Two-Year Results of Injectable Matrix-Associated Autologous Chondrocyte Transplantation in the Hip Joint: Significant Improvement in Clinical and Radiological Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Surgical Technique
2.3. Rehabilitation
2.4. Manufacturing Process of NOVOCART® Inject
2.5. Magnetic Resonance Imaging and Evaluation
2.6. Statistical Analysis
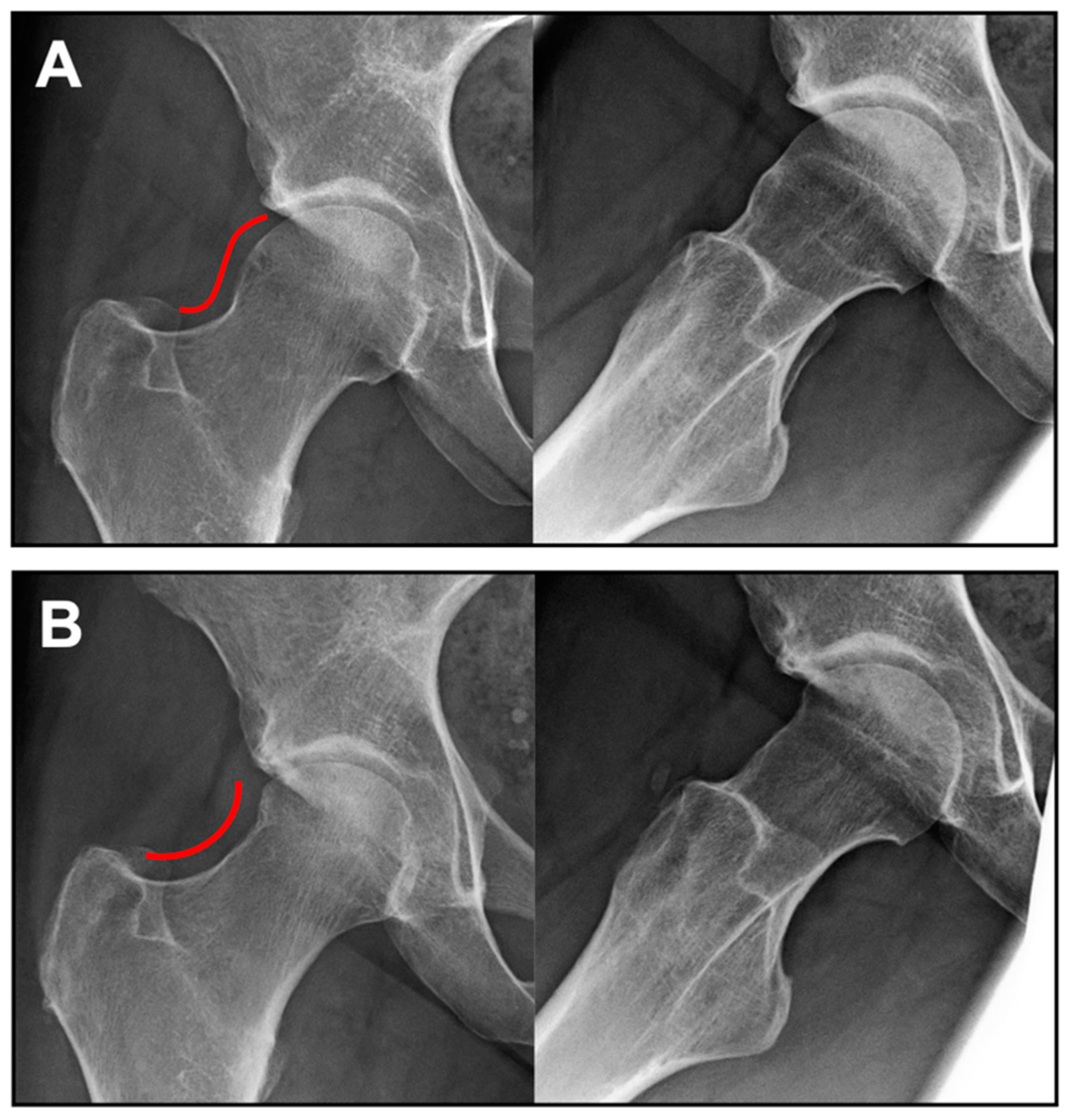
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ganz, R.; Parvizi, J.; Beck, M.; Leunig, M.; Nötzli, H.; Siebenrock, K.A. Femoroacetabular impingement: A cause for osteoarthritis of the hip. Clin. Orthop. Relat. Res. 2003, 417, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Ganz, R.; Leunig, M.; Leunig-Ganz, K.; Harris, W.H. The etiology of osteoarthritis of the hip: An integrated mechanical concept. Clin. Orthop. Relat. Res. 2008, 466, 264–272. [Google Scholar] [CrossRef]
- Jordan, M.A.; Thiel, G.S.V.; Chahal, J.; Nho, S.J. Operative treatment of chondral defects in the hip joint: A systematic review. Curr. Rev. Musculoskelet. Med. 2012, 5, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Fickert, S.; Aurich, M.; Albrecht, D.; Angele, P.; Büchler, L.; Dienst, M.; Erggelet, C.; Fritz, J.; Gebhart, C.; Gollwitzer, H.; et al. Biologic Reconstruction of Full Sized Cartilage Defects of the Hip: A Guideline from the DGOU Group ‘Clinical Tissue Regeneration’ and the Hip Committee of the AGA. Z. Orthop. Unfallchir. 2017, 155, 670–682. [Google Scholar] [CrossRef]
- Brittberg, M.; Winalski, C.S. Evaluation of cartilage injuries and repair. J. Bone Jt. Surg. Am. 2003, 85 (Suppl. 2), 58–69. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.A.; Peters, C.L.; Park, B.B.; Stoddard, G.J.; Erickson, J.A.; Crim, J.R. Acetabular cartilage delamination in femoroacetabular impingement. Risk factors and magnetic resonance imaging diagnosis. J. Bone Jt. Surg. Am. 2009, 91, 305–313. [Google Scholar] [CrossRef]
- Meyer, D.C.; Beck, M.; Ellis, T.; Ganz, R.; Leunig, M. Comparison of six radiographic projections to assess femoral head/neck asphericity. Clin. Orthop. Relat. Res. 2006, 445, 181–185. [Google Scholar] [CrossRef]
- Mohtadi, N.G.; Griffin, D.R.; Pedersen, M.E.; Chan, D.; Safran, M.R.; Parsons, N.; Sekiya, J.K.; Kelly, B.T.; Werle, J.R.; Leunig, M.; et al. The Development and validation of a self-administered quality-of-life outcome measure for young, active patients with symptomatic hip disease: The International Hip Outcome Tool (iHOT-33): The International Hip Outcome Tool (iHOT-33). Arthroscopy 2012, 28, 595–605. [Google Scholar] [CrossRef]
- The EuroQol Group. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Benz, K.; Freudigmann, C.; Müller, J.; Wurst, H.; Albrecht, D.; Badke, A.; Gaissmaier, C.; Mollenhauer, J. A Polyethylene Glycol-Crosslinked Serum Albumin/Hyaluronan Hydrogel for the Cultivation of Chondrogenic Cell Types. Adv. Eng. Mater. 2010, 12, B539–B551. [Google Scholar] [CrossRef]
- Scholz, B.; Kinzelmann, C.; Benz, K.; Mollenhauer, J.; Wurst, H.; Schlosshauer, B. Suppression of adverse angiogenesis in an albumin-based hydrogel for articular cartilage and intervertebral disc regeneration. Eur. Cells Mater. 2010, 20, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Lazik, A.; Körsmeier, K.; Claßen, T.; Jäger, M.; Kamminga, M.; Kraff, O.; Lauenstein, T.C.; Theysohn, J.M.; Landgraeber, S. 3 Tesla high-resolution and delayed gadolinium enhanced MR imaging of cartilage (dGEMRIC) after autologous chondrocyte transplantation in the hip. J. Magn. Reson. Imaging 2015, 42, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Trattnig, S.; Ba-Ssalamah, A.; Pinker, K.; Plank, C.; Vecsei, V.; Marlovits, S. Matrix-based autologous chondrocyte implantation for cartilage repair: Noninvasive monitoring by high-resolution magnetic resonance imaging. Magn. Reson. Imaging 2005, 23, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Bretschneider, H.; Trattnig, S.; Landgraeber, S.; Hartmann, A.; Günther, K.-P.; Dienst, M.; Schröder, J.; Fickert, S. Arthroscopic matrix-associated, injectable autologous chondrocyte transplantation of the hip: Significant improvement in patient-related outcome and good transplant quality in MRI assessment. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Marlovits, S.; Striessnig, G.; Resinger, C.T.; Aldrian, S.M.; Vecsei, V.; Imhof, H.; Trattnig, S. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur. J. Radiol. 2004, 52, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Marlovits, S.; Singer, P.; Zeller, P.; Mandl, I.; Haller, J.; Trattnig, S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: Determination of interobserver variability and correlation to clinical outcome after 2 years. Eur. J. Radiol. 2006, 57, 16–23. [Google Scholar] [CrossRef]
- De Windt, T.S.; Welsch, G.H.; Brittberg, M.; Vonk, L.A.; Marlovits, S.; Trattnig, S.; Saris, D.B. Is magnetic resonance imaging reliable in predicting clinical outcome after articular cartilage repair of the knee? A systematic review and meta-analysis. Am. J. Sports Med. 2013, 41, 1695–1702. [Google Scholar] [CrossRef]
- McCarthy, H.S.; McCall, I.W.; Williams, J.M.; Mennan, C.; Dugard, M.N.; Richardson, J.B.; Roberts, S. Magnetic Resonance Imaging Parameters at 1 Year Correlate with Clinical Outcomes Up to 17 Years After Autologous Chondrocyte Implantation. Orthop. J. Sports Med. 2018, 6, 2325967118788280. [Google Scholar] [CrossRef]
- Welsch, G.H.; Mamisch, T.C.; Zak, L.; Mauerer, A.; Apprich, S.; Stelzeneder, D.; Marlovits, S.; Trattnig, S. Morphological and biochemical T2 evaluation of cartilage repair tissue based on a hybrid double echo at steady state (DESS-T2d) approach. J. Magn. Reson. Imaging 2011, 34, 895–903. [Google Scholar] [CrossRef]
- Aldrian, S.; Zak, L.; Wondrasch, B.; Albrecht, C.; Stelzeneder, B.; Binder, H.; Kovar, F.; Trattnig, S.; Marlovits, S. Clinical and radiological long-term outcomes after matrix-induced autologous chondrocyte transplantation: A prospective follow-up at a minimum of 10 years. Am. J. Sports Med. 2014, 42, 2680–2688. [Google Scholar] [CrossRef]
- Mancini, D.; Fontana, A. Five-year results of arthroscopic techniques for the treatment of acetabular chondral lesions in femoroacetabular impingement. Int. Orthop. 2014, 38, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Biant, L.C.; Simons, M.; Gillespie, T.; McNicholas, M.J. Cell Viability in Arthroscopic Versus Open Autologous Chondrocyte Implantation. Am. J. Sports Med. 2017, 45, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Pietschmann, M.F.; Horng, A.; Niethammer, T.; Pagenstert, I.; Sievers, B.; Jansson, V.; Glaser, C.; Müller, P.E. Cell quality affects clinical outcome after MACI procedure for cartilage injury of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2009, 17, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.B.; Stähli, A. Surgical suturing of articular cartilage induces osteoarthritis-like changes. Osteoarthr. Cartil. 2008, 16, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Fickert, S.; Schattenberg, T.; Niks, M.; Weiss, C.; Thier, S. Feasibility of arthroscopic 3-dimensional, purely autologous chondrocyte transplantation for chondral defects of the hip: A case series. Arch. Orthop. Trauma Surg. 2014, 134, 971–978. [Google Scholar] [CrossRef]
- Thier, S.; Baumann, F.; Weiss, C.; Fickert, S. Feasibility of arthroscopic autologous chondrocyte implantation in the hip using an injectable hydrogel. Hip Int. 2018, 28, 442–449. [Google Scholar] [CrossRef]
- Körsmeier, K.; Claßen, T.; Kamminga, M.; Rekowski, J.; Jäger, M.; Landgraeber, S. Arthroscopic three-dimensional autologous chondrocyte transplantation using spheroids for the treatment of full-thickness cartilage defects of the hip joint. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 2032–2037. [Google Scholar] [CrossRef]
- Thier, S.; Weiss, C.; Fickert, S. Arthroscopic autologous chondrocyte implantation in the hip for the treatment of full-thickness cartilage defects—A case series of 29 patients and review of the literature. SICOT-J 2017, 3, 72. [Google Scholar] [CrossRef]
- Krueger, D.R.; Gesslein, M.; Schuetz, M.; Perka, C.; Schroeder, J.H. Injectable autologous chondrocyte implantation (ACI) in acetabular cartilage defects-three-year results. J. Hip Preserv. Surg. 2018, 5, 386–392. [Google Scholar] [CrossRef]
- Fontana, A.; Bistolfi, A.; Crova, M.; Rosso, F.; Massazza, G. Arthroscopic treatment of hip chondral defects: Autologous chondrocyte transplantation versus simple debridement—A pilot study. Arthroscopy 2012, 28, 322–329. [Google Scholar] [CrossRef]
- Jannelli, E.; Fontana, A. Arthroscopic treatment of chondral defects in the hip: AMIC, MACI, microfragmented adipose tissue transplantation (MATT) and other options. SICOT-J 2017, 3, 43. [Google Scholar] [CrossRef] [PubMed]


| Sex | ||
| Female | n (%) | 4 (19.1) |
| Male | n (%) | 17 (81.0) |
| Age (years) | Mean ± SD | 32.3 ± 10.0 |
| BMI (kg/m2) | Mean ± SD | 25.5 ± 3.6 |
| Smoking status | ||
| Yes | n (%) | 5 (23.8) |
| No | n (%) | 16 (76.2) |
| Defect side | ||
| Right hip | n (%) | 14 (66.7) |
| Left hip | n (%) | 7 (33.3) |
| Number of lesions | ||
| 1 | n (%) | 19 (90.5) |
| 2 | n (%) | 2 (9.5) |
| Total defect size (cm2) | Mean ± SD | 3.0 ± 1.4 |
| Range | 1.5–8.0 | |
| Defect localization | ||
| Acetabulum only | n (%) | 19 (90.5) |
| Acetabulum + head | n (%) | 2 (9.5) |
| ICRS grading | ||
| Grade III | n (%) | 17 (81.0) |
| Grade IV | n (%) | 4 (19.1) |
| Previous hip surgery | ||
| Yes | n (%) | 3 (14.3) |
| No | n (%) | 18 (85.7) |
| Preoperative | 6 Month Follow-Up | 12 Month Follow-Up | 24 Month Follow-Up | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| iHOT 33 | 52.9 | 21.1 | 76.8 | 19.8 | 82.0 | 21.9 | 85.8 | 14.8 |
| EQ-5D-5L | 0.75 | 0.17 | 0.90 | 0.08 | 0.92 | 0.08 | 0.95 | 0.05 |
| EQ-5D VAS | 67.0 | 20.1 | 83.5 | 14.0 | 85.9 | 11.6 | 86.7 | 9.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riedl, M.; Bretschneider, H.; Dienst, M.; Günther, K.-P.; Landgraeber, S.; Schröder, J.; Trattnig, S.; Fickert, S. Two-Year Results of Injectable Matrix-Associated Autologous Chondrocyte Transplantation in the Hip Joint: Significant Improvement in Clinical and Radiological Assessment. J. Clin. Med. 2023, 12, 5468. https://doi.org/10.3390/jcm12175468
Riedl M, Bretschneider H, Dienst M, Günther K-P, Landgraeber S, Schröder J, Trattnig S, Fickert S. Two-Year Results of Injectable Matrix-Associated Autologous Chondrocyte Transplantation in the Hip Joint: Significant Improvement in Clinical and Radiological Assessment. Journal of Clinical Medicine. 2023; 12(17):5468. https://doi.org/10.3390/jcm12175468
Chicago/Turabian StyleRiedl, Moritz, Henriette Bretschneider, Michael Dienst, Klaus-Peter Günther, Stefan Landgraeber, Jörg Schröder, Siegfried Trattnig, and Stefan Fickert. 2023. "Two-Year Results of Injectable Matrix-Associated Autologous Chondrocyte Transplantation in the Hip Joint: Significant Improvement in Clinical and Radiological Assessment" Journal of Clinical Medicine 12, no. 17: 5468. https://doi.org/10.3390/jcm12175468
APA StyleRiedl, M., Bretschneider, H., Dienst, M., Günther, K.-P., Landgraeber, S., Schröder, J., Trattnig, S., & Fickert, S. (2023). Two-Year Results of Injectable Matrix-Associated Autologous Chondrocyte Transplantation in the Hip Joint: Significant Improvement in Clinical and Radiological Assessment. Journal of Clinical Medicine, 12(17), 5468. https://doi.org/10.3390/jcm12175468






