The Current Role of Cardiopulmonary Exercise Test in the Diagnosis and Management of Pulmonary Hypertension
Abstract
1. Introduction
2. Pathophysiological Model of PAH and Causes of Effort Dyspnea in Affected Patients
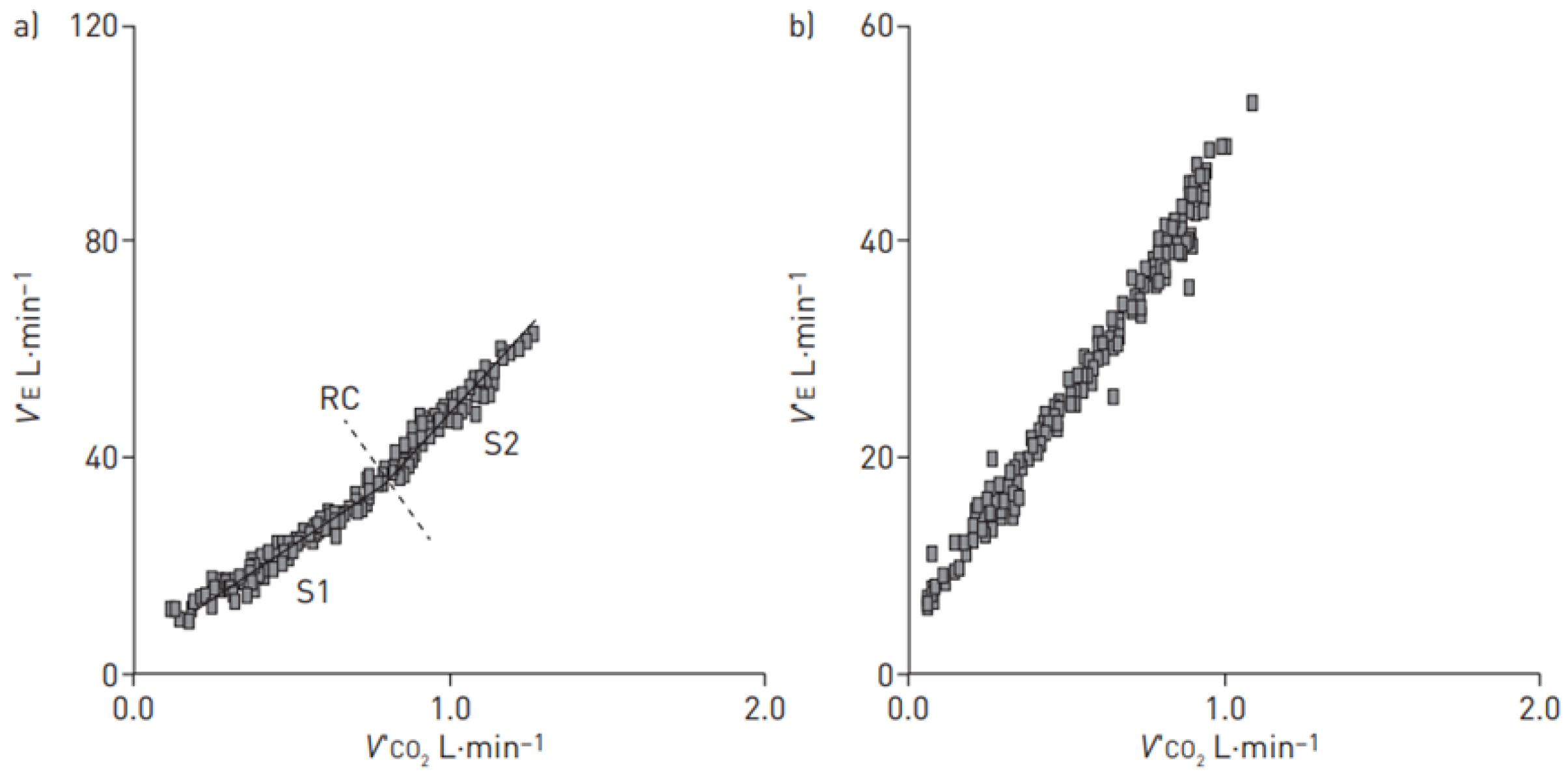
3. Characteristics of CPET in PAH Patients
4. Role of CPET in PH Diagnostic Phase and PH Differential Diagnosis
5. Role of CPET in Staging Disease Severity
6. Role of CPET in Prognostic Evaluation and Follow-Up of PAH
7. Role of CPET in Clinical Trials on PAH
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- McLaughlin, V.V.; McGoon, M.D. Pulmonary arterial hypertension. Circulation 2006, 114, 1417–1431. [Google Scholar] [CrossRef] [PubMed]
- D’Alonzo, G.E.; Barst, R.J.; Ayres, S.M.; Bergofsky, E.H.; Brundage, B.H.; Detre, K.M.; Fishman, A.P.; Goldring, R.M.; Groves, B.M.; Kernis, J.T.; et al. Survival in patients with primary pulmonary hypertension: Results from a national prospective registry. Ann. Intern. Med. 1991, 115, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Rubin, L.; Hoeper, M.; Jansa, P.; Al-Hiti, H.; Meyer, G.; Chiossi, E.; Kusic-Pajic, A.; Simonneau, G.G. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): A double-blind, randomised controlled trial. Lancet 2008, 371, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Yaici, A.; De Groote, P.; Montani, D.; Sitbon, O.; Launay, D.; Gressin, V.; Guillevin, L.; Clerson, P.; Simonneau, G.; et al. Screening for pulmonary arterial hypertension in patients with systemic sclerosis: Clinical characteristics at diagnosis and long-term survival. Arthritis Rheum. 2011, 63, 3522–3530. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.M.T.; Humbert, M.; Celermajer, D.S. Early detection of pulmonary arterial hypertension. Nat. Rev. Cardiol. 2015, 12, 143–155. [Google Scholar] [PubMed]
- Vonk-Noordegraaf, A.; Haddad, F.; Chin, K.M.; Forfia, P.R.; Kawut, S.M.; Lumens, J.; Naeije, R.; Newman, J.; Oudiz, R.J.; Provencher, S.; et al. Right heart adaptation to pulmonary arterial hypertension: Physiology and pathobiology. J. Am. Coll. Cardiol. 2013, 62 (Suppl. S25), D22–D33. [Google Scholar] [CrossRef]
- Pinkstaff, S.O.; Burger, C.D.; Daugherty, J.; Bond, S.; Arena, R. Cardiopulmonary exercise testing in patients with pulmonary hypertension: Clinical recommendations based on a reeview of the evidence. Expert. Rev. Respir. Med. 2016, 10, 279–295. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar]
- Naeije, R.; Manes, A. The right ventricle in pulmonary arterial hypertension. Eur. Respir. Rev. 2014, 23, 476–487. [Google Scholar] [CrossRef]
- Naeije, R.; Brimioulle, S.; Dewachter, C. Biomechanics of the right ventricle. Pulm. Circ. 2014, 4, 395–406. [Google Scholar] [CrossRef]
- Vonk-Noordegraaf, A.; Westerhof, N. Describing right ventricular function. Eur. Respir. J. 2013, 41, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Janicki, J.S.; Weber, K.T.; Likoff, M.J.; Fishman, A.P. The pressure-flow response of the pulmonary circulation in patients with heart failure and pulmonary vascular disease. Circulation 1985, 72, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Groepenhoff, H.; Westerhof, N.; Jacobs, W.; Boonstra, A.; Postmus, P.E.; Vonk-Noordegraaf, A. Exercise stroke volume and heart rate response differ in right and left heart failure. Eur. J. Heart Fail. 2010, 12, 716–720. [Google Scholar] [CrossRef]
- Chemla, D.; Castelain, V.; Hoette, S.; Creuzé, N.; Provencher, P.; Zhu, K.; Humbert, M.; Herve, P. Strong linear relationship between heart rate and mean pulmonary artery pressure in exercising patients with severe precapillary pulmonary hypertension. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H769–H777. [Google Scholar] [CrossRef] [PubMed]
- Weatherald, J.; Farina, S.; Bruno, N.; Laveneziana, P. Cardiopulmonary exercise testing in pulmonary hypertension. Ann. Am. Thorac. Soc. 2017, 14 (Suppl. S1), S84–S92. [Google Scholar] [CrossRef] [PubMed]
- Holverda, S.; Gan, C.T.; Marcus, J.T.; Postmus, P.E.; Boonstra, A.; Vonk-Noordegraaf, A. Impaired stroke volume response to exercise in pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2006, 47, 1732–1733. [Google Scholar] [CrossRef]
- Ferrazza, A.M.; Martolini, D.; Valli, G.; Palange, P. Cardiopulmonary exercise testing in the functional and prognostic evaluation of patients with pulmonary diseases. Respiration 2009, 77, 3–17. [Google Scholar] [CrossRef]
- Velez-Roa, S.; Ciarka, A.; Najem, B.; Vachiery, J.L.; Naeije, R.; van de Borne, P. Increased sympathetic nerve activity in pulmonary artery hypertension. Circulation 2004, 110, 1308–1312. [Google Scholar] [CrossRef]
- Farina, S.; Bruno, N.; Agalbato, C.; Contini, M.; Cassandro, R.; Elia, D.; Harari, S.; Agostoni, P. Physiological insights of exercise hyperventilation in arterial and chronic thromboembolic pulmonary hypertension. Int. J. Cardiol. 2018, 259, 178–182. [Google Scholar] [CrossRef]
- Parati, G.; Lombardi, C.; Castagna, F.; Mattaliano, P.; Filardi, P.; Agostoni, P. Heart failure and sleep disorders. Nat. Rev. Cardiol. 2016, 13, 389–403. [Google Scholar]
- Chang, A.J.; Ortega, F.E.; Riegler, J.; Madison, D.V.; Krasnow, M.A. Oxygen regulation of breathing through an olfactory receptor activated by lactate. Nature 2015, 527, 240–244. [Google Scholar] [CrossRef]
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Stringer, W.; Whipp, B. Principles of Exercise Testing and Interpretation, 3rd ed.; Lippincott, Williams & Wilkins: Baltimore, MD, USA, 1999. [Google Scholar]
- Sun, X.G.; Hansen, J.E.; Oudiz, R.J.; Wasserman, K. Pulmonary function in primary pulmonary hypertension. J. Am. Coll. Cardiol. 2003, 41, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Laveneziana, P.; Garcia, G.; Joureau, B.; Nicolas-Jilwan, F.; Brahimi, T.; Laviolette, L.; Sitbon, O.; Simonneau, G.; Humbert, M.; Similowski, T. Dynamic respiratory mechanics and exertional dyspnoea in pulmonary arterial hypertension. Eur. Respir. J. 2013, 41, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.J.; Ewert, R.; Hoeper, M.M.; Olschewski, H.; Behr, J.; Winkler, J.; Wilkens, H.; Breuer, C.; Kübler, W.; Borst, M.M.; et al. Peripheral airway obstruction in primary pulmonary hypertension. Thorax 2002, 57, 473–476. [Google Scholar] [CrossRef]
- Potus, F.; Malenfant, S.; Graydon, C.; Mainguy, V.; Tremblay, È.; Breuils-Bonnet, S.; Ribeiro, F.; Porlier, A.; Maltais, F.; Bonnet, S.; et al. Impaired angiogenesis and peripheral muscle microcirculation loss contribute to exercise intolerance in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2014, 190, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Robertson, H.T. Dead space: The physiology of wasted ventilation. Eur. Respir. J. 2015, 45, 1704–1716. [Google Scholar] [CrossRef]
- Farina, S.; Correale, M.; Bruno, N.; Paolillo, S.; Salvioni, E.; Badagliacca, R.; Agostoni, P. The role of cardiopulmonary exercise tests in pulmonary arterial hypertension. Eur. Respir. Rev. 2018, 27, 170134. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, P.; Apostolo, A.; Perrone-Filardi, P.; Sciomer, S.; Palange, P.; Agostoni, P. A non invasive estimate of dead space ventilation from exercise measurements. PLoS ONE 2014, 9, e87395. [Google Scholar] [CrossRef][Green Version]
- Apostolo, A.; Laveneziana, P.; Palange, P.; Agalbato, C.; Molle, R.; Popovic, D.; Bussotti, M.; Internullo, M.; Sciomer, S.; Bonini, M.; et al. Impact of chronic obstructive pulmonary disease on exercise ventilatory efficiency in heart failure. Int. J. Cardiol. 2015, 189, 134–140. [Google Scholar] [CrossRef]
- Yasunobu, Y.; Oudiz, R.J.; Sun, X.G.; Hansen, J.E.; Wasserman, K. End-tidal PCO2 abnormality and exercise limitation in patients with primary pulmonary hypertension. Chest 2005, 127, 1637–1646. [Google Scholar] [CrossRef]
- Jones, N.L.; Robertson, D.G.; Kane, J.W. Difference between end-tidal and arterial PCO2 in exercise. J. Appl. Physiol. 1979, 47, 954–960. [Google Scholar] [CrossRef]
- Smith, D.D.; Agostoni, P.G. The discriminatory value of the P(A-a)O2 during exercise in the detection of asbestosis in asbestos exposed workers. Chest 1989, 95, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Morosin, M.; Vignati, C.; Novi, A.; Salvioni, E.; Veglia, F.; Alimento, M.; Merli, G.; Sciomer, S.; Sinagra, G.; Agostoni, P. The alveolar to arterial oxygen partial pressure difference is associated with pulmonary diffusing capacity in heart failure patients. Respir. Physiol. Neurobiol. 2016, 233, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.G.; Hansen, J.E.; Oudiz, R.J.; Wasserman, K. Exercise pathophysiology in patients with primary pulmonary hypertension. Circulation 2001, 104, 429–435. [Google Scholar] [CrossRef]
- D’Alonzo, G.E.; Gianotti, L.A.; Pohil, R.L.; Reagle, R.R.; DuRee, S.L.; Fuentes, F.; Dantzker, D.R. Comparison of progressive exercise performance of normal subjects and patients with primary pulmonary hypertension. Chest 1987, 92, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.S.; Porszasz, J.; Engelen, M.P.; Brundage, B.H.; Wasserman, K. Gas exchange responses to continuous incremental cycle ergometry exercise in primary pulmonary hypertension in humans. Eur. J. Appl. Physiol. 2000, 83, 63–70. [Google Scholar] [CrossRef]
- Deboeck, G.; Niset, G.; Lamotte, M.; Vachiéry, J.L.; Naeije, R. Exercise testing in pulmonary arterial hypertension and in chronic heart failure. Eur. Respir. J. 2004, 23, 747–751. [Google Scholar] [CrossRef]
- Guazzi, M.; Cahalin, L.P.; Arena, R. Cardiopulmonary exercise testing as a diagnostic tool for the detection of left-sided pulmonary hypertension in heart failure. J. Card. Fail. 2013, 19, 461–467. [Google Scholar] [CrossRef]
- Vicenzi, M.; Deboeck, G.; Faoro, V.; Loison, J.; Vachiéry, J.L.; Naeije, R. Exercise oscillatory ventilation in pulmonary arterial hypertension versus heart failure. Int. J. Cardiol. 2016, 202, 736–740. [Google Scholar] [CrossRef]
- Murphy, R.M.; Shah, R.V.; Malhotra, R.; Pappagianopoulos, P.P.; Hough, S.S.; Systrom, D.M.; Semigran, M.J.; Lewis, G.D. Exercise oscillatory ventilation in systolic heart failure: An indicator of impaired hemodynamic response to exercise. Circulation 2011, 124, 1442–1451. [Google Scholar] [CrossRef]
- Solin, P.; Bergin, P.; Richardson, M.; Kaye, D.M.; Walters, E.H.; Naughton, M.T. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation 1999, 99, 1574–1579. [Google Scholar] [CrossRef]
- Caravita, S.; Faini, S.; Deboeck, G.; Bondue, A.; Naeije, R.; Parati, G.; Vachiéry, J.L. Pulmonary hypertension and ventilation during exercise: Role of the precapillary component. J. Heart Lung Transplant. 2017, 36, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Caravita, S.; Faini, A.; Lombardi, C.; Valentini, M.; Gregorini, F.; Rossi, J.; Meriggi, P.; Di Rienzo, M.; Bilo, G.; Agostoni, P.; et al. Sex and acetazolamide effects on chemoreflex and periodic breathing during sleep at altitude. Chest 2015, 147, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Righini, F.M.; Apostolo, A.; Heck, P.B.; Farina, S.; Hager, A.; Correale, M.; Badagliacca, R.; Barbieri, S.; Sciomer, S.; Agostoni, P. Exercise physiology in pulmonary hypertension patients with and without congenital heart disease. Eur. J. Prev. Cardiol. 2019, 26, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Valli, G.; Vizza, C.D.; Onorati, P.; Badagliacca, R.; Ciuffa, R.; Poscia, R.; Brandimarte, F.; Fedele, F.; Serra, P.; Palange, P. Pathophysiological adaptations to walking and cycling in primary pulmonary hypertension. Eur. J. Appl. Physiol. 2008, 102, 417–424. [Google Scholar] [CrossRef]
- Laveneziana, P.; Montani, D.; Dorfmüller, P.; Girerd, B.; Sitbon, O.; Jaïs, X.; Savale, L.; Eyries, M.; Soubrier, F.; Similowski, T.; et al. Mechanisms of exertional dyspnoea in pulmonary veno-occlusive disease with EIF2AK4 mutations. Eur. Respir. J. 2014, 44, 1069–1072. [Google Scholar] [CrossRef]
- Pérez-Olivares, C.; Segura de la Cal, T.; Flox-Camacho, Á.; Nuche, J.; Tenorio, J.; Martínez Meñaca, A.; Cruz-Utrilla, A.; de la Cruz-Bertolo, J.; Pérez Núñez, M.; Spanish Pah Consortium; et al. The role of cardiopulmonary exercise test in identifying pulmonary veno-occlusive disease. Eur. Respir. J. 2021, 57, 2100115. [Google Scholar] [CrossRef]
- Dumitrescu, D.; Nagel, C.; Kovacs, G.; Bollmann, T.; Halank, M.; Winkler, J.; Hellmich, M.; Grünig, E.; Olschewski, H.; Ewert, R.; et al. Cardiopulmonary exercise testing for detecting pulmonary arterial hypertension in systemic sclerosis. Heart 2017, 103, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Santaniello, A.; Casella, R.; Vicenzi, M.; Rota, I.; Montanelli, G.; De Santis, M.; Bellocchi, C.; Lombardi, F.; Beretta, L. Cardiopulmonary exercise testing in a combined screening approach to individuate pulmonary arterial hypertension in systemic sclerosis. Rheumatology 2020, 59, 1581–1586. [Google Scholar] [CrossRef]
- Coghlan, J.G.; Denton, C.P.; Grunig, E.; Bonderman, D.; Distler, O.; Khanna, D.; Müller-Ladner, U.; Pope, J.E.; Vonk, M.C.; Doelberg, M.; et al. DETECT study group. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: The DETECT study. Ann. Rheum. Dis. 2014, 73, 1340–1349. [Google Scholar] [CrossRef]
- Bellan, M.; Giubertoni, A.; Piccinino, C.; Buffa, M.; Cromi, D.; Sola, D.; Pedrazzoli, R.; Gagliardi, I.; Calzaducca, E.; Zecca, E.; et al. Cardiopulmonary exercise testing is an accurate tool for the diagnosis of pulmonary arterial hypertension in scleroderma related diseases. Pharmaceuticals 2021, 14, 342. [Google Scholar] [CrossRef] [PubMed]
- Pudasaini, B.; Yang, G.L.; Yang, C.; Guo, J.; Yuan, P.; Wen-Ian, Y.; Zhang, R.; Wang, L.; Zhao, Q.H.; Gong, S.G.; et al. Characteristics of exercise capacity in female systemic lupus erythematosus associated pulmonary arterial hypertension patients. BMC Cardiovasc. Disord. 2018, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- McCabe, C.; Deboeck, G.; Harvey, I.; Ross, R.M.; Gopalan, D.; Screaton, N.; Pepke-Zaba, J. Inefficient exercise gas exchange identifies pulmonary hypertension in chronic thromboembolic obstruction following pulmonary embolism. Thromb. Res. 2013, 132, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Hirashiki, A.; Kondo, T.; Okumura, T.; Kamimura, Y.; Nakano, Y.; Fukaya, K.; Sawamura, A.; Morimoto, R.; Adachi, S.; Takeshita, K.; et al. Cardiopulmonary exercise testing as a tool for diagnosing pulmonary hypertension in patients with dilated cardiomyopathy. Ann. Noninvasive Electrocardiol. 2016, 21, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Boerrigter, B.G.; Bogaard, H.J.; Trip, P.; Groepenhoff, H.; Rietema, H.; Holverda, S.; Boonstra, A.; Postmus, P.E.; Westerhof, N.; Vonk-Noordegraaf, A. Ventilatory and cardiocirculatory exercise profiles in COPD: The role of pulmonary hypertension. Chest 2012, 142, 1166–1174. [Google Scholar] [CrossRef]
- van der Plas, M.N.; van Kan, C.; Blumenthal, J.; Jansen, H.M.; Wells, A.U.; Bresser, P. Pulmonary vascular limitation to exercise and survival in idiopathic pulmonary fibrosis. Respirology 2014, 19, 269–275. [Google Scholar] [CrossRef]
- Gläser, S.; Obst, A.; Koch, B.; Henkel, B.; Grieger, A.; Felix, S.B.; Halank, M.; Bruch, L.; Bollmann, T.; Warnke, C.; et al. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. The predictive value of exercise capacity and gas exchange efficiency. PLoS ONE 2013, 8, e65643. [Google Scholar] [CrossRef][Green Version]
- Westhoff, M.; Litterst, P.; Ewert, R. Cardiopulmonary exercise testing in combined pulmonary fibrosis and emphysema. Respiration 2021, 100, 395–403. [Google Scholar] [CrossRef]
- Pezzuto, B.; Badagliacca, R.; Muratori, F.; Farina, S.; Bussotti, M.; Correale, M.; Bonomi, A.; Vignati, C.; Sciomer, S.; Papa, S.; et al. Role of cardiopulmonary exercise test in the prediction of hemodynamic impairment in patients with pulmonary arterial hypertension. Pulm. Circ. 2022, 12, e12044. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef]
- Wensel, R.; Opitz, C.F.; Anker, S.D.; Winkler, J.; Höffken, G.; Kleber, F.X.; Sharma, R.; Hummel, M.; Hetzer, R.; Ewert, R. Assessment of survival in patients with primary pulmonary hypertension: Importance of cardiopulmonary exercise testing. Circulation 2002, 106, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Groepenhoff, H.; Vonk-Noordegraaf, A.; Boonstra, A.; Spreeuwenberg, M.D.; Postmus, P.E.; Bogaard, H.J. Exercise testing to estimate survival in pulmonary hypertension. Med. Sci. Sports Exerc. 2008, 40, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Schwaiblmair, M.; Faul, C.; von Scheidt, W.; Berghaus, T.M. Ventilatory efficiency testing as prognostic value in patients with pulmonary hypertension. BMC Pulm. Med. 2012, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Deboeck, G.; Scoditti, C.; Huez, S.; Vachiéry, J.L.; Lamotte, M.; Sharples, L.; Melot, C.; Naeije, R. Exercise testing to predict outcome in idiopathic versus associated pulmonary arterial hypertension. Eur. Respir. J. 2012, 40, 1410–1419. [Google Scholar] [CrossRef]
- Blumberg, F.C.; Arzt, M.; Lange, T.; Schroll, S.; Pfeifer, M.; Wensel, R. Impact of right ventricular reserve on exercise capacity and survival in patients with pulmonary hypertension. Eur. J. Heart Fail. 2013, 15, 771–775. [Google Scholar] [CrossRef]
- Wensel, R.; Francis, D.P.; Meyer, F.J.; Opitz, C.F.; Bruch, L.; Halank, M.; Winkler, J.; Seyfarth, H.J.; Gläser, S.; Blumberg, F.; et al. Incremental prognostic value of cardiopulmonary exercise testing and resting haemodynamics in pulmonary arterial hypertension. Int. J. Cardiol. 2013, 167, 1193–1198. [Google Scholar] [CrossRef]
- Groepenhoff, H.; Vonk-Noordegraaf, A.; van de Veerdonk, M.C.; Boonstra, A.; Westerhof, N.; Bogaard, H.J. Prognostic relevance of changes in exercise test variables in pulmonary arterial hypertension. PLoS ONE 2013, 8, e72013. [Google Scholar] [CrossRef]
- Ferreira, E.V.; Ota-Arakaki, J.S.; Ramos, R.P.; Barbosa, P.B.; Almeida, M.; Treptow, E.C.; Valois, F.M.; Nery, L.E.; Neder, J.A. Optimizing the evaluation of excess exercise ventilation for prognosis assessment in pulmonary arterial hypertension. Eur. J. Prev. Cardiol. 2014, 21, 1409–1419. [Google Scholar] [CrossRef]
- Rausch, C.M.; Taylor, A.L.; Ross, H.; Sillau, S.; Ivy, D.D. Ventilatory efficiency slope correlates with functional capacity, outcomes, and disease severity in pediatric patients with pulmonary hypertension. Int. J. Cardiol. 2013, 169, 445–448. [Google Scholar] [CrossRef]
- Badagliacca, R.; Papa, S.; Valli, G.; Pezzuto, B.; Poscia, R.; Manzi, G.; Giannetta, E.; Sciomer, S.; Palange, P.; Naeije, R.; et al. Echocardiography combined with cardiopulmonary exercise testing for the prediction of outcome in idiopathic pulmonary arterial hypertension. Chest 2016, 150, 1313–1322. [Google Scholar] [CrossRef]
- Badagliacca, R.; Papa, S.; Poscia, R.; Valli, G.; Pezzuto, B.; Manzi, G.; Torre, R.; Gianfrilli, D.; Sciomer, S.; Palange, P.; et al. The added value of cardiopulmonary exercise testing in the follow-up of pulmonary arterial hypertension. J. Heart Lung Transplant. 2019, 38, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Badagliacca, R.; Rischard, F.; Lo Giudice, F.; Howard, L.; Papa, S.; Valli, G.; Manzi, G.; Sciomer, S.; Palange, P.; Garcia, J.G.N.; et al. Incremental value of cardiopulmonary exercise testing in intermediate-risk pulmonary arterial hypertension. J. Heart Lung Transplant. 2022, 41, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Luo, Q.; Liu, Z.; Ma, X.; Zhao, Z.; Huang, Z.; Gao, L.; Jin, Q.; Xiong, C.; Ni, X. Oxygen uptake efficiency slope predicts poor outcome in patients with idiopathic pulmonary arterial hypertension. J. Am. Heart Assoc. 2017, 6, e005037. [Google Scholar] [CrossRef] [PubMed]
- Oudiz, R.J.; Midde, R.; Hovenesyan, A.; Sun, X.G.; Roveran, G.; Hansen, J.E.; Wasserman, K. Usefulness of right-to-left shunting and poor exercise gas exchange for predicting prognosis in patients with pulmonary arterial hypertension. Am. J. Cardiol. 2010, 105, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.P.; Arakaki, J.S.O.; Barbosa, P.; Treptow, E.; Valois, F.M.; Ferreia, E.V.M.; Nery, L.E.; Neder, A. Heart rate recovery in pulmonary arterial hypertension: Relationship with exercise capacity and prognosis. Am. Heart J. 2012, 163, 580–588. [Google Scholar] [CrossRef]
- Yuan, P.; Ni, H.J.; Chen, T.X.; Pudasaini, B.; Jiang, R.; Liu, H.; Zhao, Q.H.; Wang, L.; Gong, S.G.; Liu, J.M. Sex-specific cardiopulmonary exercise testing parameters as predictors in patients with idiopathic pulmonary arterial hypertension. Hypertens. Res. 2017, 40, 868–875. [Google Scholar] [CrossRef]
- Diller, G.P.; Dimopoulos, K.; Okonko, D.; Li, W.; Babu-Narayan, S.V.; Broberg, C.S.; Johansson, B.; Bouzas, B.; Mullen, M.J.; Poole-Wilson, P.A.; et al. Exercise Intolerance in adult congenital heart disease comparative severity, correlates, and prognostic implication. Circulation 2005, 112, 828–835. [Google Scholar] [CrossRef]
- Dimopoulos, K.; Okonko, D.O.; Diller, G.P.; Broberg, C.S.; Salukhe, T.V.; Babu-Narayan, S.V.; Li, W.; Uebing, A.; Bayne, S.; Wensel, R.; et al. Abnormal ventilatory response to exercise in adults with congenital heart disease relates to cyanosis and predicts survival. Circulation 2006, 113, 2796–2802. [Google Scholar] [CrossRef]
- Richter, M.J.; Pader, P.; Gall, H.; Reichenberger, F.; Seeger, W.; Mayer, E.; Guth, S.; Kramm, T.; Grimminger, F.; Ghofrani, H.A.; et al. The prognostic relevance of oxygen uptake in inoperable chronic thromboembolic pulmonary hypertension. Clin. Respir. J. 2017, 11, 682–690. [Google Scholar] [CrossRef]
- Zhong, X.J.; Jiang, R.; Yang, L.; Yuan, P.; Gong, S.G.; Zhao, Q.H.; Luo, C.J.; Qiu, H.L.; Li, H.T.; Zhang, R.; et al. Peak oxygen uptake is a strong prognostic predictor for pulmonary hypertension due to left heart disease. BMC Cardiovasc. Disord. 2022, 22, 137. [Google Scholar] [CrossRef]
- Barst, R.J.; Langleben, D.; Frost, A.; Horn, E.M.; Oudiz, R.; Shapiro, S.; McLaughlin, V.; Hill, N.; Tapson, V.F.; Robbins, I.M.; et al. Sitaxsentan therapy for pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2004, 169, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Oudiz, R.J.; Barst, R.J.; Hansen, J.E.; Sun, X.G.; Garofano, R.; Wu, X.; Wasserman, K. Cardiopulmonary exercise testing and six-minute walk correlations in pulmonary arterial hypertension. Am. J. Cardiol. 2006, 97, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Oudiz, R.J.; Roveran, G.; Hansen, J.E.; Sun, X.G.; Wasserman, K. Effect of sildenafil on ventilatory efficiency and exercise tolerance in pulmonary hypertension. Eur. J. Heart Fail. 2007, 9, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Barst, R.J.; Ivy, D.D.; Gaitan, G.; Szatmari, A.; Rudzinski, A.; Garcia, A.E.; Sastry, B.K.S.; Pulido, T.; Layton, G.R.; Serdarevic-Pehar, M.; et al. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation 2012, 125, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Rahaghi, F.N.; Naeije, R.; Oliveira, R.K.F.; Vanderpool, R.V.; Waxman, A.B.; Systrom, D.M. Dynamic right ventricular-pulmonary arterial uncoupling during maximum incremental exercise in exercise pulmonary hypertension and pulmonary arterial hypertension. Pulm. Circ. 2019, 9, 2045894019862435. [Google Scholar] [CrossRef]


| Reference | Patients | Main Results |
|---|---|---|
| McCabe C et al., Thromb. Res. 2013, 132, 659–665 [54] | 15 CTEPH 15 CTED |
|
| Guazzi et al., J. Card. Fail. 2013, 19, 461–467 [39] | 293 HF |
|
| Caravita et al., J. Heart Lung Transplant. 2017, 36, 754–762 [43] | 29 IpcPH 12 CpcPH 29Idiopathic/Heritable PAH |
|
| Hirashiki A et al., Ann. Noninvasive. Electrocardiol. 2016, 21, 263–271 [55] | 90 DCM |
|
| Boerrigter BG et al., Chest 2012, 142, 1166–1174 [56] | 47 COPD |
|
| van der Plas MN et al., Respirology 2014, 19, 269–275 [57] | 38 IPF |
|
| Gläser S et al., PLoS ONE 2013, 8, e65643 [58] | 135 IPF |
|
| Westhoff M et al., Respiration 2021, 100, 395–403 [59] | 41 CPFE |
|
| Reference | Patients | Main Results |
|---|---|---|
| Wensel et al., Circulation 2002, 106, 319–324 [62] | 76 PPH |
|
| Groepenhoff H et al., Med. Sci. Sports Exerc. 2008, 40, 1725–1732 [63] | 127 PAH and CTEPH |
|
| Schwaiblmair et al., BMC Pulm. Med. 2012, 12, 23 [64] | 116 PAH and CTEPH |
|
| Deboeck et al., Eur. Respir. J. 2012, 40, 1410–1419 [65] | 136 PAH (idiopathic and associated) |
|
| Blumberg et al., Eur. J. Heart Fail. 2013, 15, 771–775 [66] | 36 PAH and CTEPH |
|
| Wensel et al., Int. J. Cardiol. 2013, 167, 1193–1198 [67] | 226 idiopathic and familial PAH |
|
| Groepenhoff H. et al., PloS ONE 2013, 8, e72013 [68] | 65 idiopathic and heritable PAH |
|
| Ferreira EV et al., Eur. J. Prev. Cardiol. 2014, 21, 1409–1419 [69] | 84 idiopathic and associated PAH |
|
| Rausch CM et al., Int. J. Cardiol. 2013, 169, 445–448 [70] | 76 pediatric PH |
|
| Badagliacca R et al., Chest 2016, 150, 1313–1322 [71] | 102 IPAH |
|
| Badagliacca R et al., J. Heart Lung. Transplant. 2019, 38, 306–314 [72] | 80 IPAH, HPAH, drug-induced PAH 80 HPAH, drug-induced PAH (validation cohort) |
|
| Badagliacca R et al., J. Heart Lung Transplant. 2022, 41, 780–790 [73] | 124 IPAH 143 IPAH (validation cohort) |
|
| Tang Y et al., J. Am. Heart Assoc. 2017, 6, e005037 [74] | 210 PAH |
|
| Oudiz RJ et al., Am. J. Cardiol. 2010, 105, 1186–1191 [75] | 103 PAH |
|
| Ramos R.P. et al., Am. Heart J. 2012, 163, 580–588 [76] | 72 PAH |
|
| Yuan P. et al., Hypertens Res. 2017, 40, 868–875 [77] | 57 (21 male/36female) IPAH |
|
| Diller G.P. et al., Circulation 2005, 112, 828–835 [78] | 335 ACHD 40 non-congenital HF 23 healthy subjects |
|
| Dimopoulos K. et al., Circulation 2006, 113, 2796–2802 [79] | 560 ACHD 50 healthy subjects |
|
| Richter M.J. et al., Clin. Respir. J. 2017, 11, 682–690 [80] | 151 CTEPH |
|
| Zhong X.J. et al., BMC Cardiovasc, Disord. 2002, 22, 137 [81] | 89 PH-LHD |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pezzuto, B.; Agostoni, P. The Current Role of Cardiopulmonary Exercise Test in the Diagnosis and Management of Pulmonary Hypertension. J. Clin. Med. 2023, 12, 5465. https://doi.org/10.3390/jcm12175465
Pezzuto B, Agostoni P. The Current Role of Cardiopulmonary Exercise Test in the Diagnosis and Management of Pulmonary Hypertension. Journal of Clinical Medicine. 2023; 12(17):5465. https://doi.org/10.3390/jcm12175465
Chicago/Turabian StylePezzuto, Beatrice, and Piergiuseppe Agostoni. 2023. "The Current Role of Cardiopulmonary Exercise Test in the Diagnosis and Management of Pulmonary Hypertension" Journal of Clinical Medicine 12, no. 17: 5465. https://doi.org/10.3390/jcm12175465
APA StylePezzuto, B., & Agostoni, P. (2023). The Current Role of Cardiopulmonary Exercise Test in the Diagnosis and Management of Pulmonary Hypertension. Journal of Clinical Medicine, 12(17), 5465. https://doi.org/10.3390/jcm12175465





