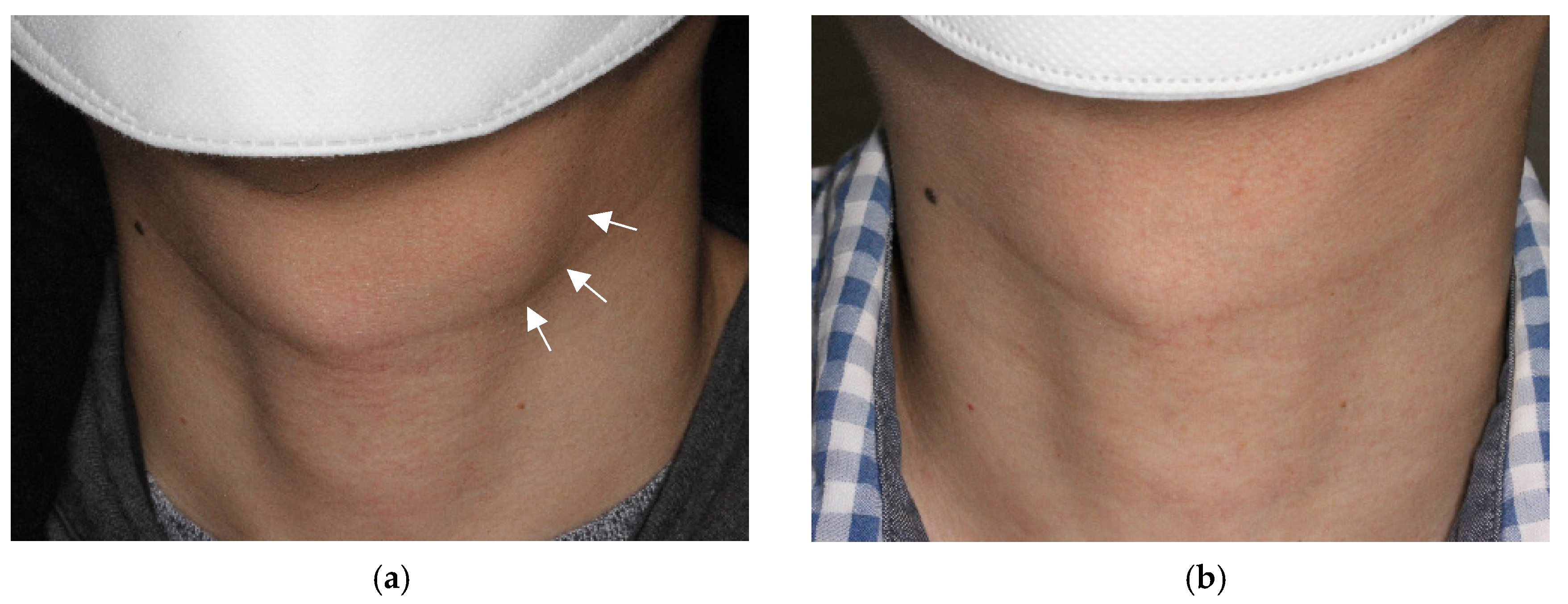
3.1. Treatment Efficacy
3.2. Outcome-Related Factors
3.3. Complications
3.4. Cost
4. Discussion
4.1. Needs for Local Anesthesia
4.2. Needle Size Used for EA
4.3. Amount of Ethanol Injected
4.4. Retention or Aspiration of Injected Ethanol
4.5. Number of Treatment Sessions
4.6. Potential of EA as a Primary Treatment of TGDC in Terms of Feasibility, Safety, and Treatment Efficacy
4.7. Practical Limitations of EA for TGDC
5. Study Limitations
6. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Flint, P.W.; Haughey, B.H.; Robbins, K.T.; Thomas, J.R.; Niparko, J.K.; Lund, V.J.; Lesperance, M.M. Cummings Otolaryngology-Head and Neck Surgery E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Gioacchini, F.M.; Alicandri-Ciufelli, M.; Kaleci, S.; Magliulo, G.; Presutti, L.; Re, M. Clinical presentation and treatment outcomes of thyroglossal duct cysts: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, F.; Pignataro, L.; Gaini, R.M.; Hartley, B.; Garavello, W. Risk of recurrence in children operated for thyroglossal duct cysts: A systematic review. J. Pediatr. Surg. 2013, 48, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Goldsztein, H.; Khan, A.; Pereira, K.D. Thyroglossal duct cyst excision—The Sistrunk procedure. Oper. Tech. Otolaryngol.-Head. Neck Surg. 2009, 20, 256–259. [Google Scholar] [CrossRef]
- Shah, R.; Gow, K.; Sobol, S.E. Outcome of thyroglossal duct cyst excision is independent of presenting age or symptomatology. Int. J. Pediatr. Otorhinolaryngol. 2007, 71, 1731–1735. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.D.R.; Herrera, H.B.; Lau, S.K. Thyroglossal Duct Cyst Carcinomas: A Clinicopathologic Series of 22 Cases with Staging Recommendations. Head. Neck Pathol. 2017, 11, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.; Kwak, J.H.; Lee, G.J.; Sohn, J.H. Ultrasound-Guided Ethanol Ablation as a Primary Treatment for Thyroglossal Duct Cyst: Feasibility, Characteristics, and Outcomes. Otolaryngol. Head. Neck Surg. 2023, 168, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Rayess, H.M.; Monk, I.; Svider, P.F.; Gupta, A.; Raza, S.N.; Lin, H.-S. Thyroglossal duct cyst carcinoma: A systematic review of clinical features and outcomes. Otolaryngol.–Head. Neck Surg. 2017, 156, 794–802. [Google Scholar] [CrossRef]
- Malka Yosef, L.; Lahav, Y.; Hazout, C.; Zloczower, E.; Halperin, D.; Cohen, O. Impact of age on surgical outcomes and failure rates in patients with thyroglossal duct cysts. Am. J. Otolaryngol. 2021, 42, 102902. [Google Scholar] [CrossRef]
- Ross, J.; Manteghi, A.; Rethy, K.; Ding, J.; Chennupati, S.K. Thyroglossal duct cyst surgery: A ten-year single institution experience. Int. J. Pediatr. Otorhinolaryngol. 2017, 101, 132–136. [Google Scholar] [CrossRef]
- Park, S.I.; Baek, J.H.; Suh, C.H.; Chung, S.R.; Choi, Y.J.; Kim, T.Y.; Lee, Y.-M.; Lee, J.H. Chemical ablation using ethanol or OK-432 for the treatment of thyroglossal duct cysts: A systematic review and meta-analysis. Eur. Radiol. 2021, 31, 9048–9056. [Google Scholar] [CrossRef]
- Karatay, E.; Javadov, M. The effectiveness of ethanol ablation in the treatment of thyroglossal duct cysts in adult cases and evaluation with cosmetic scoring. Jpn. J. Radiol. 2021, 39, 994–999. [Google Scholar] [CrossRef]
- Kim, S.M.; Baek, J.H.; Kim, Y.S.; Sung, J.Y.; Lim, H.K.; Choi, H.; Lee, J. Efficacy and safety of ethanol ablation for thyroglossal duct cysts. Am. J. Neuroradiol. 2011, 32, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Seo, J.W.; Park, H.S.; Kang, M.K.; Jang, A.L.; Lee, J.H.; Hong, J.C. Efficacy of ethanol ablation for thyroglossal duct cyst. Ann. Otol. Rhinol. Laryngol. 2015, 124, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.S.; Baek, J.H.; Lee, J.H.; Choi, Y.J.; Yoon, J.H.; Nam, S.Y.; Kim, S.C.; Sung, J.Y.; Baek, S.M.; Na, D.G. Treatment Efficacy and Safety of Ethanol Ablation for Thyroglossal Duct Cysts: A Comparison with Surgery. Eur. Radiol. 2017, 27, 2708–2716. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Baek, J.H.; Chung, S.R.; Choi, Y.J.; Lee, J.H.; Kim, T.Y.; Lee, Y.M.; Baek, S.M. Ethanol ablation for the treatment of thyroglossal duct cysts: Follow-up results for longer than 2 years. Eur. Radiol. 2022, 32, 3525–3531. [Google Scholar] [CrossRef] [PubMed]
- Aculate, N.R.; Jones, H.B.; Bansal, A.; Ho, M.W. Papillary carcinoma within a thyroglossal duct cyst: Significance of a central solid component on ultrasound imaging. Br. J. Oral Maxillofac. Surg. 2014, 52, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Chow, T.L.; Choi, C.Y.; Yee-Hing Hui, J. Thyroglossal duct cysts in adults treated by ethanol sclerotherapy: A pilot study of a nonsurgical technique. Laryngoscope 2012, 122, 1262–1264. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Park, I.; Elzomor, A.; Li, L.; Lloyd, A.; Benito, D.A.; Goodman, J.F.; Thakkar, P.G.; Joshi, A. Efficacy of ethanol ablation as a treatment of benign head and neck cystic lesions. Am. J. Otolaryngol. 2021, 42, 103082. [Google Scholar] [CrossRef]
- Yang, C.C.; Hsu, Y.; Liou, J.Y. Efficacy of Ethanol Ablation for Benign Thyroid Cysts and Predominantly Cystic Nodules: A Systematic Review and Meta-Analysis. Endocrinol. Metab. 2021, 36, 81–95. [Google Scholar] [CrossRef]
- Cesareo, R.; Tabacco, G.; Naciu, A.M.; Crescenzi, A.; Bernardi, S.; Romanelli, F.; Deandrea, M.; Trimboli, P.; Palermo, A.; Castellana, M. Long-term efficacy and safety of percutaneous ethanol injection (PEI) in cystic thyroid nodules: A systematic review and meta-analysis. Clin. Endocrinol. 2022, 96, 97–106. [Google Scholar] [CrossRef]
- Mulita, F.; Tchabashvili, L.; Verras, G.I.; Liolis, E.; Siouti, S.; Panagopoulos, K.; Vailas, M. Thyroid abscess as a complication of percutaneous ethanol ablation of cystic thyroid nodules. Endokrynol. Pol. 2021, 72, 284–285. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.Y.; Shin, J.H.; Na, D.G.; Ha, E.J.; Ahn, H.S.; Lim, H.K.; Lee, J.H.; Park, J.S.; Kim, J.H.; Sung, J.Y.; et al. Ethanol Ablation of the Thyroid Nodules: 2018 Consensus Statement by the Korean Society of Thyroid Radiology. Korean J. Radiol. 2019, 20, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Orloff, L.A.; Noel, J.E.; Stack, B.C., Jr.; Russell, M.D.; Angelos, P.; Baek, J.H.; Brumund, K.T.; Chiang, F.Y.; Cunnane, M.B.; Davies, L.; et al. Radiofrequency ablation and related ultrasound-guided ablation technologies for treatment of benign and malignant thyroid disease: An international multidisciplinary consensus statement of the American Head and Neck Society Endocrine Surgery Section with the Asia Pacific Society of Thyroid Surgery, Associazione Medici Endocrinologi, British Association of Endocrine and Thyroid Surgeons, European Thyroid Association, Italian Society of Endocrine Surgery Units, Korean Society of Thyroid Radiology, Latin American Thyroid Society, and Thyroid Nodules Therapies Association. Head Neck 2022, 44, 633–660. [Google Scholar]
- Papini, E.; Monpeyssen, H.; Frasoldati, A.; Hegedus, L. 2020 European Thyroid Association Clinical Practice Guideline for the Use of Image-Guided Ablation in Benign Thyroid Nodules. Eur. Thyroid J. 2020, 9, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Feldkamp, J.; Grunwald, F.; Luster, M.; Lorenz, K.; Vorlander, C.; Fuhrer, D. Non-Surgical and Non-Radioiodine Techniques for Ablation of Benign Thyroid Nodules: Consensus Statement and Recommendation. Exp. Clin. Endocrinol. Diabetes 2020, 128, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.H.; Lee, J.H.; Lee, J.Y.; Chung, S.R.; Chung, M.S.; Kim, H.W.; Choi, Y.J.; Baek, J.H. Ethanol Ablation of Ranulas: Short-Term Follow-Up Results and Clinicoradiologic Factors for Successful Outcome. Am. J. Neuroradiol. 2017, 38, 1794–1798. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.J.; Baek, S.M.; Baek, J.H.; Shin, S.Y.; Han, M.; Kim, C.H. Efficacy and Safety of Ethanol Ablation for Branchial Cleft Cysts. Am. J. Neuroradiol. 2017, 38, 2351–2356. [Google Scholar] [CrossRef]
- Cho, W.; Sim, J.S.; Jung, S.L. Ultrasound-guided ethanol ablation for cystic thyroid nodules: Effectiveness of small amounts of ethanol in a single session. Ultrasonography 2021, 40, 417–427. [Google Scholar] [CrossRef]
- Park, H.S.; Yim, Y.; Baek, J.H.; Choi, Y.J.; Shong, Y.K.; Lee, J.H. Ethanol ablation as a treatment strategy for benign cystic thyroid nodules: A comparison of the ethanol retention and aspiration techniques. Ultrasonography 2019, 38, 166. [Google Scholar] [CrossRef]
- Kim, D.W.; Rho, M.H.; Kim, H.J.; Kwon, J.S.; Sung, Y.S.; Lee, S.W. Percutaneous ethanol injection for benign cystic thyroid nodules: Is aspiration of ethanol-mixed fluid advantageous? Am. J. Neuroradiol. 2005, 26, 2122–2127. [Google Scholar]
- Hirshoren, N.; Neuman, T.; Udassin, R.; Elidan, J.; Weinberger, J.M. The imperative of the Sistrunk operation: Review of 160 thyroglossal tract remnant operations. Otolaryngol. Head Neck Surg. 2009, 140, 338–342. [Google Scholar] [CrossRef]
- Maddalozzo, J.; Venkatesan, T.K.; Gupta, P. Complications associated with the Sistrunk procedure. Laryngoscope 2001, 111, 119–123. [Google Scholar] [CrossRef]
- Lekkerkerker, I.; van Heurn, E.L.; van der Steeg, A.F.; Derikx, J.P. Pediatric thyroglossal duct cysts: Post-operative complications. Int. J. Pediatr. Otorhinolaryngol. 2019, 124, 14–17. [Google Scholar] [CrossRef]
| Author (Publication Year) | Study Design | Study Period | Patient Number | Age (Years) | Male:Female | Initial Volume (mL) | Local Anesthesia | Needle | Aspirate Volume (mL) | Injection Amount |
|---|---|---|---|---|---|---|---|---|---|---|
| Kim [13] (2011) | RCS | 2005–2008 | 11 | 34.9 (23–44) | 3:08 | 6.0 (0.7–29.4) | N/A | 21 | 4.6 (0.5–25) | 50–80% of aspirated volume |
| Chow [17] (2012) | RCS | NA | 6 | 44.8 (37–61) | 2:04 | 4.9 (1.5–9.8) | No | 20–22 | N/A | 50–90% of aspirated volume |
| Lee [14] (2015) | RCS | 2012–2013 | 9 | 36 (14–58) | 7:02 | 8.9 (0.2–36.9) | Yes | 18 | N/A | 70–80% of cystic volume |
| Chung [15] (2017) | RCC | 2005–2014 | 56 | 37.5 a (14–71) | 23:33 | 3.9 a (0.3–26.6) | N/A | 18 or 21 | 4.4 (0–25) | 50% of aspirated volume |
| Karatay [12] (2021) | RCS | 2018–2020 | 28 | 42 a (19–0–76) | 14:14 | 4.1 a (1.0–15.1) | N/A | 18 | N/A | ≤5 mL |
| Park [16] (2022) | RCS | 2008–2018 | 68 | 38 ± 16 | 21:47 | 7.3 ± 19.0 | Yes | 18 or 21 | N/A | 50% of aspirated volume |
| Ahn [7] (2023) | RCS | 2016–2021 | 28 | 47 (20–69) | 16:12 | 6.7 a (1.4–20.1) | No | 21–23 | 5.0 a (0–18) | ≤2.5 mL |
| Author (Publication Year) | Number of EA Sessions | Multiple EA Sessions | Aspiration of Injected Ethanol | Retention Time (min) | Volume at Last Follow-Up (mL) | VRR at Last Follow-Up | TSR | Follow-Up (Months) | Complications | |
| Kim [13] (2011) | 1.4 (1–3) | 3 (27%) | Yes | 10 | 0.36 (0–1.08) | 81.3% (43.9–98.3%) | 80% b | 13.6 (3–29) | Mild pain | |
| Chow [17] (2012) | 1.3 (1–3) | 1 (16.8%) | N/A | N/A | N/A | N/A | N/A (relapse in one patient) | 34.5 (17–72) | Moderate pain in two patients | |
| Lee [14] (2015) | 1.7 (1–3) | 4 (44.4%) | Yes | 5 | 1.9 | 76.6% (−4–100%) | 77.8% c | 13.1 (12–15) | No | |
| Chung [15] (2017) | 1.5 (1–6) | 15 (26.8%) | Yes | 10 | 0.8 a (0–14.9) | 82.30% | 80.4% b | 10 a (1–94) | Temporary inspiratory stridor in one patient | |
| Karatay [12] (2021) | 1 | 0 (0.0%) | No | N/A | 0.01 a (0–1.2) | 95.10% | N/A | 12 | Temporary pain in three patients | |
| Park [16] (2022) | 1.1 (1–3) | 8 (11.2%) | Yes | 10 | N/A | 81% | Immediate, 81% b; long-term, 83% b | 69 a (24–131) for 42 patients | Wound infection in one patient | |
| Ahn [7] (2023) | 1.4 (1–3) | 10 (35.7%) | No | N/A | 0.2 a (0.2–2.8) | 96.2% a (58.1–100) | 96.4%c | 22 a (9–65) | Mild-to-moderate pain | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, D. Ultrasound-Guided Ethanol Ablation for Thyroglossal Duct Cyst: A Review of Technical Issues and Potential as a New Standard Treatment. J. Clin. Med. 2023, 12, 5445. https://doi.org/10.3390/jcm12175445
Ahn D. Ultrasound-Guided Ethanol Ablation for Thyroglossal Duct Cyst: A Review of Technical Issues and Potential as a New Standard Treatment. Journal of Clinical Medicine. 2023; 12(17):5445. https://doi.org/10.3390/jcm12175445
Chicago/Turabian StyleAhn, Dongbin. 2023. "Ultrasound-Guided Ethanol Ablation for Thyroglossal Duct Cyst: A Review of Technical Issues and Potential as a New Standard Treatment" Journal of Clinical Medicine 12, no. 17: 5445. https://doi.org/10.3390/jcm12175445





