The Impact of Growth Hormone Therapy on Sleep-Related Health Outcomes in Children with Prader–Willi Syndrome: A Review and Clinical Analysis
Abstract
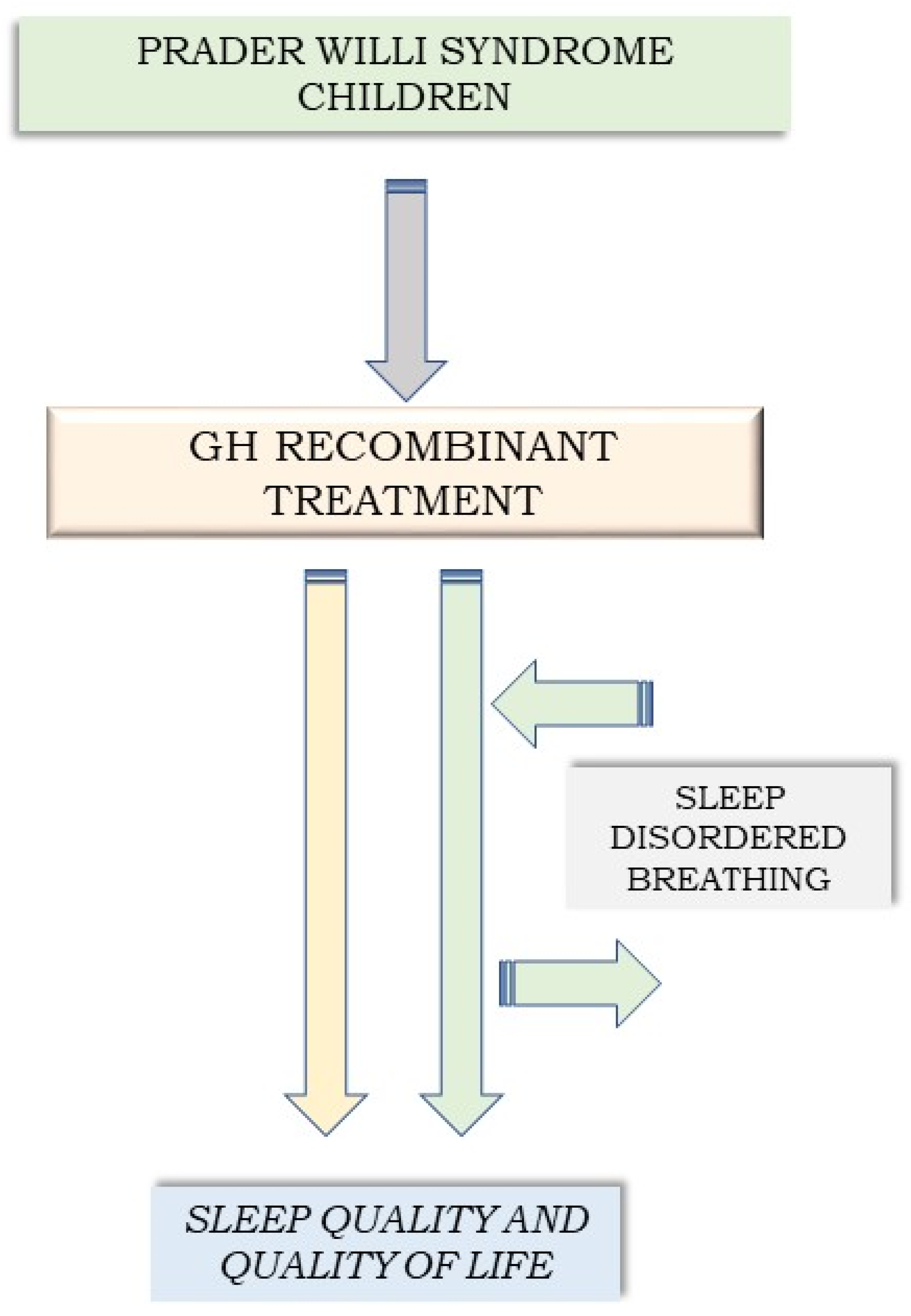
:1. Introduction
Study Aims
2. Materials and Methods
- (1)
- (children OR infants OR pediatric) AND (“growth hormone” OR GH OR “GH deficiency” OR “GH treatment”) AND (“sleep quality” OR “sleep pattern” OR “sleep duration and efficiency” OR (rest OR “sleep depth” OR “good sleep quality” OR “sleep satisfaction” OR “sleep health”) AND (“Prader–Willi syndrome”);
- (2)
- (children OR infants OR pediatric) AND (polysomnography OR “nighttime apnea” OR “disrupted breathing during sleep” OR “sleep-related respiratory disorders” OR “obstructive sleep apnea syndrome”) AND (“GH treatment” OR “GH therapy” OR “GH replacement therapy”) AND (“Prader–Willi syndrome”);
- (3)
- (children OR infants OR pediatric) AND (polysomnography OR “nighttime apnea” OR “disrupted breathing during sleep” OR “sleep-related respiratory disorders” OR “obstructive sleep apnea syndrome”) AND (“growth hormone” OR GH OR “GH deficiency” OR “GH treatment”) AND (“Prader–Willi syndrome”).
3. Results
| Author | Year Published | Country | Study Design | PWS Patients—No. | Age | Methods | SDB Severity | rhGH | Outcome | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|
| Haqq et al. | 2003 | USA | RCT | 14 (7 males) | 4.5–14.5 yrs | rhGH therapy (or placebo for 6 mths, then alternative intervention for 6 mths), PSG (at 0, 6, and 12 mths) | PWS vs. normals: apnea events 50.6 ± 69.0/h vs. 27.3 ± 20.4/h (p = 0.26); hypopneic events 146 ± 55/h vs. 114 ± 65/h, p = 0.18 | 0.043 mg/kg/day in 6 patients | rhGH therapy improved sleep, behavior, cognition | Hypopnea and apnea improved after rhGH therapy |
| Miller et al. [34] | 2006 | USA | Longitudinal | 25 (15 males) | 6 mths to 39 yrs | rhGH therapy (baseline and after 6 wks), PSG (repeat PSG after 6 mths of GH therapy) | GH improved AHI by mean of 1.2/h (p = 0.02); CA by median of 1.7/h (p < 0.001) | 0.24 mg/kg/wk | AHI improved after rhGH therapy; OA worsened in 6 patients (3 males) | AHI improved after rhGH therapy; initial transient worsening of SDB in patient subset |
| Festen et al. [31] | 2006 | Netherlands | RCT | 53 prepubertal (30 males) | Median of 5.4 yrs (IQR 2.1–7.2) | rhGH therapy, PSG (repeat PSG after 6 mths of GH therapy) in 39 patients | AHI 5.1 (2.8–8.7)/h; CA 2.8 (1.5–5.4)/h | Somatropin 1 mg/m2/day | No differences in SDB and SpO2 before and after 6 mths of rhGH therapy; AHI decreased after 6 mths of rhGH therapy | No worsening of SDB during rhGH therapy |
| Craig et al. [41] | 2006 | Australia | Prospective | 328 (83.5% prepubertal) | Median of 6.0 yrs (prepubertal median 12.7 yrs) | rhGH therapy, PSG (repeat PSG after 6 mths of GH therapy) | Case 1 died following presumed SA 3 mths after rhGH initiated | 0.23 (0.15–0.31) mg/kg/wk in prepubertal and 0.22 (0.12–0.30) mg/kg/wk in pubertal children | Sudden death (bronchopneumonia, respiratory failure, SA) in 5/675 cases | GH therapy to be used with caution in patients with extreme obesity or SDB |
| Williams et al. [30] | 2008 | USA | Observational | 37 (54% males) | 9 ± 6 (range of 15 mths to 24 yrs) | rhGH therapy, PSG, Multiple Sleep Latency Test (MSLT) | Mean AHI of 17/h (n = 37); mean CA of 1.7/h (n = 37) | Mean of 0.22 mg/kg/wk (n = 16), treated for 4.8 yrs (range of 1.5–12) | All patients had SA; no difference in AHI, CA, SpO2 between the rhGH and the non-rhGH groups | rhGH therapy did not affect SDBs |
| Miller et al. [29] | 2009 | USA | Pilot | 20 infants (12 males) | 2–21 mths | rhGH therapy, PSG (repeat sleep studies at 6 wks after GH therapy initiated) | Pre-OA: median of 35.8/h; post-OA: 34.8/h; pre-CA: median of 25.2/h; post-CA: 27.1/h | Start 1 mg/m2/day | rhGH therapy did not affect SDBs; increased OA associated with respiratory infections or GER in 12 children after rhGH therapy | rhGH therapy did not affect SDBs |
| Fillion et al. [24] | 2009 | Canada | Retrospective | 23 (14 males) | 8.6 yrs (range of 1.3–13.5) in GH group; 5.0 yrs (range of 2.0–13.0) in no-GH group (p = 0.43) | rhGH therapy (10 patients treated for 0.1 to 5.5 yrs), PSG (patients with signs and symptoms of OSA) | 2/10 died, 1 developed OSA 2 mths after starting rhGH; OSA disappeared after GH discontinued | 0.25 mg/kg/wk (range of 0.14 to 0.42) | rhGH associated with OSA | Did not directly evaluate correlation between GH therapy and sleep |
| Salvatoni et al. [28] | 2009 | Italy | Longitudinal observational | 34 non-severely obese (20 males) (children with OA and/or severe obesity excluded) | 0.94–11.8 yrs; median of 2.24 | rhGH therapy, PSG, ENT evaluation | OAHI increased in 8/16 (50%) children and decreased in 5/16 (31%) | 0.03 mg/kg/day | AHI increased in 50% of patients (not significant) after 6 wks of rhGH therapy; rhGH did not cause upper-airway obstruction | Short-term rhGH therapy did not cause upper airway obstruction. No increase in AHI in patients receiving rhGH vs. controls |
| DeMarcantonio et al. [27] | 2010 | USA | Retrospective | 5 | Median of 5.1 yrs (range of 1.1–16.7) | OSA surgery; effect of rhGH therapy on OSA; PSG (post-operative PSG performed at median of 8.7 mths (range of 1.75–33.3) after surgery | Median AHI decreased from 16.4/h to 4.4/h (p = 0.274) | NA, (3 patients received therapy and 2 received treatment prior to surgery) | Complete resolution of OSA challenging to achieve with upper-airway surgery | PSG evaluation for OSA in children considered for rhGH therapy |
| Meyer et al. [42] | 2012 | USA | Comparative | 13 (7 males) | rhGH therapy initiated at median of 8.5 mths (range of 2 mths to 6 yrs) | rhGH, A&T, PSG (PSG repeated if upper-airway surgery performed) | 9/13 patients with mild to moderate OSA; breathing normalized after A&T in 8/9 | NA | Increase in CAs may occur; 2 children with highest postoperative AHI aged 2 yrs and 6 mths, respectively, had initiated rhGH at age 4 and 2 mths, respectively | Starting rhGH therapy at younger age may have contributed to development of OSA at younger age |
| Katz-Salamon et al. [35] | 2012 | Sweden | Clinical case series | 16 (7 males) | rhGH initiated at median age of 30 mths (range of 5–63 mths), | rhGH therapy follow-up at 6 mths (range of 2–32) after initiating GH, basal PSG | Before GH treatment: AHI (h): 1.32 (0.3–2.8)/h; On GH treatment: AHI: 0.8 (0.1–26)/h, p = 0.06 | NA (start at median age 30 mths (range of 5–63) | Gender, age at initiating rhGH therapy, and duration did not influence cardio-respiratory responsiveness to CO2/O2 | rhGH therapy improved circulatory stress and function; saturation in GH-treated children better, although AHI remained unchanged |
| Berini et al. [43] | 2013 | Italy | Observational | 75 | 1.9 yrs, IQR 2.2 (0.4–7.8) | rhGH therapy, PSG (prior to treatment, up to 4 yrs) | AHI > 1 during treatment in 11/50 (22%) patients | 0.010 to 0.030 mg/kg/day | AHI improved with progressive decrease in CAI during rhGH therapy; therapy temporarily discontinued in 3 patients | rhGH therapy did not impair respiratory function during sleep |
| Vandeleur et al. [23] | 2013 | Australia | Observational | 34 prepubertal children (17 males) | Mean of 7.3 yrs (range of 3 mths–16.3 yrs) | PSG prior to rhGH therapy | OSA in 15/34 (44%) patients | NA | CAI > 5/h in 4/34 children (range of 0.7–15.6 yrs); rhGH therapy deferred in 38% | OSA diagnosed in 44% before starting GH therapy; more likely in older children |
| Meinhardt et al. [39] | 2013 | Switzerland | Clinical trial | 41 prepubertal children | Mean of 3.8 ± 3.0 yrs | Medical records of children receiving rhGH therapy for over 12 mths, (PSG not stated) | SA recorded in 3 (7.3%) patients | 0.03–0.06 mg/kg/day for 4.1 yrs (range of 0.9–9.5) | Apnea recorded in 1/41 patients on long-term rhGH | No serious adverse effects reported |
| Cohen et al. [44] | 2014 | Canada | Observational | 44 (20 males) | Median of 1.9 yrs (range of 0.3–15.6) | rhGH therapy PSG | Median CAI: 10.6 (range of 5.0–68.3)/h. Median oAHI in those with OSA: 4.0 (range of 1.5–57.0)/h); OSA predominated in older children (52% vs. 5% p = 0.001) | NA | CA more likely to occur in infants; OA more prevalent than CA in older children | Most common indication for referral was evaluation prior to initiation of GH therapy |
| Pavone et al. [38] | 2015 | Italy | Multicentric | 88 (44 males) | Median of 5.1 yrs (range of 0.3–44.3) (IQR 1.0–14.5) | rhGH therapy, overnight respiratory polygraphy (before GH initiated) | Mixed AHI of 1.8 [0.6–5.0]/h, and CAI of 0.1 [0.0–0.6]/h | NA | PGs performed in routine care before initiating rhGH therapy; rhGH started in 48 patients | High prevalence of SDB and number of therapeutic interventions after PG; aim of rhGH therapy to improve natural course of disease |
| Khayat et al. [37] | 2017 | Canada | Observational | 28 infants (12 males) | Median of 0.9 yrs (IQR 0.5–1.1); median at follow-up PSG of 2.1 yrs (1.5–2.6) | rhGH therapy, PSG (baseline PSG before age 2 yrs and follow-up PSG) | Median CAI at baseline: 6.6 (IQR 2.6, 12.1)/h improved to 2.3/h (p < 0.0001) | Initial GH dose 0.03 mg/kg/day | rhGH therapy initiated after baseline assessment in 19 infants; 10 infants with CSA at baseline started on rhGH therapy. Follow-up PSG revealed resolution of CSA in 7/10 infants | GH therapy did not appear to potentiate CSA |
| Scheermeyer et al. [26] | 2017 | Australia | Observational | 31 infants (55% males); 42 toddlers (48% males) | Infants aged 2–12 mths; toddlers 13–24 mths | Low-dose rhGH in children (2–24 mths); PSG (before and within 6 mths after GH therapy initiated) | Mild to severe central and/or OSA in 40% of children prior to rhGH; onset or worsening of OSA in 2 infants in first mths and in 6 after 6–24 mths. | 4.5 mg/m2/wk | rhGH therapy discontinued in 8 (11%) children due to increase in OSA in first 24 mths of therapy; severe OSA developed in first mths in 2 children | Possible increased risk of OSA during first wks of therapy |
| Lecka-Ambroziak et al. [45] | 2017 | Poland | Observational | 36 | Group 1: before rhGH 3.0 ± 3.0 yrs; Group 1a: after short-term rhGH 2.5 ± 0.8) yrs; Group 2: on rhGH 8.8 ± 5.1 yrs; Group 3: without rhGH 13.1 ± 4.4 yrs (severely obese) | rhGH therapy, PSG before (Group 1) and after initiating rhGH (Group 1a), on rhGH therapy for 4 ± 3.0 years (Group 2) and patients not treated due to severe obesity (Group 3) | OSA, AHI (/h): Group 1: 10.2 (±6.9); Group 1a: 12.0 (±5.8); Group 2: 9.0 (±6.5); Group 3: 8.2 (±5.4) | 0.019 mg/kg/day (±0.006) | No difference in AHI before and after initiation of rhGH therapy between patients on rhGH therapy and untreated severely obese patients | Did not validate the hypothesis that initiation of rhGH therapy worsens OSA due to growth of lymphoid tissue; limitation: wide range in patient age |
| Zimmermann et al. [8] | 2020 | Germany | Longitudinal | 62 (31 males); 21 initiated GH-therapy during and 41 after first year of life | 0–2.5 yrs at baseline Group A (21 children): initiated GH-therapy during and Group B (41 children): after first year of life | rhGH, overnight respiratory polygraphy (before therapy (t0), after 3 (t1), 6 (t2) mths, and 1.2 (t3), 2.2 (t4), and 3.2 yrs (t5) after GH therapy initiated | Group A (5/21, 23.8%) patients versus Group B (15/41, 36.6%) patients with obstructive sleep apnea (OAHI ≥ 1.5) | 0.028 mg/kg/day (range of 0.000–0.037) | No significant differences in OSA and CA, regardless of age at initiation of rhGH therapy; OSA increased during first 3 mths of therapy but decreased after 1 year; ODI changed during rhGH therapy | Increased incidence of severe OSA from baseline to 6 mths after GH therapy initiated |
| Caudri et al. [36] | 2022 | Australia | Multicenter | 112 | Median of 1.9 yrs (range of 0.1–13.5) at start of GH therapy | PSG before and after rhGH initiated | PSG (n = 94) included in analysis prior to initiating GH; median obstructive AHI 0.40 (range 0–4.9)/h, PSG after rhGH therapy 0.50 (0–51.7)/h; p = 0.13 | NA | Worsening of OSA severity in 13% of children | Early identification of worsening OSA may prevent severe sequelae in subset of children; PSG should be performed after initiation of rhGH therapy to monitor for worsening OSA |
| Schaefer et al. [32] | 2022 | Australia | Retrospective single-center chart review | 17 (8 males) on rhGH | Median of 11.6 yrs (range of 6.6–16.1) | rhGH, PSG (routine PSG pre- or post-GH therapy) | Total RDI/h: 9.8 (3.9–14.8); central AHI 4.2 (1.9–11.1)/h) | 9 (53%) children on rhGH therapy at time of index PSG, and on therapy for median of 6.8 yrs (IQR 3.2–8.0) | 15 (88%) had SDB, including CSA (n = 3, 18%), OSA (n = 4, 24%), both OSA and CSA (n = 5, 29%) | SDBs frequently recorded, also in patients on long-term rhGH therapy |
| Tan et al. [33] | 2022 | Canada | Retrospective | 29 (41% males); 87 controls (46% males) | PWS, 4.4 ± 5.2 yrs; controls 4.4 ± 5.1 yrs | rhGH, 24 (82%) patients underwent PSG prior to rhGH; 12 before and after starting rhGH | AHI: PWS: 7.3 (IQR 11.8)/h; controls 6.0 (IQR 13.8); CI 2.9 (IQR 10.1)/h in patients with PWS vs. controls 1.8 (5.4)/h | rhGH therapy in 6 (21%) children | 24/29 (82%) children with PWS underwent PSG prior to initiating rhGH therapy. No change in PSG parameters at 6.8 mths (95% CI 2.0, 11.7) after starting rhGH with a mean difference of 1.0 yrs (95% CI 1.3, 0.80) between PSG | Increases and decreases in respiratory events recorded after initiation of rhGH therapy, which did not affect respiratory parameters |
4. Discussion
4.1. GH Administration and Sleep-Disordered Breathing
4.2. Influencing Variables
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwartz, J.R.; Roth, T. Neurophysiology of sleep and wakefulness: Basic science and clinical implications. Curr. Neuropharmacol. 2008, 6, 367–378. [Google Scholar] [CrossRef]
- Alrousan, G.; Hassan, A.; Pillai, A.A.; Atrooz, F.; Salim, S. Early Life Sleep Deprivation and Brain Development: Insights From Human and Animal Studies. Front. Neurosci. 2022, 16, 833786. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Mastorakos, G.; Bixler, E.O.; Kales, A.; Gold, P.W.; Chrousos, G.P. Sleep deprivation effects on the activity of the hypothalamic-pituitary-adrenal and growth axes: Potential clinical implications. Clin. Endocrinol. 1999, 51, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Iughetti, L.; Tornese, G.; Street, M.E.; Napoli, F.; Giavoli, C.; Antoniazzi, F.; Stagi, S.; Luongo, C.; Azzolini, S.; Ragusa, L.; et al. Long-term safety and efficacy of Omnitrope®, a somatropin biosimilar, in children requiring growth hormone treatment: Italian interim analysis of the PATRO Children study. Ital. J. Pediatr. 2016, 42, 93. [Google Scholar] [CrossRef]
- Grimberg, A.; DiVall, S.A.; Polychronakos, C.; Allen, D.B.; Cohen, L.E.; Quintos, J.B.; Rossi, W.C.; Feudtner, C.; Murad, M.H. Guidelines for Growth Hormone and Insulin-Like Growth Factor-I Treatment in Children and Adolescents: Growth Hormone Deficiency, Idiopathic Short Stature, and Primary Insulin-Like Growth Factor-I Deficiency. Horm. Res. Paediatr. 2016, 86, 361–397. [Google Scholar] [CrossRef]
- Antoniazzi, F.; Cavarzere, P.; Gaudino, R. Growth hormone and early treatment. Minerva Endocrinol. 2015, 40, 129–143. [Google Scholar] [PubMed]
- Cavarzere, P.; Gaudino, R.; Sandri, M.; Ramaroli, D.A.; Pietrobelli, A.; Zaffanello, M.; Guzzo, A.; Salvagno, G.L.; Piacentini, G.; Antoniazzi, F. Growth hormone retesting during puberty: A cohort study. Eur. J. Endocrinol. 2020, 182, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Laemmer, C.; Woelfle, J.; Fimmers, R.; Gohlke, B. Sleep-Disordered Breathing in Children with Prader-Willi Syndrome in Relation to Growth Hormone Therapy Onset. Horm. Res. Paediatr. 2020, 93, 85–93. [Google Scholar] [CrossRef]
- Goldstone, A.P.; Holland, A.J.; Hauffa, B.P.; Hokken-Koelega, A.C.; Tauber, M. Recommendations for the diagnosis and management of Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 4183–4197. [Google Scholar] [CrossRef]
- Deal, C.L.; Tony, M.; Höybye, C.; Allen, D.B.; Tauber, M.; Christiansen, J.S. GrowthHormone Research Society workshop summary: Consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2013, 98, E1072–E1087. [Google Scholar] [CrossRef]
- Lyons, M.M.; Bhatt, N.Y.; Pack, A.I.; Magalang, U.J. Global burden of sleep-disordered breathing and its implications. Respirology 2020, 25, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Zaffanello, M.; Lippi, G.; Arman, N.; Piazza, M.; Tenero, L.; Piacentini, G. Popularity of sleep disordered breathing in childhood: An analysis of worldwide search using Google Trends. Transl. Pediatr. 2019, 8, 383–390. [Google Scholar] [CrossRef]
- Zaffanello, M.; Ferrante, G.; Zoccante, L.; Ciceri, M.L.; Nosetti, L.; Tenero, L.; Piazza, M.; Piacentini, G. Predictive Power of Oxygen Desaturation Index (ODI) and Apnea-Hypopnea Index (AHI) in Detecting Long-Term Neurocognitive and Psychosocial Outcomes of Sleep-Disordered Breathing in Children: A Questionnaire-Based Study. J. Clin. Med. 2023, 12, 3060. [Google Scholar] [CrossRef]
- Zaffanello, M.; Piacentini, G.; La Grutta, S. Beyond the growth delay in children with sleep-related breathing disorders: A systematic review. Panminerva Med. 2020, 62, 164–175. [Google Scholar] [CrossRef]
- Lagravère, M.O.; Zecca, P.A.; Caprioglio, A.; Fastuca, R. Metabolic effects of treatment in patients with obstructive sleep apnea: A systematic review. Minerva Pediatr. 2019, 71, 380–389. [Google Scholar] [CrossRef]
- Tagetti, A.; Bonafini, S.; Zaffanello, M.; Benetti, M.V.; Vedove, F.D.; Gasperi, E.; Cavarzere, P.; Gaudino, R.; Piacentini, G.; Minuz, P.; et al. Sleep-disordered breathing is associated with blood pressure and carotid arterial stiffness in obese children. J. Hypertens. 2016, 35, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Lo Bue, A.; Salvaggio, A.; Insalaco, G. Obstructive sleep apnea in developmental age. A narrative review. Eur. J. Pediatr. 2020, 179, 357–365. [Google Scholar] [CrossRef]
- Brockmann, P.E.; Gozal, D. Neurocognitive Consequences in Children with Sleep Disordered Breathing: Who Is at Risk? Children 2022, 9, 1278. [Google Scholar] [CrossRef]
- Todd, C.A.; Bareiss, A.K.; McCoul, E.D.; Rodriguez, K.H. Adenotonsillectomy for Obstructive Sleep Apnea and Quality of Life: Systematic Review and Meta-analysis. Otolaryngol.–Head. Neck Surg. Off. J. Am. Acad. Otolaryngol.-Head. Neck Surg. 2017, 157, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Zaffanello, M.; Antoniazzi, F.; Tenero, L.; Nosetti, L.; Piazza, M.; Piacentini, G. Sleep-disordered breathing in paediatric setting: Existing and upcoming of the genetic disorders. Ann. Transl. Med. 2018, 6, 17. [Google Scholar] [CrossRef]
- Gerard, J.M.; Garibaldi, L.; Myers, S.E.; Aceto, T., Jr.; Kotagal, S.; Gibbons, V.P.; Stith, J.; Weber, C. Sleep apnea in patients receiving growth hormone. Clin. Pediatr. 1997, 36, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Mistretta, A.; Modica, D.M.; Pitruzzella, A.; Burgio, S.; Lorusso, F.; Billone, S.; Valenti, C.; Vita, G.; Poma, S.; Amata, M.; et al. OSAHS Growth Impairment and Resolution after Adenotonsillectomy in Children. Iran. J. Otorhinolaryngol. 2022, 34, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Vandeleur, M.; Davey, M.J.; Nixon, G.M. Are sleep studies helpful in children with Prader-Willi syndrome prior to commencement of growth hormone therapy? J. Paediatr. Child. Health 2013, 49, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Fillion, M.; Deal, C.; Van Vliet, G. Retrospective study of the potential benefits and adverse events during growth hormone treatment in children with Prader-Willi syndrome. J. Pediatr. 2009, 154, 230–233. [Google Scholar] [CrossRef]
- Haqq, A.M.; Stadler, D.D.; Jackson, R.H.; Rosenfeld, R.G.; Purnell, J.Q.; LaFranchi, S.H. Effects of growth hormone on pulmonary function, sleep quality, behavior, cognition, growth velocity, body composition, and resting energy expenditure in Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 2206–2212. [Google Scholar] [CrossRef]
- Scheermeyer, E.; Harris, M.; Hughes, I.; Crock, P.A.; Ambler, G.; Verge, C.F.; Bergman, P.; Werther, G.; Craig, M.E.; Choong, C.S.; et al. Low dose growth hormone treatment in infants and toddlers with Prader-Willi syndrome is comparable to higher dosage regimens. Growth Horm. IGF Res. 2017, 34, 1–7. [Google Scholar] [CrossRef]
- DeMarcantonio, M.A.; Darrow, D.H.; Gyuricsko, E.; Derkay, C.S. Obstructive sleep disorders in Prader-Willi syndrome: The role of surgery and growth hormone. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 1270–1272. [Google Scholar] [CrossRef]
- Salvatoni, A.; Veronelli, E.; Nosetti, L.; Berini, J.; de Simone, S.; Iughetti, L.; Bosio, L.; Chiumello, G.; Grugni, G.; Delu, G.; et al. Short-term effects of growth hormone treatment on the upper airways of non severely obese children with Prader-Willi syndrome. J. Endocrinol. Investig. 2009, 32, 601–605. [Google Scholar] [CrossRef]
- Miller, J.L.; Shuster, J.; Theriaque, D.; Driscoll, D.J.; Wagner, M. Sleep disordered breathing in infants with Prader-Willi syndrome during the first 6 weeks of growth hormone therapy: A pilot study. J. Clin. Sleep. Med. 2009, 5, 448–453. [Google Scholar] [CrossRef]
- Williams, K.; Scheimann, A.; Sutton, V.; Hayslett, E.; Glaze, D.G. Sleepiness and sleep disordered breathing in Prader-Willi syndrome: Relationship to genotype, growth hormone therapy, and body composition. J. Clin. Sleep. Med. 2008, 4, 111–118. [Google Scholar] [CrossRef]
- Festen, D.A.; de Weerd, A.W.; van den Bossche, R.A.; Joosten, K.; Hoeve, H.; Hokken-Koelega, A.C. Sleep-related breathing disorders in prepubertal children with Prader-Willi syndrome and effects of growth hormone treatment. J. Clin. Endocrinol. Metab. 2006, 91, 4911–4915. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, J.; Davey, M.J.; Nixon, G.M. Sleep-disordered breathing in school-aged children with Prader-Willi syndrome. J. Clin. Sleep. Med. 2022, 18, 1055–1061. [Google Scholar] [CrossRef]
- Tan, Q.; He, X.T.T.; Kang, S.; Haqq, A.M.; MacLean, J.E. Preserved Sleep for the Same Level of Respiratory Disturbance in Children with Prader-Willi Syndrome. Int. J. Mol. Sci. 2022, 23, 580. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Silverstein, J.; Shuster, J.; Driscoll, D.J.; Wagner, M. Short-term effects of growth hormone on sleep abnormalities in Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Katz-Salamon, M.; Lindgren, A.C.; Cohen, G. The effect of growth hormone on sleep-related cardio-respiratory control in Prader-Willi syndrome. Acta Paediatr. Int. J. Paediatr. 2012, 101, 643–648. [Google Scholar] [CrossRef]
- Caudri, D.; Nixon, G.M.; Nielsen, A.; Mai, L.; Hafekost, C.R.; Kapur, N.; Seton, C.; Tai, A.; Blecher, G.; Ambler, G.; et al. Sleep-disordered breathing in Australian children with Prader-Willi syndrome following initiation of growth hormone therapy. J. Paediatr. Child. Health 2022, 58, 248–255. [Google Scholar] [CrossRef]
- Khayat, A.; Narang, I.; Bin-Hasan, S.; Amin, R.; Al-Saleh, S. Longitudinal evaluation of sleep disordered breathing in infants with Prader-Willi syndrome. Arch. Dis. Child. 2017, 102, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Pavone, M.; Caldarelli, V.; Khirani, S.; Colella, M.; Ramirez, A.; Aubertin, G.; Crinò, A.; Brioude, F.; Gastaud, F.; Beydon, N.; et al. Sleep disordered breathing in patients with Prader-Willi syndrome: A multicenter study. Pediatr. Pulmonol. 2015, 50, 1354–1359. [Google Scholar] [CrossRef]
- Meinhardt, U.; Christiansen, J.S.; Farholt, S.; Lammer, C.; Ostergaard, J.R.; Schmidt, F.; Kappelgaard, A.M.; Eiholzer, U. The efficacy and safety of long-term Norditropin(R) treatment in children with Prader-Willi syndrome. Horm. Metab. Res. 2013, 45, 532–536. [Google Scholar] [CrossRef]
- Lecka-Ambroziak, A.; Wysocka-Mincewicz, M.; Świercz, A.; Jędrzejczak, M.; Szalecki, M. Comparison of Frequency and Severity of Sleep-Related Breathing Disorders in Children with Simple Obesity and Paediatric Patients with Prader-Willi Syndrome. J. Pers. Med. 2021, 11, 141. [Google Scholar] [CrossRef]
- Craig, M.E.; Cowell, C.T.; Larsson, P.; Zipf, W.B.; Reiter, E.O.; Albertsson Wikland, K.; Ranke, M.B.; Price, D.A. Growth hormone treatment and adverse events in Prader-Willi syndrome: Data from KIGS (the Pfizer International Growth Database). Clin. Endocrinol. 2006, 65, 178–185. [Google Scholar] [CrossRef]
- Meyer, S.L.; Splaingard, M.; Repaske, D.R.; Zipf, W.; Atkins, J.; Jatana, K. Outcomes of adenotonsillectomy in patients with Prader-Willi syndrome. Arch. Otolaryngol. Head. Neck Surg. 2012, 138, 1047–1051. [Google Scholar] [CrossRef]
- Berini, J.; Spica Russotto, V.; Castelnuovo, P.; Di Candia, S.; Gargantini, L.; Grugni, G.; Iughetti, L.; Nespoli, L.; Nosetti, L.; Padoan, G.; et al. Growth hormone therapy and respiratory disorders: Long-term follow-up in PWS children. J. Clin. Endocrinol. Metab. 2013, 98, E1516–E1523. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Hamilton, J.; Narang, I. Clinically important age-related differences in sleep related disordered breathing in infants and children with Prader-Willi Syndrome. PLoS ONE 2014, 9, e101012. [Google Scholar] [CrossRef]
- Lecka-Ambroziak, A.; Jedrzejczak, M.; Wysocka-Mincewicz, M.; Szalecki, M. Sleep-related breathing disorders in patients with Prader-Willi syndrome depending on the period of growth hormone treatment. Endokrynol. Pol. 2017, 68, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Stafler, P.; Wallis, C. Prader-Willi syndrome: Who can have growth hormone? Arch. Dis. Child. 2008, 93, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Garnier, P.; Raynaud, F.; Job, J.C. Growth hormone secretion during sleep. I. Comparison with GH responses to conventional pharmacologic stimuli in pubertal and early pubertal short subjects. Effects of treatment with human GH in patients with discrepant measurements of GH secretion. Horm. Res. 1988, 29, 133–139. [Google Scholar] [CrossRef]
- Morris, C.J.; Aeschbach, D.; Scheer, F.A. Circadian system, sleep and endocrinology. Mol. Cell Endocrinol. 2012, 349, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.C.; Mong, J.A. Neuroendocrine Control of Sleep. Curr. Top. Behav. Neurosci. 2019, 43, 353–378. [Google Scholar] [CrossRef] [PubMed]
- Carrel, A.L.; Myers, S.E.; Whitman, B.Y.; Eickhoff, J.; Allen, D.B. Long-term growth hormone therapy changes the natural history of body composition and motor function in children with prader-willi syndrome. J. Clin. Endocrinol. Metab. 2010, 95, 1131–1136. [Google Scholar] [CrossRef]
- Reus, L.; Zwarts, M.; van Vlimmeren, L.A.; Willemsen, M.A.; Otten, B.J.; Nijhuis-van der Sanden, M.W. Motor problems in Prader-Willi syndrome: A systematic review on body composition and neuromuscular functioning. Neurosci. Biobehav. Rev. 2011, 35, 956–969. [Google Scholar] [CrossRef]
- Bridges, N. What is the value of growth hormone therapy in Prader Willi syndrome? Arch. Dis. Child. 2014, 99, 166–170. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaffanello, M.; Pietrobelli, A.; Piacentini, G.; Guzzo, A.; Antoniazzi, F. The Impact of Growth Hormone Therapy on Sleep-Related Health Outcomes in Children with Prader–Willi Syndrome: A Review and Clinical Analysis. J. Clin. Med. 2023, 12, 5504. https://doi.org/10.3390/jcm12175504
Zaffanello M, Pietrobelli A, Piacentini G, Guzzo A, Antoniazzi F. The Impact of Growth Hormone Therapy on Sleep-Related Health Outcomes in Children with Prader–Willi Syndrome: A Review and Clinical Analysis. Journal of Clinical Medicine. 2023; 12(17):5504. https://doi.org/10.3390/jcm12175504
Chicago/Turabian StyleZaffanello, Marco, Angelo Pietrobelli, Giorgio Piacentini, Alessandra Guzzo, and Franco Antoniazzi. 2023. "The Impact of Growth Hormone Therapy on Sleep-Related Health Outcomes in Children with Prader–Willi Syndrome: A Review and Clinical Analysis" Journal of Clinical Medicine 12, no. 17: 5504. https://doi.org/10.3390/jcm12175504
APA StyleZaffanello, M., Pietrobelli, A., Piacentini, G., Guzzo, A., & Antoniazzi, F. (2023). The Impact of Growth Hormone Therapy on Sleep-Related Health Outcomes in Children with Prader–Willi Syndrome: A Review and Clinical Analysis. Journal of Clinical Medicine, 12(17), 5504. https://doi.org/10.3390/jcm12175504






