Cellular and Molecular Mechanisms of Vestibular Ageing
Abstract
:1. Cellular and Molecular Mechanisms of Vestibular Ageing
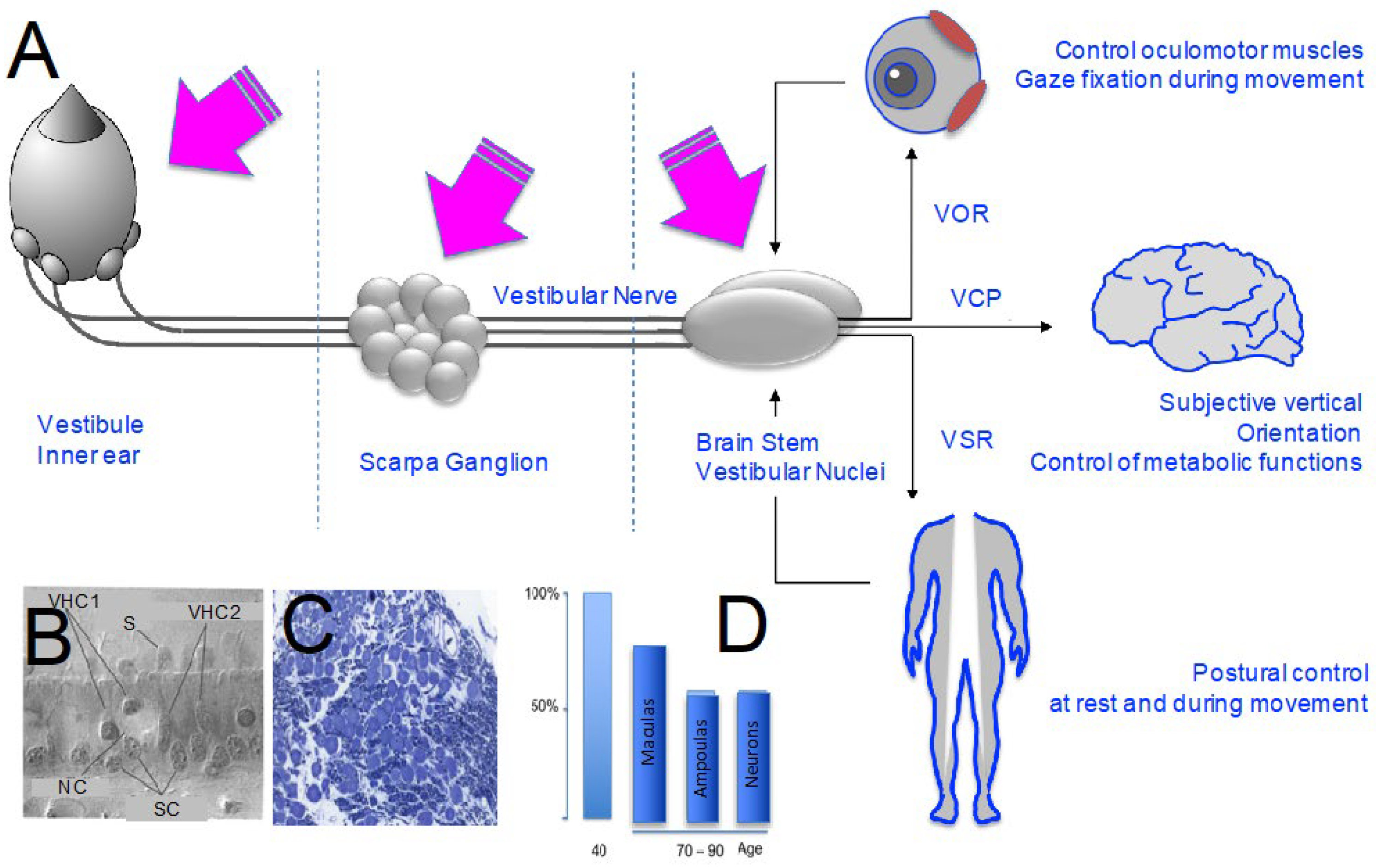
2. Ageing of the Peripheral Vestibular System
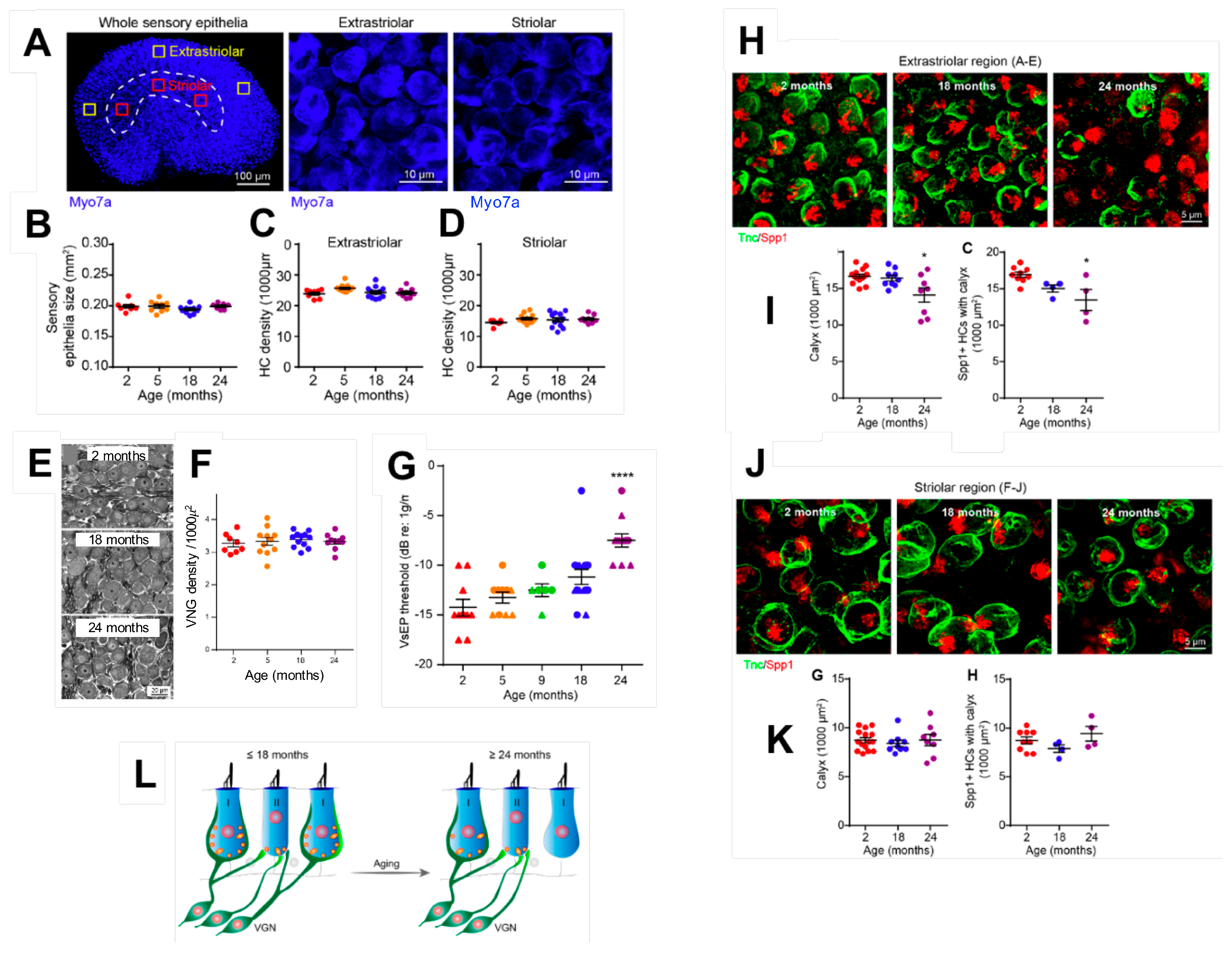
2.1. Age-Related Cell Loss in the Vestibule
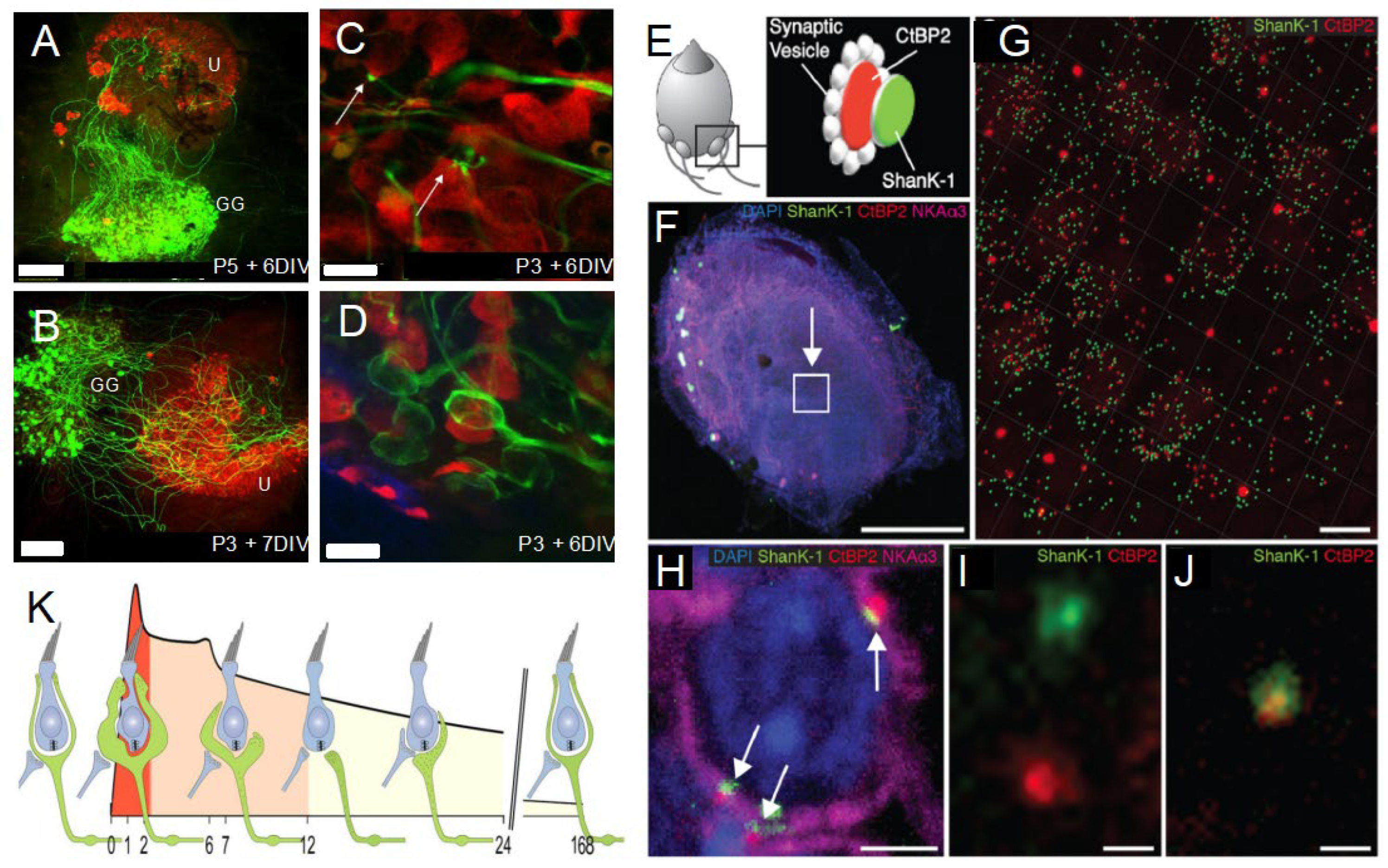
2.2. Age-Related Synaptic Loss and Vestibular Ageing
2.3. Conditions That Accelerate Age-Related Synaptic Loss
2.4. Spontaneous Endogenous Mechanisms to Slow down Age-Related Synaptic Loss
2.5. Current Data on Ageing Affecting Otoconia Metabolism
2.6. Functional Consequences of Age-Related Damages to the Peripheral Vestibular System
3. Ageing of Central Vestibular System
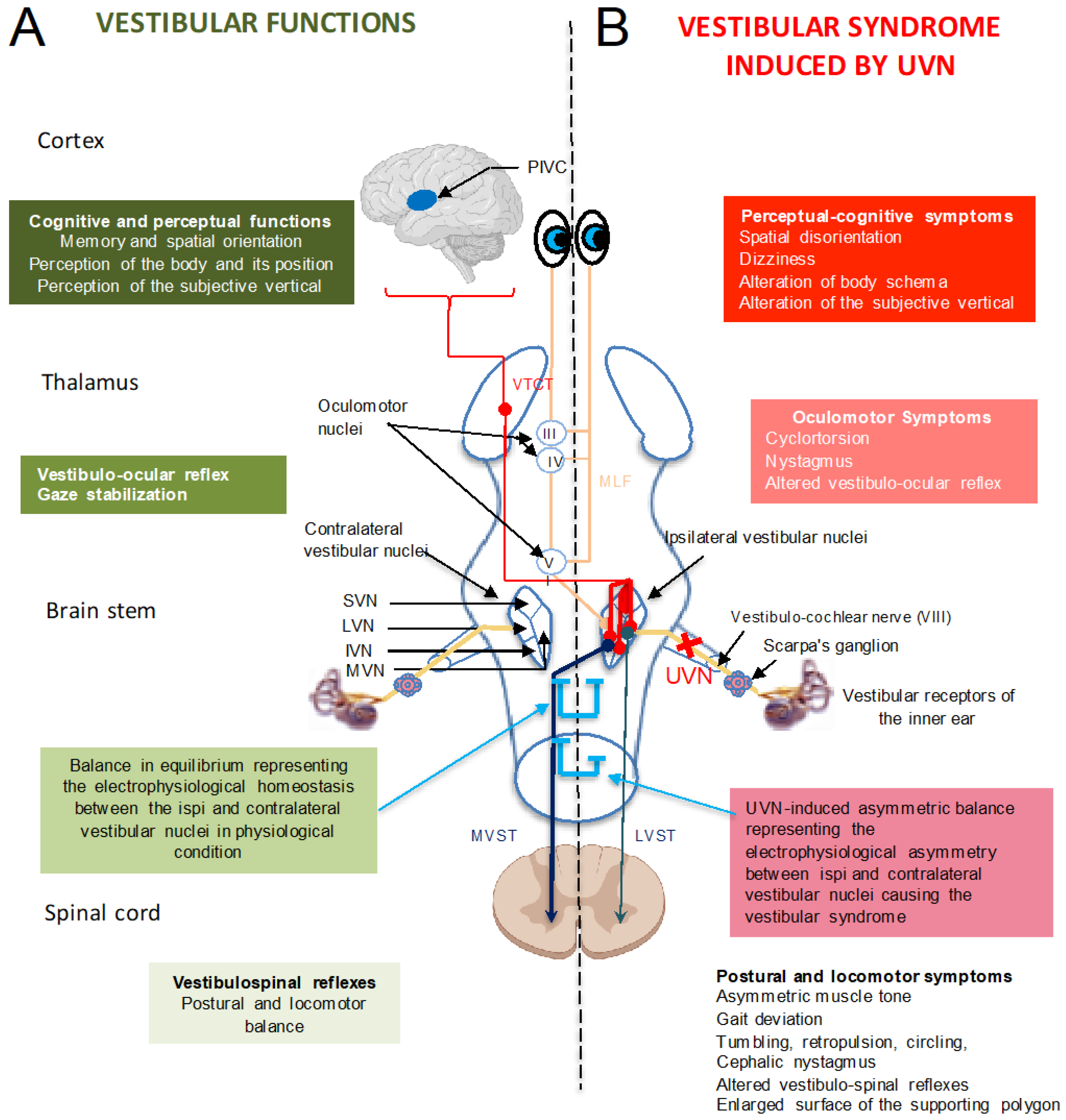
3.1. Central Integration of Vestibular Information: The Vestibular Nuclei, a Central Information Processing Hub
3.2. Vestibular Syndrome: A Range of Oculomotor, Postural, Perceptual, Cognitive and Vegetative Symptoms
3.3. Vestibular Compensation upon Ageing
3.4. Anatomical Changes in the Vestibular Nuclei during Ageing
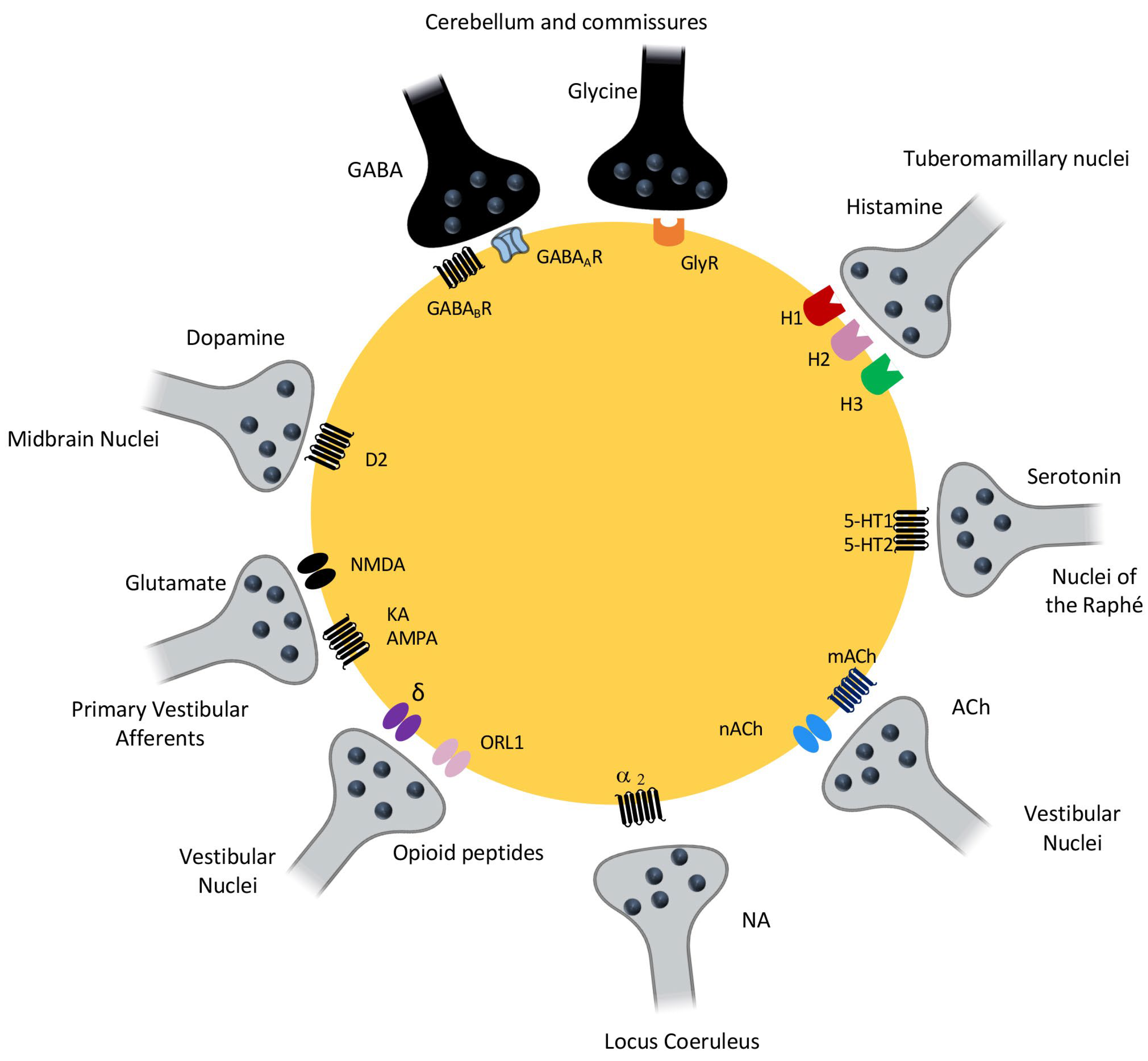
3.5. Neurochemical Changes in the Vestibular Nuclei during Ageing
3.5.1. Changes in the Glutamatergic and GABAergic Systems
3.5.2. Changes in Monoaminergic Systems
3.6. Other Changes in Neurobiological Factors during Aging That Are Essential for Vestibular Compensation
3.7. Example of Vestibular Compensation in Older Adults and Animals
3.8. Re-Emergence of a Critical Post-Injury Period Reproducing Developmental Plasticity Processes
3.9. Future Insights into the Study of Vestibular Aging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Merchant, S.N.; Velázquez-Villaseñor, L.; Tsuji, K.; Glynn, R.J.; Wall, C.; Rauch, S.D. Temporal Bone Studies of the Human Peripheral Vestibular System. Normative Vestibular Hair Cell Data. Ann. Otol. Rhinol. Laryngol. Suppl. 2000, 181, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Vignaux, G.; Chabbert, C.; Gaboyard-Niay, S.; Travo, C.; Machado, M.L.; Denise, P.; Comoz, F.; Hitier, M.; Landemore, G.; Philoxène, B.; et al. Evaluation of the Chemical Model of Vestibular Lesions Induced by Arsanilate in Rats. Toxicol. Appl. Pharmacol. 2012, 258, 61–71. [Google Scholar] [CrossRef]
- Rosenhall, U. Degenerative Patterns in The Aging Human Vestibular Neuro-Epithelia. Acta Otolaryngol. 1973, 76, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Anniko, M. The Aging Vestibular Hair Cell. Am. J. Otolaryngol. 1983, 4, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Matheson, A.J.; Darlington, C.L.; Smith, P.F. Dizziness in the Older adults and Age-Related Degeneration of the Vestibular System. N. Z. J. Psychol. 1999, 28, 10–16. [Google Scholar]
- Campos, A.; Cañizares, F.J.; Sánchez-Quevedo, M.C.; Romero, P.J. Otoconial Degeneration in the Aged Utricle and Saccule. Adv. Otorhinolaryngol. 1990, 45, 143–153. [Google Scholar] [CrossRef]
- Furman, J.M.; Redfern, M.S. Effect of Aging on the Otolith-Ocular Reflex. J. Vestib. Res. Equilib. Orientat. 2001, 11, 91–103. [Google Scholar] [CrossRef]
- Lim, D.J. Otoconia in Health and Disease. A Review. Ann. Otol. Rhinol. Laryngol. Suppl. 1984, 112, 17–24. [Google Scholar] [CrossRef]
- Maes, L.; Dhooge, I.; D’haenens, W.; Bockstael, A.; Keppler, H.; Philips, B.; Swinnen, F.; Vinck, B.M. The Effect of Age on the Sinusoidal Harmonic Acceleration Test, Pseudorandom Rotation Test, Velocity Step Test, Caloric Test, and Vestibular-Evoked Myogenic Potential Test. Ear Hear. 2010, 31, 84–94. [Google Scholar] [CrossRef]
- Walther, L.E.; Westhofen, M. Presbyvertigo-Aging of Otoconia and Vestibular Sensory Cells. J. Vestib. Res. Equilib. Orientat. 2007, 17, 89–92. [Google Scholar] [CrossRef]
- Tighilet, B.; Chabbert, C. Adult Neurogenesis Promotes Balance Recovery after Vestibular Loss. Prog. Neurobiol. 2019, 174, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Cassel, R.; Bordiga, P.; Carcaud, J.; Simon, F.; Beraneck, M.; Le Gall, A.; Benoit, A.; Bouet, V.; Philoxene, B.; Besnard, S.; et al. Morphological and Functional Correlates of Vestibular Synaptic Deafferentation and Repair in a Mouse Model of Acute-Onset Vertigo. Dis. Model. Mech. 2019, 12, dmm039115. [Google Scholar] [CrossRef]
- Rosenhall, U.; Rubin, W. Degenerative Changes in the Human Vestibular Sensory Epithelia. Acta Otolaryngol. 1975, 79, 67–80. [Google Scholar] [CrossRef]
- Richter, E. Quantitative Study of Human Scarpa’s Ganglion and Vestibular Sensory Epithelia. Acta Otolaryngol. 1980, 90, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Bergström, B. Morphology of the Vestibular Nerve. II. The Number of Myelinated Vestibular Nerve Fibers in Man at Various Ages. Acta Otolaryngol. 1973, 76, 173–179. [Google Scholar] [CrossRef]
- Velázquez-Villaseñor, L.; Merchant, S.N.; Tsuji, K.; Glynn, R.J.; Wall, C.; Rauch, S.D. Temporal Bone Studies of the Human Peripheral Vestibular System. Normative Scarpa’s Ganglion Cell Data. Ann. Otol. Rhinol. Laryngol. Suppl. 2000, 181, 14–19. [Google Scholar] [CrossRef]
- Zalewski, C.K. Aging of the Human Vestibular System. Semin. Hear. 2015, 36, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Ji, L.; Schrepfer, T.; Gong, S.; Wang, G.-P.; Corfas, G. Synaptopathy as a Mechanism for Age-Related Vestibular Dysfunction in Mice. Front. Aging Neurosci. 2019, 11, 156. [Google Scholar] [CrossRef]
- Gaboyard-Niay, S.; Travo, C.; Saleur, A.; Broussy, A.; Brugeaud, A.; Chabbert, C. Correlation between Afferent Rearrangements and Behavioral Deficits after Local Excitotoxic Insult in the Mammalian Vestibule: A Rat Model of Vertigo Symptoms. Dis. Model. Mech. 2016, 9, 1181–1192. [Google Scholar] [CrossRef]
- Cassel, R.; Bordiga, P.; Pericat, D.; Hautefort, C.; Tighilet, B.; Chabbert, C. New Mouse Model for Inducing and Evaluating Unilateral Vestibular Deafferentation Syndrome. J. Neurosci. Methods 2018, 293, 128–135. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Adding Insult to Injury: Cochlear Nerve Degeneration after “Temporary” Noise-Induced Hearing Loss. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef] [PubMed]
- Halmagyi, G.M.; Weber, K.P.; Curthoys, I.S. Vestibular Function after Acute Vestibular Neuritis. Restor. Neurol. Neurosci. 2010, 28, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Brugeaud, A.; Travo, C.; Demêmes, D.; Lenoir, M.; Llorens, J.; Puel, J.-L.; Chabbert, C. Control of Hair Cell Excitability by Vestibular Primary Sensory Neurons. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 3503–3511. [Google Scholar] [CrossRef]
- Travo, C.; Gaboyard-Niay, S.; Chabbert, C. Plasticity of Scarpa’s Ganglion Neurons as a Possible Basis for Functional Restoration within Vestibular Endorgans. Front. Neurol. 2012, 3, 91. [Google Scholar] [CrossRef]
- Cassel, R.; Wiener-Vacher, S.; El Ahmadi, A.; Tighilet, B.; Chabbert, C. Reduced Balance Restoration Capacities Following Unilateral Vestibular Insult in Older adults Mice. Front. Neurol. 2018, 9, 462. [Google Scholar] [CrossRef]
- Dror, A.A.; Politi, Y.; Shahin, H.; Lenz, D.R.; Dossena, S.; Nofziger, C.; Fuchs, H.; Hrabé de Angelis, M.; Paulmichl, M.; Weiner, S.; et al. Calcium Oxalate Stone Formation in the Inner Ear as a Result of an Slc26a4 Mutation. J. Biol. Chem. 2010, 285, 21724–21735. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, Y.W.; Xu, Y.; Thiessen, K.D.; Kramer, K.L. Mechanisms of Otoconia and Otolith Development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2015, 244, 239–253. [Google Scholar] [CrossRef]
- Lundberg, Y.W.; Zhao, X.; Yamoah, E.N. Assembly of the Otoconia Complex to the Macular Sensory Epithelium of the Vestibule. Brain Res. 2006, 1091, 47–57. [Google Scholar] [CrossRef]
- Thalmann, R.; Ignatova, E.; Kachar, B.; Ornitz, D.M.; Thalmann, I. Development and Maintenance of Otoconia: Biochemical Considerations. Ann. N. Y. Acad. Sci. 2001, 942, 162–178. [Google Scholar] [CrossRef]
- Igarashi, M.; Saito, R.; Mizukoshi, K.; Alford, B.R. Otoconia in Young and Elderly Persons: A Temporal Bone Study. Acta Oto-Laryngol. Suppl. 1993, 504, 26–29. [Google Scholar] [CrossRef]
- Ross, M.D.; Peacor, D.; Johnsson, L.G.; Allard, L.F. Observations on Normal and Degenerating Human Otoconia. Ann. Otol. Rhinol. Laryngol. 1976, 85, 310–326. [Google Scholar] [CrossRef]
- Jang, Y.S.; Hwang, C.H.; Shin, J.Y.; Bae, W.Y.; Kim, L.S. Age-Related Changes on the Morphology of the Otoconia. Laryngoscope 2006, 116, 996–1001. [Google Scholar] [CrossRef]
- Johnsson, L.G.; Hawkins, J.E. Otolithic Membranes of the Saccule and Utricle in Man. Science 1967, 157, 1454–1456. [Google Scholar] [CrossRef]
- Vibert, D.; Kompis, M.; Häusler, R. Benign Paroxysmal Positional Vertigo in Older Women May Be Related to Osteoporosis and Osteopenia. Ann. Otol. Rhinol. Laryngol. 2003, 112, 885–889. [Google Scholar] [CrossRef]
- Vibert, D.; Sans, A.; Kompis, M.; Travo, C.; Muhlbauer, R.C.; Tschudi, I.; Boukhaddaoui, H.; Häusler, R. Ultrastructural Changes in Otoconia of Osteoporotic Rats. Audiol. Neurootol. 2008, 13, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Arshad, Q.; Seemungal, B.M. Age-Related Vestibular Loss: Current Understanding and Future Research Directions. Front. Neurol. 2016, 7, 231, Erratum in Front Neurol. 2017, 8, 391. [Google Scholar] [CrossRef]
- Paplou, V.; Schubert, N.M.A.; Pyott, S.J. Age-Related Changes in the Cochlea and Vestibule: Shared Patterns and Processes. Front. Neurosci. 2021, 15, 680856. [Google Scholar] [CrossRef] [PubMed]
- Angelaki, D.E.; Cullen, K.E. Vestibular System: The Many Facets of a Multimodal Sense. Annu. Rev. Neurosci. 2008, 31, 125–150. [Google Scholar] [CrossRef]
- Lopez, C. The Vestibular System: Balancing More than Just the Body. Curr. Opin. Neurol. 2016, 29, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Balaban, C.D. Vestibular Autonomic Regulation (Including Motion Sickness and the Mechanism of Vomiting). Curr. Opin. Neurol. 1999, 12, 29–33. [Google Scholar] [CrossRef]
- MacKinnon, C.D. Chapter 1-Sensorimotor Anatomy of Gait, Balance, and Falls. In Handbook of Clinical Neurology; Day, B.L., Lord, S.R., Eds.; Balance, Gait, and Falls; Elsevier: Amsterdam, The Netherlands, 2018; Volume 159, pp. 3–26. [Google Scholar]
- Cullen, K.E. The Vestibular System: Multimodal Integration and Encoding of Self-Motion for Motor Control. Trends Neurosci. 2012, 35, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Strupp, M.; Arbusow, V. Acute Vestibulopathy. Curr. Opin. Neurol. 2001, 14, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt-Henn, A.; Best, C.; Bense, S.; Breuer, P.; Diener, G.; Tschan, R.; Dieterich, M. Psychiatric Comorbidity in Different Organic Vertigo Syndromes. J. Neurol. 2008, 255, 420–428. [Google Scholar] [CrossRef]
- Balaban, C.D. Neural Substrates Linking Balance Control and Anxiety. Physiol. Behav. 2002, 77, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Lacour, M.; Helmchen, C.; Vidal, P.-P. Vestibular Compensation: The Neuro-Otologist’s Best Friend. J. Neurol. 2016, 263, 54–64. [Google Scholar] [CrossRef]
- Dieringer, N. “Vestibular Compensation”: Neural Plasticity and Its Relations to Functional Recovery after Labyrinthine Lesions in Frogs and Other Vertebrates. Prog. Neurobiol. 1995, 46, 97–129. [Google Scholar]
- Darlington, C.L.; Smith, P.F. Molecular Mechanisms of Recovery from Vestibular Damage in Mammals: Recent Advances. Prog. Neurobiol. 2000, 62, 313–325. [Google Scholar] [CrossRef]
- Smith, P.F.; Curthoys, I.S. Mechanisms of Recovery Following Unilateral Labyrinthectomy: A Review. Brain Res. Rev. 1989, 14, 155–180. [Google Scholar] [CrossRef]
- Darlington, C.L.; Dutia, M.B.; Smith, P.F. The Contribution of the Intrinsic Excitability of Vestibular Nucleus Neurons to Recovery from Vestibular Damage: Recovery from Vestibular Damage: Role of Vestibular Nucleus. Eur. J. Neurosci. 2002, 15, 1719–1727. [Google Scholar] [CrossRef]
- Dani, S.U.; Hori, A.; Walter, G.F. Principles of Neural Aging; Elsevier Science BV: Amsterdam, The Netherlands, 1997; ISBN 0-444-82329-8. [Google Scholar]
- Mozolic, J.L.; Hugenschmidt, C.E.; Peiffer, A.M.; Laurienti, P.J. Multisensory Integration and Aging. In The Neural Bases of Multisensory Processes; Murray, M.M., Wallace, M.T., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2012; Chapter 20. [Google Scholar]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Alvarez, J.C.; Díaz, C.; Suárez, C.; Fernández, J.A.; González Del Rey, C.; Navarro, A.; Tolivia, J. Neuronal Loss in Human Medial Vestibular Nucleus. Anat. Rec. 1998, 251, 431–438. [Google Scholar] [CrossRef]
- Alvarez, J.C.; Díaz, C.; Suárez, C.; Fernández, J.A.; González del Rey, C.; Navarro, A.; Tolivia, J. Aging and the Human Vestibular Nuclei: Morphometric Analysis. Mech. Ageing Dev. 2000, 114, 149–172. [Google Scholar] [CrossRef] [PubMed]
- Lopez, I.; Honrubia, V.; Baloh, R.W. Aging and the Human Vestibular Nucleus. J. Vestib. Res. Equilib. Orientat. 1997, 7, 77–85. [Google Scholar] [CrossRef]
- Tang, Y.; Lopez, I.; Baloh, R.W. Age-Related Change of the Neuronal Number in the Human Medial Vestibular Nucleus: A Stereological Investigation. J. Vestib. Res. Equilib. Orientat. 2001, 11, 357–363. [Google Scholar] [CrossRef]
- Sturrock, R.R. Age Related Changes in Neuron Number in the Mouse Lateral Vestibular Nucleus. J. Anat. 1989, 166, 227–232. [Google Scholar]
- Fernández, J.A.; Suárez, C.; Navarro, A.; Díaz, C.; Alvarez, J.C.; González del Rey, C.; Tolivia, J. Aging in the Vestibular Nuclear Complex of the Male Golden Hamster (Mesocricetus Auratus): Anatomic and Morphometric Study. Histol. Histopathol. 2007, 22, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.B.; Gundersen, H.J.G.; Pakkenberg, B. Aging of the Human Cerebellum: A Stereological Study. J. Comp. Neurol. 2003, 466, 356–365. [Google Scholar] [CrossRef]
- Buchman, A.S.; Leurgans, S.E.; VanderHorst, V.G.J.M.; Nag, S.; Schneider, J.A.; Bennett, D.A. Spinal motor neurons and motor function in older adults. J. Neurol. 2019, 266, 174–182. [Google Scholar] [CrossRef]
- Johnson, J.E.; Miquel, J. Fine Structural Changes in the Lateral Vestibular Nucleus of Aging Rats. Mech. Ageing Dev. 1974, 3, 203–224. [Google Scholar] [CrossRef]
- Takeuchi, Y.K.; Takeuchi, I.K.; Murashima, Y.; Seto-Ohshima, A. Age-Related Appearance of Dystrophic Axon Terminals in Cerebellar and Vestibular Nuclei of Mongolian Gerbils. Exp. Anim. 1997, 46, 59–65. [Google Scholar] [CrossRef]
- Him, A.; Guneser, R.; Cengiz, N.; Oztürk, G. Glutamate Responsiveness of Medial Vestibular Nucleus Neurons in Aged Rats. Brain Res. Bull. 2010, 81, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, H.; Devaraj, R.; Ganesalingam, G.S.; Smith, P.F. A Multivariate Analysis of the Effects of Aging on Glutamate, GABA and Arginine Metabolites in the Rat Vestibular Nucleus. Hear. Res. 2010, 269, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Gupta, N.; Jing, Y.; Collie, N.D.; Zhang, H.; Smith, P.F. Further Studies of the Effects of Aging on Arginine Metabolites in the Rat Vestibular Nucleus and Cerebellum. Neuroscience 2017, 348, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Him, A.; Johnston, A.R.; Yau, J.L.; Seckl, J.; Dutia, M.B. Tonic Activity and GABA Responsiveness of Medial Vestibular Nucleus Neurons in Aged Rats. Neuroreport 2001, 12, 3965–3968. [Google Scholar] [CrossRef]
- Giardino, L.; Zanni, M.; Fernandez, M.; Battaglia, A.; Pignataro, O.; Calzà, L. Plasticity of GABA(a) System during Ageing: Focus on Vestibular Compensation and Possible Pharmacological Intervention. Brain Res. 2002, 929, 76–86. [Google Scholar] [CrossRef]
- Nakayama, M.; Caspary, D.M.; Konrad, H.R.; Milbrandt, J.C.; Helfert, R.H. Age-Related Changes in [3H]Strychnine Binding in the Vestibular Nuclei of Rats. Hear. Res. 1999, 127, 103–107. [Google Scholar] [CrossRef]
- Godfrey, D.A.; Mikesell, N.L.; Godfrey, T.G.; Kaltenbach, J.A. Amino Acid and Acetylcholine Chemistry in Mountain Beaver Cochlear Nucleus and Comparisons to Pocket Gopher, Other Rodents, and Cat. Hear. Res. 2020, 385, 107841. [Google Scholar] [CrossRef]
- Cransac, H.; Peyrin, L.; Cottet-Emard, J.M.; Farhat, F.; Pequignot, J.M.; Reber, A. Aging Effects on Monoamines in Rat Medial Vestibular and Cochlear Nuclei. Hear. Res. 1996, 100, 150–156. [Google Scholar] [CrossRef]
- Di Mauro, M.; Bronzi, D.; Li Volsi, G.; Licata, F.; Lombardo, P.; Santangelo, F. Noradrenaline Modulates Neuronal Responses to GABA in Vestibular Nuclei. Neuroscience 2008, 153, 1320–1331. [Google Scholar] [CrossRef]
- Bernal, G.M.; Peterson, D.A. Neural Stem Cells as Therapeutic Agents for Age-Related Brain Repair. Aging Cell 2004, 3, 345–351. [Google Scholar] [CrossRef]
- Enwere, E.; Shingo, T.; Gregg, C.; Fujikawa, H.; Ohta, S.; Weiss, S. Aging Results in Reduced Epidermal Growth Factor Receptor Signaling, Diminished Olfactory Neurogenesis, and Deficits in Fine Olfactory Discrimination. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 8354–8365. [Google Scholar] [CrossRef]
- Toni, N.; Laplagne, D.A.; Zhao, C.; Lombardi, G.; Ribak, C.E.; Gage, F.H.; Schinder, A.F. Neurons Born in the Adult Dentate Gyrus Form Functional Synapses with Target Cells. Nat. Neurosci. 2008, 11, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; Tward, D.J.; Resnick, S.; Smith, P.F.; Lopez, C.; Rebello, E.; Wei, E.X.; Tilak Ratnanather, J.; Agrawal, Y. Vestibular Function and Cortical and Sub-Cortical Alterations in an Aging Population. Heliyon 2020, 6, e04728. [Google Scholar] [CrossRef] [PubMed]
- Brawek, B.; Skok, M.; Garaschuk, O. Changing Functional Signatures of Microglia along the Axis of Brain Aging. Int. J. Mol. Sci. 2021, 22, 1091. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.L.; Ousman, S.S. Astrocytes and Aging. Front. Aging Neurosci. 2018, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Mercado, N.; Collier, T.; Sortwell, C.; Steece-Collier, K. BDNF in the Aged Brain: Translational Implications for Parkinson’s Disease. Austin Neurol. Neurosci. 2017, 2, 1021. [Google Scholar]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.J.O.; Bromberg, E.; de Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef]
- Dunn, A.R.; Kaczorowski, C.C. Regulation of Intrinsic Excitability: Roles for Learning and Memory, Aging and Alzheimer’s Disease, and Genetic Diversity. Neurobiol. Learn. Mem. 2019, 164, 107069. [Google Scholar] [CrossRef]
- Scheltinga, A.; Honegger, F.; Timmermans, D.P.H.; Allum, J.H.J. The Effect of Age on Improvements in Vestibulo-Ocular Reflexes and Balance Control after Acute Unilateral Peripheral Vestibular Loss. Front. Neurol. 2016, 7, 18. [Google Scholar] [CrossRef]
- Xerri, C.; Lacour, M. Compensation deficits in posture and kinetics following unilateral vestibular neurectomy in cats. The role of sensorimotor activity. Acta Otolaryngol. 1980, 90, 414–424. [Google Scholar] [CrossRef]
- Lacour, M.; Roll, J.P.; Appaix, M. Modifications and Development of Spinal Reflexes in the Alert Baboon (Papio Papio) Following an Unilateral Vestibular Neurotomy. Brain Res. 1976, 113, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Dutheil, S.; Watabe, I.; Sadlaoud, K.; Tonetto, A.; Tighilet, B. BDNF Signaling Promotes Vestibular Compensation by Increasing Neurogenesis and Remodeling the Expression of Potassium-Chloride Cotransporter KCC2 and GABAA Receptor in the Vestibular Nuclei. J. Neurosci. 2016, 36, 6199–6212. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, J.R.; Heynen, A.J.; Shuler, M.G.; Bear, M.F. Learning Induces Long-Term Potentiation in the Hippocampus. Science 2006, 313, 1093–1097. [Google Scholar] [CrossRef]
- Racine, R.J.; Wilson, D.A.; Gingell, R.; Sunderland, D. Long-Term Potentiation in the Interpositus and Vestibular Nuclei in the Rat. Exp. Brain Res. 1986, 63, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Pettorossi, V.E.; Dieni, C.V.; Scarduzio, M.; Grassi, S. Long-Term Potentiation of Synaptic Response and Intrinsic Excitability in Neurons of the Rat Medial Vestibular Nuclei. Neuroscience 2011, 187, 1–14. [Google Scholar] [CrossRef]
- Pettorossi, V.E.; Dutia, M.; Frondaroli, A.; Dieni, C.; Grassi, S. Long-Term Potentiation and Depression after Unilateral Labyrinthectomy in the Medial Vestibular Nucleus of Rats. Acta Otolaryngol. 2003, 123, 182–186. [Google Scholar] [CrossRef]
- Smith, P.F. Vestibular-Hippocampal Interactions. Hippocampus 1997, 7, 465–471. [Google Scholar] [CrossRef]
- Rastoldo, G.; Marouane, E.; El Mahmoudi, N.; Péricat, D.; Bourdet, A.; Timon-David, E.; Dumas, O.; Chabbert, C.; Tighilet, B. Quantitative Evaluation of a New Posturo-Locomotor Phenotype in a Rodent Model of Acute Unilateral Vestibulopathy. Front. Neurol. 2020, 11, 536963. [Google Scholar] [CrossRef]
- Ernfors, P.; Lee, K.-F.; Jaenisch, R. Mice Lacking Brain-Derived Neurotrophic Factor Develop with Sensory Deficits. Nature 1994, 368, 147–150. [Google Scholar] [CrossRef]
- Lacour, M.; Tighilet, B. Plastic Events in the Vestibular Nuclei during Vestibular Compensation: The Brain Orchestration of a “Deafferentation” Code. Restor. Neurol. Neurosci. 2010, 28, 19–35. [Google Scholar] [CrossRef]
- Maingay, M.G.; Sansom, A.J.; Kerr, D.R.; Smith, P.F.; Darlington, C.L. The Effects of Intra-Vestibular Nucleus Administration of Brain-Derived Neurotrophic Factor (BDNF) on Recovery from Peripheral Vestibular Damage in Guinea Pig. Neuroreport 2000, 11, 2429–2432. [Google Scholar] [CrossRef]
- Erickson, K.I.; Miller, D.L.; Roecklein, K.A. The Aging Hippocampus: Interactions between Exercise, Depression, and BDNF. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2012, 18, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Manchishi, S.M.; Cui, R.J.; Zou, X.H.; Cheng, Z.Q.; Li, B.J. Effect of Caloric Restriction on Depression. J. Cell. Mol. Med. 2018, 22, 2528–2535. [Google Scholar] [CrossRef] [PubMed]
- Venditti, S.; Verdone, L.; Reale, A.; Vetriani, V.; Caserta, M.; Zampieri, M. Molecules of Silence: Effects of Meditation on Gene Expression and Epigenetics. Front. Psychol. 2020, 11, 1767. [Google Scholar] [CrossRef] [PubMed]
- Besnard, S.; Lopez, C.; Brandt, T.; Denise, P.; Smith, P.F. Editorial: The Vestibular System in Cognitive and Memory Processes in Mammalians. Front. Integr. Neurosci. 2015, 9, 55. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tighilet, B.; Chabbert, C. Cellular and Molecular Mechanisms of Vestibular Ageing. J. Clin. Med. 2023, 12, 5519. https://doi.org/10.3390/jcm12175519
Tighilet B, Chabbert C. Cellular and Molecular Mechanisms of Vestibular Ageing. Journal of Clinical Medicine. 2023; 12(17):5519. https://doi.org/10.3390/jcm12175519
Chicago/Turabian StyleTighilet, Brahim, and Christian Chabbert. 2023. "Cellular and Molecular Mechanisms of Vestibular Ageing" Journal of Clinical Medicine 12, no. 17: 5519. https://doi.org/10.3390/jcm12175519





