The Management of Ruptured Abdominal Aortic Aneurysms: An Ongoing Challenge
Abstract
:1. Introduction
2. Materials and Methods
3. Results
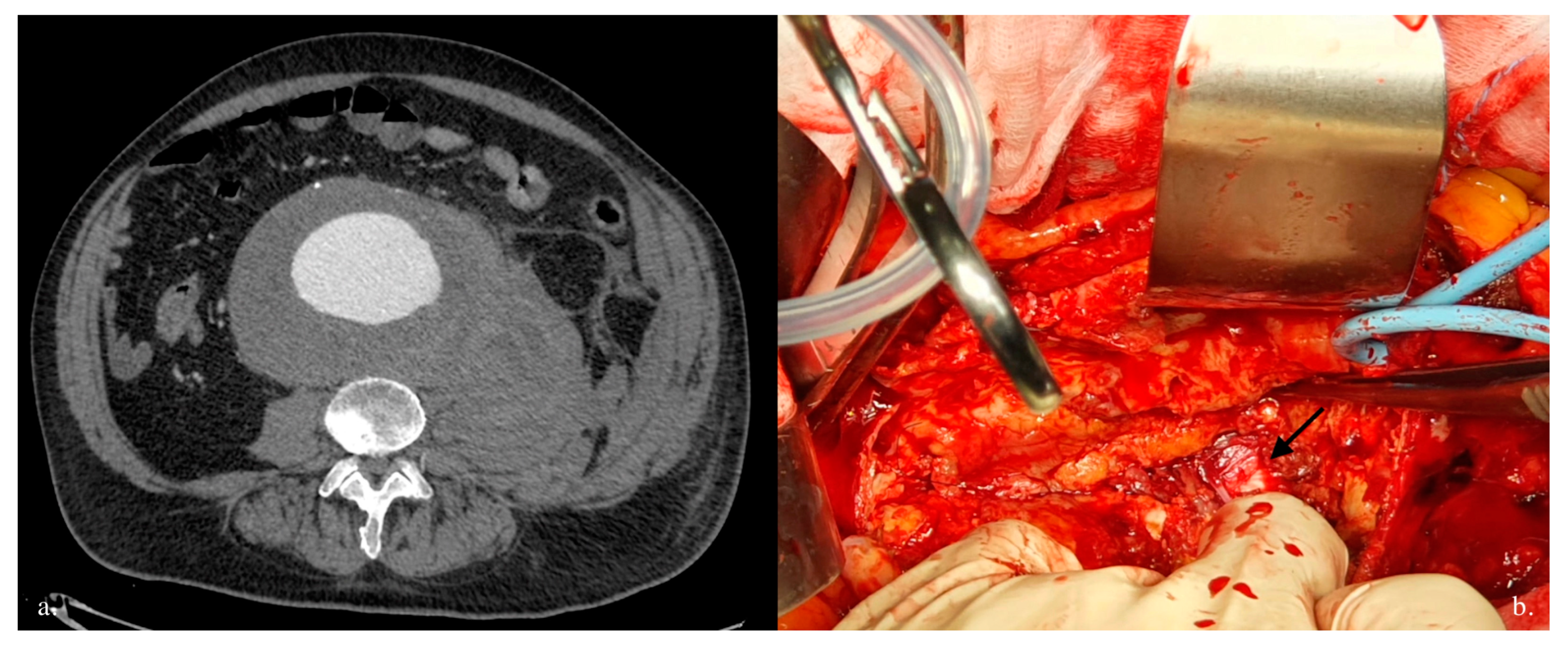
4. Discussion
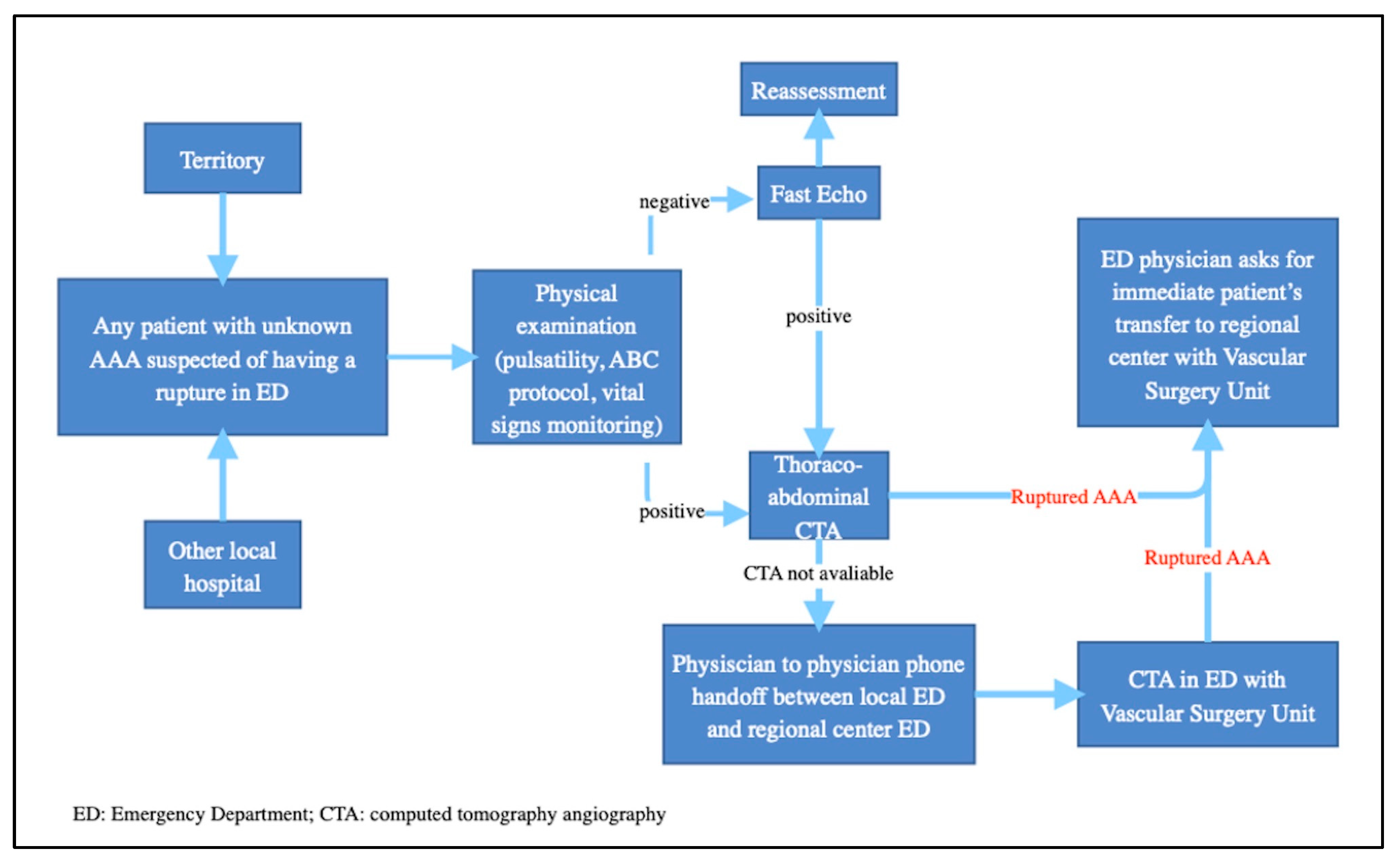
- Standardizing the approach: this ensures that all patients receive uniform care, based on best clinical practice, regardless of the hospital facility in which they are treated.
- Reducing time for intervention: this involves the rapid diagnosis and efficient planning of either surgical or endovascular intervention. Timely intervention is crucial to improve short- and long-term outcomes and to increase the chances of a patient’s survival.
- Improving clinical outcomes: this may include reducing intraoperative and postoperative mortality, decreasing complications, improving pain management, and quicker recovery. Centralization also enables the systematic collection of clinical data, which helps to monitor outcomes and further improve clinical practice.
- Quality assurance and standardization: these protocols are developed on the basis of evidence-based practice, national guidelines, and clinical expertise. Implementation of standardized protocols through multiple hospitals enhances care delivery and reduces discrepancies in treatment approaches. Regular audits and quality control measures help to identify areas that require improvement and to refine protocols for further enhancing patient care.
- Data collection and research opportunities: standardized protocols ease the collection of comprehensive clinical data, including patient demographic features, treatment outcomes, and long-term follow-up. These data serve as a valuable resource for research studies, evaluating the effectiveness of different interventions, and identifying trends or areas that require further investigation.
- Training and education: high-volume centers offer training programs, fellowships, and educational opportunities for medical students, residents, and fellows. In the context of a learning environment, centralization promotes expertise and encourages the dissemination of knowledge throughout the medical community.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashton, H.A.; Buxton, M.J.; Day, N.E.; Kim, L.G.; Marteau, T.M.; Scott, R.A.P.; Thompson, S.G.; Walker, N.M.; Multicentre Aneurysm Screening Study Group. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: A randomised controlled trial. Lancet 2002, 360, 1531–1539. [Google Scholar] [PubMed]
- Mureebe, L.; Egorova, N.; Giacovelli, J.K.; Gelijns, A.; Kent, K.C.; McKinsey, J.F. National trends in the repair of ruptured abdominal aortic aneurysms. J. Vasc. Surg. 2008, 48, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Acosta, S.; Ogren, M.; Bengtsson, H.; Bergqvist, D.; Lindblad, B.; Zdanowski, Z. Increasing incidence of ruptured abdominal aortic aneurysm: A population-based study. J. Vasc. Surg. 2006, 44, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Karthikesalingam, A.; Holt, P.J.; Vidal-Diez, A.; Ozdemir, B.A.; Poloniecki, J.D.; Hinchliffe, R.J.; Thompson, M.M. Mortality from ruptured abdominal aortic aneurysms: Clinical lessons from a comparison of outcomes in England and the USA. Lancet 2014, 383, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Jacomelli, J.; Summers, L.; Stevenson, A.; Lees, T.; Earnshaw, J.J. Impact of the first 5 years of a national abdominal aortic aneurysm screening programme. Br. J. Surg. 2016, 103, 1125–1131. [Google Scholar] [CrossRef]
- Lilja, F.; Mani, K.; Wanhainen, A. Editor’s choice—Trend-break in abdominal aortic aneurysm repair with decreasing surgical workload. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 811–819. [Google Scholar] [CrossRef]
- Budtz-Lilly, J.; Björck, M. Editor’s Choice—The Impact of Centralisation and Endovascular Aneurysm Repair on Treatment of Ruptured Abdominal Aortic Aneurysms Based on International Registries. Eur. J. Vasc. Endovasc. 2018, 2, 181–188. [Google Scholar] [CrossRef]
- Harris, L.M.; Faggioli, G.L.; Fiedler, R.; Curl, G.; Ricotta, J.J. Ruptured abdominal aortic aneurysms: Factors affecting mortality rates. J. Vasc. Surg. 1991, 14, 812–818. [Google Scholar] [CrossRef]
- Reimerink, J.J.; van der Laan, M.J.; Koelemay, M.J.; Balm, R.; Legemate, D.A. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br. J. Surg. 2013, 100, 1405–1413. [Google Scholar] [CrossRef]
- Wakefield, T.W.; Whitehouse, W.M.; Wu, S.C. Abdominal aortic aneurysm rupture: Statistical analysis of factors affecting outcome of surgical treatment. Surgery 1982, 91, 586–589. [Google Scholar]
- Rayt, H.S.; Sutton, A.J.; London, N.J.; Sayers, R.D.; Bown, M.J. A systematic review and meta-analysis of endovascular repair (EVAR) for ruptured abdominal aortic aneurysm. Eur. J. Vasc. Endovasc. Surg. 2008, 36, 536–544. [Google Scholar] [PubMed]
- Beck, A.W.; Sedrakyan, A.; Mao, J.; Venermo, M.; Faizer, R.; Debus, S.; Behrendt, C.-A.; Scali, S.T.; Altreuther, M.; Schermerhorn, M.; et al. Variations in abdominal aortic aneurysm care: A report from the international consortium of vascular registries. Circulation 2016, 134, 1948–1958. [Google Scholar]
- Badger, S.A.; Harkin, D.W.; Blair, P.H.; Ellis, P.K.; Kee, F.; Forster, R. Endovascular repair or open repair for ruptured abdominal aortic aneurysm: A Cochrane systematic review. BMJ Open 2016, 6, e008391. [Google Scholar] [PubMed]
- Canning, P.; Tawfick, W.; Kamel, K.; Hynes, N.; Sultan, S. Q-TWiST and Cost-Effectiveness Analysis of Endovascular versus Open Repair for Ruptured Abdominal Aortic Aneurysms in a High Deliberate Practice Volume Center. Ann. Vasc. Surg. 2019, 56, 163–174. [Google Scholar] [PubMed]
- Singh, K.; Bønaa, K.H.; Jacobsen, B.K.; Bjørk, L.; Solberg, S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: The Tromsø Study. Am. J. Epidemiol. 2001, 154, 236–244. [Google Scholar] [CrossRef] [PubMed]
- McPhee, J.; Eslami, M.H.; Arous, E.J.; Messina, L.M.; Schanzer, A. Endovascular treatment of ruptured abdominal aortic aneurysms in the United States (2001–2006): A significant survival benefit over open repair is independently associated with increased institutional volume. J. Vasc. Surg. 2009, 49, 817–826. [Google Scholar]
- Zettervall, S.L.; Schermerhorn, M.L.; Soden, P.A.; McCallum, J.C.; Shean, K.E.; Deery, S.E.; O’Malley, A.J.; Landon, B. The effect of surgeon and hospital volume on mortality after open and endovascular repair of abdominal aortic aneurysms. J. Vasc. Surg. 2017, 65, 626–634. [Google Scholar]
- Kontopodis, N.; Galanakis, N.; Akoumianakis, E.; Ioannou, C.V.; Tsetis, D.; Antoniou, G.A. Editor’s Choice—Systematic Review and Meta-Analysis of the Impact of Institutional and Surgeon Procedure Volume on Outcomes After Ruptured Abdominal Aortic Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 388–398. [Google Scholar]
- Marcaccio, C.L.; Schermerhorn, M.L. Epidemiology of abdominal aortic aneurysms. Semin. Vasc. Surg. 2021, 34, 29–37. [Google Scholar]
- Laukontaus, S.J.; Aho, P.-S.; Pettilä, V.; Albäck, A.; Kantonen, I.; Railo, M.; Hynninen, M.; Lepäntalo, M. Decrease of mortality of ruptured abdominal aortic aneurysm after centralization and in-hospital quality improvement of vascular service. Ann. Vasc. Surg. 2007, 21, 580–585. [Google Scholar]
- Li, B.; Eisenberg, N.; Witheford, M.; Lindsay, T.F.; Forbes, T.L.; Roche-Nagle, G. Sex Differences in Outcomes Following Ruptured Abdominal Aortic Aneurysm Repair. JAMA Netw. Open 2022, 5, e2211336. [Google Scholar] [PubMed]
- Sonesson, B.; Dias, N.; Resch, T. Is there an age limit for abdominal aortic aneurysm repair? J. Cardiovasc. Surg. 2018, 59, 190–194. [Google Scholar]
- Roosendaal, L.C.; Kramer, G.M.; Wiersema, A.M.; Wisselink, W.; Jongkind, V. Outcome of Ruptured Abdominal Aortic Aneurysm Repair in Octogenarians: A Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 16–22. [Google Scholar] [PubMed]
- Korhonen, S.J.; Ylönen, K.; Biancari, F.; Heikkinen, M.; Salenius, J.P.; Lepäntalo, M. Glasgow Aneurysm Score as a predictor of immediate outcome after surgery for ruptured abdominal aortic aneurysm. Br. J. Surg. 2004, 91, 1449–1452. [Google Scholar] [PubMed]
- Hardman, D.T.A.; Fisher, C.M.; Patel, M.I.; Neale, M.; Chambers, J.; Lane, R.; Appleberg, M. Ruptured abdominal aortic aneurysms: Who should be offered surgery? J. Vasc. Surg. 1996, 23, 123–129. [Google Scholar]
- Chen, J.C.; Hildebrand, H.D.; Salvian, A.J.; Taylor, D.C.; Strandberg, S.; Myckatyn, T.M.; Hsiang, Y.N. Predictors of death in nonruptured and ruptured abdominal aortic aneurysms. J. Vasc. Surg. 1996, 24, 614–623. [Google Scholar]
- Prytherch, D.R.; Sutton, G.L.; Boyle, J.R. Portsmouth POSSUM models for abdominal aortic aneurysm surgery. Br. J. Surg. 2001, 88, 958–963. [Google Scholar]
- Neary, W.D.; Crow, P.; Foy, C.; Prytherch, D.; Heather, B.P.; Earnshaw, J.J. Comparison of POSSUM scoring and the Hardman Index in selection of patients for repair of ruptured abdominal aortic aneurysm. Br. J. Surg. 2003, 90, 421–425. [Google Scholar]
- Tambyraja, A.L.; Murie, J.A.; Chalmers, R.T.A. Prediction of outcome after abdominal aortic aneurysm rupture. J. Vasc. Surg. 2008, 47, 222–230. [Google Scholar]
- Thompson, P.C.; Dalman, R.L.; Harris, E.J.; Chandra, V.; Lee, J.T.; Mell, M.W. Predictive models for mortality after ruptured aortic aneurysm repair do not predict futility and are not useful for clinical decision making. J. Vasc. Surg. 2016, 64, 1617–1622. [Google Scholar]
- Robinson, W.P.; Schanzer, A.; Li, Y.; Goodney, P.P.; Nolan, B.W.; Eslami, M.H.; Cronenwett, J.L.; Messina, L.M. Derivation and validation of a practical risk score for prediction of mortality after open repair of ruptured abdominal aortic aneurysms in a US regional cohort and comparison to existing scoring systems. J. Vasc. Surg. 2013, 57, 354–361. [Google Scholar] [PubMed]
- von Meijenfeldt, G.C.I.; van Beek, S.C.; Bastos Gonçalves, F.; Verhagen, H.J.M.; Zeebregts, C.J.; Vahl, A.C.; Wisselink, W.; van der Laan, M.J.; Balm, R. Development and External Validation of a Model Predicting Death After Surgery in Patients with a Ruptured Abdominal Aortic Aneurysm: The Dutch Aneurysm Score. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 168–174. [Google Scholar] [PubMed]
- Garland, B.T.; Danaher, P.J.; Desikan, S.; Tran, N.T.; Quiroga, E.; Singh, N.; Starnes, B.W. Preoperative risk score for the prediction of mortality after repair of ruptured abdominal aortic aneurysms. J. Vasc. Surg. 2018, 68, 991–997. [Google Scholar]
- Hemingway, J.F.; French, B.; Caps, M.; Benyakorn, T.; Quiroga, E.; Tran, N.; Singh, N.; Starnes, B.W. Preoperative risk score accuracy confirmed in a modern ruptured abdominal aortic aneurysm experience. J. Vasc. Surg. 2021, 74, 1508–1518. [Google Scholar] [PubMed]
- Vos, C.G.; de Vries, J.P.P.M.; Werson, D.A.B.; van Dongen, E.P.A.; Schreve, M.A.; Ünlü, Ç. Evaluation of five different aneurysm scoring systems to predict mortality in ruptured abdominal aortic aneurysm patients. J. Vasc. Surg. 2016, 64, 1609–1616. [Google Scholar] [PubMed]
- Visser, P.; Akkersdijk, G.J.M.; Blankensteijn, J.D. In-hospital operative mortality of ruptured abdominal aortic aneurysm: A population-based analysis of 5593 patients in The Netherlands over a 10-year period. Eur. J. Vasc. Endovasc. Surg. 2005, 30, 359–364. [Google Scholar] [PubMed]
- Heller, J.A.; Weinberg, A.; Arons, R.; Krishnasastry, K.V.; Lyon, R.T.; Deitch, J.S.; Schulick, A.H.; Bush, H.L., Jr.; Kent, K.C. Two decades of abdominal aortic aneurysm repair: Have we made any progress? J. Vasc. Surg. 2000, 32, 1091–1100. [Google Scholar]
- Greenhalgh, R.M.; Brown, L.C.; Epstein, D.; Kwong, G.P.S.; Powell, J.T.; Sculpher, M.J.; United Kingdom EVAR Trial Investigators. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): Randomised controlled trial. Lancet 2005, 365, 2179–2186. [Google Scholar]
- Yusuf, S.W.; Whitaker, S.C.; Chuter, T.A.M.; Wenham, P.W.; Hopkinson, B.R. Emergency endovascular repair of leaking aortic aneurysm. Lancet 1994, 344, 1645. [Google Scholar]
- García-Madrid, C.; Josa, M.; Riambau, V.; Mestres, C.A.; Muntaña, J.; Mulet, J. Endovascular versus open surgical repair of abdominal aortic aneurysm: A comparison of early and intermediate results in patients suitable for both techniques. Eur. J. Vasc. Endovasc. Surg. 2004, 28, 365–372. [Google Scholar]
- Rigberg, D.A.; Dorafshar, A.; Sridhar, A.; Quinones-Baldrich, W.; Moore, W.S. Abdominal aortic aneurysm: Stent graft vs clinical pathway for direct retroperitoneal repair. Arch Surg. 2004, 139, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Kontopodis, N.; Tavlas, E.; Ioannou, C.V.; Giannoukas, A.D.; Geroulakos, G.; Antoniou, G.A. Systematic Review and Meta-Analysis of Outcomes of Open and Endovascular Repair of Ruptured Abdominal Aortic Aneurysm in Patients with Hostile vs. Friendly Aortic Anatomy. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Egorova, N.; Giacovelli, J.; Greco, G.; Gelijns, A.; Kent, C.K.; McKinsey, J.F. National outcomes for the treatment of ruptured abdominal aortic aneurysm: Comparison of open versus endovascular repairs. J. Vasc. Surg. 2008, 48, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kwan, S.; Colvard, B.D.; d’Audiffret, A.; Kashyap, V.S.; Cho, J.S. Impact of interfacility transfer of ruptured abdominal aortic aneurysm patients. J. Vasc. Surg. 2022, 76, 1548–1554.e1. [Google Scholar] [CrossRef]
- Powell, J.T.; Sweeting, M.J.; Thompson, M.M.; Ashleigh, R.; Bell, R.; Gomes, M.; Greenhalgh, R.M.; Grieve, R.; Heatley, F.; Hinchliff, R.J.; et al. Endovascular or open repair strategy for ruptured abdominal aortic aneurysm: 30 day outcomes from IMPROVE randomised trial. BMJ 2014, 348, f7661. [Google Scholar] [CrossRef]
- Lundgren, F.; Troëng, T. Treatment choice and survival after ruptured abdominal aortic aneurysm: A population-based study. J. Vasc. Surg. 2020, 72, 508–517. [Google Scholar] [CrossRef]
- Morishita, Y.; Arikawa, K.; Yamashita, M.; Shimokawa, S.; Ohzono, H.; Saigenji, H.; Taira, A. Ruptured abdominal aortic aneurysm: Factors influencing operative mortality. Jpn. J. Surg. 1986, 16, 272–276. [Google Scholar] [CrossRef]
- Kunishige, H.; Ishibashi, Y.; Kawasaki, M.; Morimoto, K.; Inoue, N. Risk factors affecting survival after surgical repair of ruptured abdominal aortic aneurysm. Ann. Vasc. Dis. 2013, 6, 631–636. [Google Scholar] [CrossRef]
- Borger van der Burg, B.L.S.; van Dongen, T.T.C.; Morrison, J.J.; Hedeman Joosten, P.P.A.; DuBose, J.J.; Hörer, T.M.; Hoencamp, R. A systematic review and meta-analysis of the use of resuscitative endovascular balloon occlusion of the aorta in the management of major exsanguination. Eur. J. Trauma Emerg. Surg. 2018, 44, 535–550. [Google Scholar] [CrossRef]
- Marković, M.; Davidović, L.; Maksimović, Z.; Kostić, D.; Cinara, I.; Cvetković, S.; Sindjelic, R.; Seferović, P.M.; Ristić, A.D. Ruptured abdominal aortic aneurysm. Predictors of survival in 229 consecutive surgical patients. Herz 2004, 29, 123–129. [Google Scholar] [CrossRef]
- Troisi, N.; Bertagna, G.; Saratzis, A.; Guadagni, S.; Minichilli, F.; Adami, D.; Ferrari, M.; Berchiolli, R. Intraoperative predictors of in-hospital mortality after open repair of ruptured abdominal aortic aneurysms. Int. Angiol. 2023, 42, 310–317. [Google Scholar] [CrossRef]
- Li, H.J.; Kao, T.C.; Liu, D.W.; Yu, S.Y.; Ko, P.J.; Hsieh, H.C. Predictors of outcome after open repair of ruptured abdominal aortic aneurysms. Chang Gung Med. J. 2011, 34, 520–527. [Google Scholar]
- Gurakar, M.; Locham, S.; Alshaikh, H.N.; Malas, M.B. Risk factors and outcomes for bowel ischemia after open and endovascular abdominal aortic aneurysm repair. J. Vasc. Surg. 2019, 70, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Malina, M.; Veith, F.; Ivancev, K.; Sonesson, B. Balloon occlusion of the aorta during endovascular repair of ruptured abdominal aortic aneurysm. J. Endovasc. Ther. 2005, 12, 556–559. [Google Scholar] [CrossRef]
- Rubenstein, C.; Bietz, G.; Davenport, D.L.; Winkler, M.; Endean, E.D. Abdominal compartment syndrome associated with endovascular and open repair of ruptured abdominal aortic aneurysms. J. Vasc. Surg. 2015, 61, 648–654. [Google Scholar] [CrossRef]
- Zarkowsky, D.S.; Sorber, R.; Ramirez, J.L.; Goodney, P.P.; Iannuzzi, J.C.; Wohlauer, M.; Hicks, C.W. Aortic Neck IFU Violations During EVAR for Ruptured Infrarenal Aortic Aneurysms are Associated with Increased In-Hospital Mortality. Ann. Vasc. Surg. 2021, 75, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Koury, H.; Faris, P.; Moore, R. Impact of an emergency endovascular aneurysm repair protocol on 30-day ruptured abdominal aortic aneurysm mortality. J. Vasc. Surg. 2023, 77, 668. [Google Scholar]
- Gupta, P.K.; Ramanan, B.; Engelbert, T.L.; Tefera, G.; Hoch, J.R.; Kent, K.C. A comparison of open surgery versus endovascular repair of unstable ruptured abdominal aortic aneurysms. J. Vasc. Surg. 2014, 60, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Veith, F.J.; Lachat, M.; Mayer, D.; Malina, M.; Holst, J.; Mehta, M.; Verhoeven, E.L.; Larzon, T.; Gennai, S.; Coppi, G.; et al. Collected world and single center experience with endovascular treatment of ruptured abdominal aortic aneurysms. Ann. Surg. 2009, 250, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Kapma, M.R.; Dijksman, L.M.; Reimerink, J.J.; de Groof, A.J.; Zeebregts, C.J.; Wisselink, W.; Balm, R.; Dijkgraaf, M.G.; Vahl, A.C. Cost-effectiveness and cost-utility of endovascular versus open repair of ruptured abdominal aortic aneurysm in the Amsterdam Acute Aneurysm Trial. Br. J. Surg. 2014, 101, 208–215. [Google Scholar] [CrossRef]
- Nedeau, A.E.; Pomposelli, F.B.; Hamdan, A.D.; Wyers, M.C.; Hsu, R.; Sachs, T.; Siracuse, J.J.; Schermerhorn, M.L. Endovascular vs open repair for ruptured abdominal aortic aneurysm. J. Vasc. Surg. 2012, 56, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Hinchliffe, R.J.; Powell, J.T.; Cheshire, N.J.; Thompson, M.M. Endovascular repair of ruptured abdominal aortic aneurysm: A strategy in need of definitive evidence. J. Vasc. Surg. 2009, 49, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Mohan, P.P.; Hamblin, M.H. Comparison of endovascular and open repair of ruptured abdominal aortic aneurysm in the United States in the past decade. Cardiovasc. Intervent. Radiol. 2014, 37, 337–342. [Google Scholar] [CrossRef]
- Barakat, H.M.; Shahin, Y.; Din, W.; Akomolafe, B.; Johnson, B.F.; Renwick, P.; Chetter, I.; McCollum, P. Perioperative, Postoperative, and Long-Term Outcomes Following Open Surgical Repair of Ruptured Abdominal Aortic Aneurysm. Angiology 2020, 71, 626–632. [Google Scholar] [CrossRef]
- Williamson, J.S.; Ambler, G.K.; Twine, C.P.; Williams, I.M.; Williams, G.L. Elective Repair of Abdominal Aortic Aneurysm and the Risk of Colonic Ischaemia: Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Tsilimparis, N.; Saleptsis, V.; Rohlffs, F.; Wipper, S.; Debus, E.S.; Kölbel, T. New developments in the treatment of ruptured AAA. J. Cardiovasc. Surg. 2016, 57, 233–241. [Google Scholar]
- Meldrum, D.R.; Moore, F.A.; Moore, E.E.; Franciose, R.J.; Sauaia, A.; Burch, J.M. Prospective characterization and selective management of the abdominal compartment syndrome. Am. J. Surg. 1997, 174, 667–672. [Google Scholar] [CrossRef]
- Rasmussen, T.E.; Hallett, J.W., Jr.; Noel, A.A.; Jenkins, G.; Bower, T.C.; Cherry, K.J., Jr.; Panneton, J.M.; Gloviczki, P. Early abdominal closure with mesh reduces multiple organ failure after ruptured abdominal aortic aneurysm repair: Guidelines from a 10-year case-control study. J. Vasc. Surg. 2002, 35, 246–253. [Google Scholar] [CrossRef]
- Kimball, E.J.; Adams, D.M.; Kinikini, D.V.; Mone, M.C.; Alder, S.C. Delayed abdominal closure in the management of ruptured abdominal aortic aneurysm. Vascular 2009, 17, 309–315. [Google Scholar] [CrossRef]
- Acosta, S.; Wanhainen, A.; Björck, M. Temporary Abdominal Closure After Abdominal Aortic Aneurysm Repair: A Systematic Review of Contemporary Observational Studies. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 371–378. [Google Scholar] [CrossRef]
- Qrareya, M.; Zuhaili, B. Management of Postoperative Complications Following Endovascular Aortic Aneurysm Repair. Surg. Clin. North Am. 2021, 101, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Reite, A.; Søreide, K.; Kvaløy, J.T.; Vetrhus, M. Long-Term Outcomes After Open Repair for Ruptured Abdominal Aortic Aneurysm. World J. Surg. 2020, 44, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Shirasu, T.; Kuno, T.; Yasuhara, J.; Yokoyama, Y.; Takagi, H.; Cullen, M.J.; Kent, K.C.; Clouse, W.D. Meta-analysis finds recurrent infection is more common after endovascular than after open repair of infected abdominal aortic aneurysm. J. Vasc. Surg. 2022, 75, 348–355. [Google Scholar] [CrossRef]
- Perini, P.; Gargiulo, M.; Silingardi, R.; Bonardelli, S.; Bellosta, R.; Bonvini, S.; Michelagnoli, S.; Tusini, N.; Capelli, P.; Freyrie, A. LOCOS-1 investigators. Twenty-two Year Multicentre Experience of Late Open Conversions after Endovascular Abdominal Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 757–765. [Google Scholar] [CrossRef] [PubMed]

| PICOS Criteria to Develop the Research | |
|---|---|
| Participants | All patients with ruptured abdominal aortic aneurysms (rAAA) treated in emergency settings, with treatment performed in high-volume centers. |
| Intervention | All emergency open surgical repair (rOSR) and endovascular abdominal aortic aneurysm repair (rEVAR) performed in the analyzed period. All preoperative score models used to predict 30-day mortality and to guide decision-making process. |
| Comparison | All studies investigating the comparison between rOSR and rEVAR (e.g., IMPROVE trial). Main intraoperative factors influencing survival in patients undergoing either rOSR or rEVAR. |
| Outcomes | All studies discussing optimal treatment strategies, complications, short-term and long-term outcomes after both rOSR and rEVAR. |
| Study design | All prospective and retrospective studies (clinical cases and case series have been excluded). |
| Model | Formula | Interpretation |
|---|---|---|
| GAS (Glasgow Aneurysm Score) | Age + 17 for shock + 7 for myocardial disease + 10 for cerebrovascular disease + 14 for renal disease | Score > 95 = mortality risk > 80% |
| Hardman Index | Age > 76 years Hemoglobin < 9.0 g/dL Creatinine > 190 mmol/L, Electrocardiographic ischemia Loss of consciousness Score from 1 to 5 depending on number of five risk factors present | Score 0 = 16% mortality Score 1 = 37% mortality Score 2 = 72% mortality Score ≥ 3 = 100% mortality |
| VSS (Vancouver Scoring System) | Ex/(1 + Ex), where x = (−3.44) + [sum of coefficients of significant variables] Variable Coefficient Age 0.062 × age Reduced consciousness Yes: 1.14 Reduced consciousness No: −1.14 Cardiac arrest Yes: 0.6 Cardiac arrest No: −0.6 | Result of formula is the calculated mortality risk |
| RAA-POSSUM (RAAA Physiological and Operative Severity Score for enUmeration of Mortality and morbidity) | In(R/1 − R) = −4.9795 + (0.0913 × physiological score) + (0.0958 × operative severity score) R = risk of death | Result of formula is the mean predicted risk of death |
| ERAS (Edinburgh Ruptured Aneurysm Score) | Preoperative Glasgow Coma Scale score < 15 Preoperative systolic blood pressure < 90 mm Hg Hemoglobin level < 9 g/dL Score from 1 to 3 depending on number of three risk factors | Score ≤ 1 = 30% mortality Score 2 = 50% mortality Score 3 = 80% mortality |
| VSGNE (Vascular Study Group of New England) | Age > 76 years: 2 points Cardiac arrest: 2 points Loss of consciousness: 1 point Suprarenal aortic clamping: 1 point | Score 0 = 8% mortality Score 1 = 25% mortality Score 2 = 37% mortality Score 3 = 60% mortality Score 4 = 80% mortality Score ≥ 5 = 87% mortality |
| DAS (Dutch Aneurysm Score) | (age × 0.74) + (systolic blood pressure [mm Hg]/10 × −0.12) + (1 for cardiopulmonary resuscitation) + (hemoglobin [g/dL]/10)3 × − 1.27) ln (odds): −4.73 + DAS 30-day death rate = exp(ln(odds))/(1 + exp(ln(odds))) | Result of formula is mortality risk |
| HRS (Harborview Risk Score) | Age > 76 years pH 2 mg/dL Creatinine > 2 mg/dL Any episode of hypotension, defined as systolic blood pressure < 70 mmHg 1 point for each of four preoperative variables when present | Score 0 = 14.6% mortality Score 1 = 35.7% mortality Score 2 = 68.4% mortality Score ≥ 3 = 100% mortality |
| Study | Cohort | 30-Day Mortality OSR (%) | 30-Day Mortality EVAR (%) | p-Value |
|---|---|---|---|---|
| Veith et al. [59], Ann. Surg. 2009 | 1037 | 36.3 | 21.2 | <0.0001 |
| Nedeau et al. [61], J. Vasc. Surg. 2012 | 74 | 49 | 15.7 | 0.008 |
| Kapma et al. [60], Br. J. Surg. 2014 | 116 | 25 | 21 | 0.002 |
| Powell et al. [45], BMJ 2014 | 613 | 37.4 | 35.4 | 0.620 |
| Gupta et al. [58], J. Vasc. Surg. 2014 | 1447 | 52.8 | 35.6 | <0.0001 |
| Jones et al. [57], J. Vasc. Surg. 2022 | 376 | 29.9 | 27.7 | 0.687 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troisi, N.; Bertagna, G.; Torri, L.; Canovaro, F.; D’Oria, M.; Adami, D.; Berchiolli, R. The Management of Ruptured Abdominal Aortic Aneurysms: An Ongoing Challenge. J. Clin. Med. 2023, 12, 5530. https://doi.org/10.3390/jcm12175530
Troisi N, Bertagna G, Torri L, Canovaro F, D’Oria M, Adami D, Berchiolli R. The Management of Ruptured Abdominal Aortic Aneurysms: An Ongoing Challenge. Journal of Clinical Medicine. 2023; 12(17):5530. https://doi.org/10.3390/jcm12175530
Chicago/Turabian StyleTroisi, Nicola, Giulia Bertagna, Lorenzo Torri, Francesco Canovaro, Mario D’Oria, Daniele Adami, and Raffaella Berchiolli. 2023. "The Management of Ruptured Abdominal Aortic Aneurysms: An Ongoing Challenge" Journal of Clinical Medicine 12, no. 17: 5530. https://doi.org/10.3390/jcm12175530
APA StyleTroisi, N., Bertagna, G., Torri, L., Canovaro, F., D’Oria, M., Adami, D., & Berchiolli, R. (2023). The Management of Ruptured Abdominal Aortic Aneurysms: An Ongoing Challenge. Journal of Clinical Medicine, 12(17), 5530. https://doi.org/10.3390/jcm12175530








