Impact of Comorbidities and Previous Surgery on Mid-Term Results of Revision Total Knee Arthroplasty for Periprosthetic Joint Infection
Abstract
:1. Introduction
2. Patients and Methods
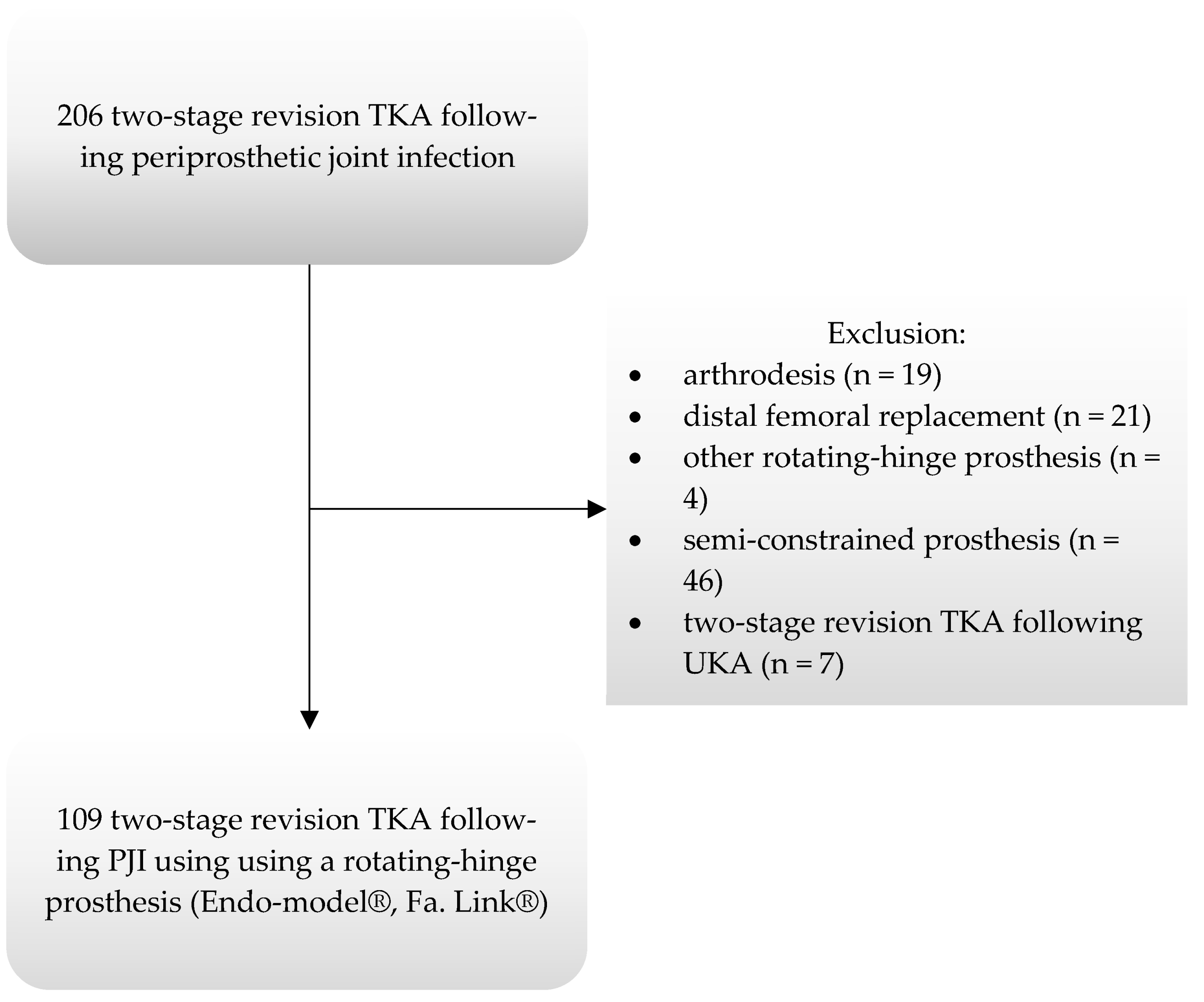
2.1. Study Cohort
2.2. Revision Protocol
2.3. Clinical Follow-Up and Radiographic Assessment
2.4. Statistical Analysis
3. Results
3.1. Survival Analysis
3.2. Mortality
3.3. Regression Analysis
3.4. Microorganisms Causing Reinfection
3.5. Clinical Outcome
3.6. Radiographic Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, A.; Pavlou, G.; Mujica-Mota, R.E.; Toms, A.D. The epidemiology of revision total knee and hip arthroplasty in England and Wales: A comparative analysis with projections for the United States. A study using the National Joint Registry dataset. Bone Jt. J. 2015, 97, 1076–1081. [Google Scholar] [CrossRef]
- Inacio, M.C.S.; Paxton, E.W.; Graves, S.E.; Namba, R.S.; Nemes, S. Projected increase in total knee arthroplasty in the United States—An alternative projection model. Osteoarthr. Cartil. 2017, 25, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. Vol. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Ong, K.L.; Lau, E.; Bozic, K.J.; Berry, D.; Parvizi, J. Prosthetic joint infection risk after TKA in the Medicare population. Clin. Orthop. Relat. Res. 2010, 468, 52–56. [Google Scholar] [CrossRef]
- Cochran, A.R.; Ong, K.L.; Lau, E.; Mont, M.A.; Malkani, A.L. Risk of Reinfection After Treatment of Infected Total Knee Arthroplasty. J. Arthroplast. 2016, 31, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Pulido, L.; Ghanem, E.; Joshi, A.; Purtill, J.J.; Parvizi, J. Periprosthetic joint infection: The incidence, timing, and predisposing factors. Clin. Orthop. Relat. Res. 2008, 466, 1710–1715. [Google Scholar] [CrossRef]
- Jamsen, E.; Huhtala, H.; Puolakka, T.; Moilanen, T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J. Bone Jt. Surg. Am. Vol. 2009, 91, 38–47. [Google Scholar] [CrossRef]
- Lenguerrand, E.; Whitehouse, M.R.; Beswick, A.D.; Kunutsor, S.K.; Foguet, P.; Porter, M.; Blom, A.W. Risk factors associated with revision for prosthetic joint infection following knee replacement: An observational cohort study from England and Wales. Lancet Infect. Dis. 2019, 19, 589–600. [Google Scholar] [CrossRef]
- Suarez, J.; Griffin, W.; Springer, B.; Fehring, T.; Mason, J.B.; Odum, S. Why do revision knee arthroplasties fail? J. Arthroplast. 2008, 23 (Suppl. S1), 99–103. [Google Scholar] [CrossRef]
- Mortazavi, S.M.; Molligan, J.; Austin, M.S.; Purtill, J.J.; Hozack, W.J.; Parvizi, J. Failure following revision total knee arthroplasty: Infection is the major cause. Int. Orthop. 2011, 35, 1157–1164. [Google Scholar] [CrossRef]
- Dai, W.L.; Lin, Z.M.; Shi, Z.J.; Wang, J. Outcomes Following Revision Total Knee Arthroplasty Septic versus Aseptic Failure: A National Propensity-Score-Matched Comparison. J. Knee Surg. 2020, 34, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Lum, Z.C.; Natsuhara, K.M.; Shelton, T.J.; Giordani, M.; Pereira, G.C.; Meehan, J.P. Mortality during Total Knee Periprosthetic Joint Infection. J. Arthroplast. 2018, 33, 3783–3788. [Google Scholar] [CrossRef]
- Boddapati, V.; Fu, M.C.; Mayman, D.J.; Su, E.P.; Sculco, P.K.; McLawhorn, A.S. Revision Total Knee Arthroplasty for Periprosthetic Joint Infection Is Associated with Increased Postoperative Morbidity and Mortality Relative to Noninfectious Revisions. J. Arthroplast. 2018, 33, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Cahill, J.L.; Shadbolt, B.; Scarvell, J.M.; Smith, P.N. Quality of life after infection in total joint replacement. J. Orthop. Surg. 2008, 16, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Beswick, A.D.; Peters, T.J.; Gooberman-Hill, R.; Whitehouse, M.R.; Blom, A.W.; Moore, A.J. Health Care Needs and Support for Patients Undergoing Treatment for Prosthetic Joint Infection following Hip or Knee Arthroplasty: A Systematic Review. PLoS ONE 2017, 12, e0169068. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.J.; Blom, A.W.; Whitehouse, M.R.; Gooberman-Hill, R. Deep prosthetic joint infection: A qualitative study of the impact on patients and their experiences of revision surgery. BMJ Open 2015, 5, e009495. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Whitehouse, M.R.; Lenguerrand, E.; Blom, A.W.; Beswick, A.D. Re-Infection Outcomes Following One-and Two-Stage Surgical Revision of Infected Knee Prosthesis: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0151537. [Google Scholar] [CrossRef]
- Petis, S.M.; Perry, K.I.; Mabry, T.M.; Hanssen, A.D.; Berry, D.J.; Abdel, M.P. Two-Stage Exchange Protocol for Periprosthetic Joint Infection Following Total Knee Arthroplasty in 245 Knees without Prior Treatment for Infection. J. Bone Jt. Surg. Am. Vol. 2019, 101, 239–249. [Google Scholar] [CrossRef]
- Haleem, A.A.; Berry, D.J.; Hanssen, A.D. Mid-term to long-term followup of two-stage reimplantation for infected total knee arthroplasty. Clin. Orthop. Relat. Res. 2004, 35–39. [Google Scholar] [CrossRef]
- Mahmud, T.; Lyons, M.C.; Naudie, D.D.; Macdonald, S.J.; McCalden, R.W. Assessing the gold standard: A review of 253 two-stage revisions for infected TKA. Clin. Orthop. Relat. Res. 2012, 470, 2730–2736. [Google Scholar] [CrossRef]
- Grimberg, A.; Jansson, V.; Melsheimer, O.; Steinbrück, A. Endoprothesenregister Deutschland [EPRD]—Jahresbericht 2019; German Arthroplasty Registry EPRD: Berlin, Germany, 2019. [Google Scholar]
- Jamsen, E.; Stogiannidis, I.; Malmivaara, A.; Pajamaki, J.; Puolakka, T.; Konttinen, Y.T. Outcome of prosthesis exchange for infected knee arthroplasty: The effect of treatment approach. Acta Orthop. 2009, 80, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.M.; Schwartzenberger, J.; Austin, M.S.; Purtill, J.J.; Parvizi, J. Revision total knee arthroplasty infection: Incidence and predictors. Clin. Orthop. Relat. Res. 2010, 468, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Sabry, F.Y.; Buller, L.; Ahmed, S.; Klika, A.K.; Barsoum, W.K. Preoperative prediction of failure following two-stage revision for knee prosthetic joint infections. J. Arthroplast. 2014, 29, 115–121. [Google Scholar] [CrossRef]
- Ma, C.Y.; Lu, Y.D.; Bell, K.L.; Wang, J.W.; Ko, J.Y.; Wang, C.J.; Kuo, F.C. Predictors of Treatment Failure After 2-Stage Reimplantation for Infected Total Knee Arthroplasty: A 2- to 10-Year Follow-Up. J. Arthroplast. 2018, 33, 2234–2239. [Google Scholar] [CrossRef] [PubMed]
- Blom, A.W.; Brown, J.; Taylor, A.H.; Pattison, G.; Whitehouse, S.; Bannister, G.C. Infection after total knee arthroplasty. J. Bone Jt. Surg. Br. Vol. 2004, 86, 688–691. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Parvizi, J.; Gehrke, T.; Chen, A.F. Proceedings of the International Consensus on Periprosthetic Joint Infection. Bone Jt. J. 2013, 95, 1450–1452. [Google Scholar] [CrossRef]
- Insall, J.N.; Dorr, L.D.; Scott, R.D.; Scott, W.N. Rationale of the Knee Society clinical rating system. Clin. Orthop. Relat. Res. 1989, 248, 13–14. [Google Scholar] [CrossRef]
- Murray, D.W.; Fitzpatrick, R.; Rogers, K.; Pandit, H.; Beard, D.J.; Carr, A.J.; Dawson, J. The use of the Oxford hip and knee scores. J. Bone Jt. Surg. Br. Vol. 2007, 89, 1010–1014. [Google Scholar] [CrossRef]
- Rolfson, O.; Eresian Chenok, K.; Bohm, E.; Lübbeke, A.; Denissen, G.; Dunn, J.; Lyman, S.; Franklin, P.; Dunbar, M.; Overgaard, S.; et al. Patient-reported outcome measures in arthroplasty registries. Acta Orthop. 2016, 87 (Suppl. S1), 3–8. [Google Scholar] [CrossRef]
- Pelt, C.E.; Grijalva, R.; Anderson, L.; Anderson, M.B.; Erickson, J.; Peters, C.L. Two-Stage Revision TKA Is Associated with High Complication and Failure Rates. Adv. Orthop. 2014, 2014, 659047. [Google Scholar] [CrossRef] [PubMed]
- Kurd, M.F.; Ghanem, E.; Steinbrecher, J.; Parvizi, J. Two-stage exchange knee arthroplasty: Does resistance of the infecting organism influence the outcome? Clin. Orthop. Relat. Res. 2010, 468, 2060–2066. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.S.; Cho, S.H.; Kim, D.H.; Yoon, H.K.; Cho, H.S.; Lee, D.Y.; Lee, S.H.; Hwang, S.C. Two-Stage Total Knee Arthroplasty for Prosthetic Joint Infection. Knee Surg. Relat. Res. 2015, 27, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Kubista, B.; Hartzler, R.U.; Wood, C.M.; Osmon, D.R.; Hanssen, A.D.; Lewallen, D.G. Reinfection after two-stage revision for periprosthetic infection of total knee arthroplasty. Int. Orthop. 2012, 36, 65–71. [Google Scholar] [CrossRef]
- Bejon, P.; Berendt, A.; Atkins, B.L.; Green, N.; Parry, H.; Masters, S.; McLardy-Smith, P.; Gundle, R.; Byren, I. Two-stage revision for prosthetic joint infection: Predictors of outcome and the role of reimplantation microbiology. J. Antimicrob. Chemother. 2010, 65, 569–575. [Google Scholar] [CrossRef]
- Namba, R.S.; Inacio, M.C.; Paxton, E.W. Risk factors associated with deep surgical site infections after primary total knee arthroplasty: An analysis of 56,216 knees. J. Bone Jt. Surg. Am. Vol. 2013, 95, 775–782. [Google Scholar] [CrossRef]
- Song, K.H.; Kim, E.S.; Kim, Y.K.; Jin, H.Y.; Jeong, S.Y.; Kwak, Y.G.; Cho, Y.K.; Sung, J.; Lee, Y.S.; Oh, H.B.; et al. Differences in the risk factors for surgical site infection between total hip arthroplasty and total knee arthroplasty in the Korean Nosocomial Infections Surveillance System (KONIS). Infect. Control Hosp. Epidemiol. 2012, 33, 1086–1093. [Google Scholar] [CrossRef]
- Dowsey, M.M.; Choong, P.F. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin. Orthop. Relat. Res. 2009, 467, 1577–1581. [Google Scholar] [CrossRef]
- Durand, F.; Berthelot, P.; Cazorla, C.; Farizon, F.; Lucht, F. Smoking is a risk factor of organ/space surgical site infection in orthopaedic surgery with implant materials. Int. Orthop. 2013, 37, 723–727. [Google Scholar] [CrossRef]
- Malinzak, R.A.; Ritter, M.A.; Berend, M.E.; Meding, J.B.; Olberding, E.M.; Davis, K.E. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J. Arthroplast. 2009, 24, 84–88. [Google Scholar] [CrossRef]
- Jamsen, E.; Nevalainen, P.; Eskelinen, A.; Huotari, K.; Kalliovalkama, J.; Moilanen, T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: A single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J. Bone Jt. Surg. Am. Vol. 2012, 94, e101. [Google Scholar] [CrossRef] [PubMed]
- Ravi, B.; Croxford, R.; Hollands, S.; Paterson, J.M.; Bogoch, E.; Kreder, H.; Hawker, G.A. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014, 66, 254–263. [Google Scholar] [CrossRef]
- Bozic, K.J.; Lau, E.; Kurtz, S.; Ong, K.; Berry, D.J. Patient-related risk factors for postoperative mortality and periprosthetic joint infection in medicare patients undergoing TKA. Clin. Orthop. Relat. Res. 2012, 470, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Dowsey, M.M.; Choong, P.F. Obesity is a major risk factor for prosthetic infection after primary hip arthroplasty. Clin. Orthop. Relat. Res. 2008, 466, 153–158. [Google Scholar] [CrossRef]
- Watts, C.D.; Wagner, E.R.; Houdek, M.T.; Osmon, D.R.; Hanssen, A.D.; Lewallen, D.G.; Mabry, T.M. Morbid obesity: A significant risk factor for failure of two-stage revision total knee arthroplasty for infection. J. Bone Jt. Surg. Am. Vol. 2014, 96, e154. [Google Scholar] [CrossRef]
- Lindberg-Larsen, M.; Pitter, F.T.; Voldstedlund, M.; Schroder, H.M.; Bagger, J. Microbiological diagnosis in revision of infected knee arthroplasties in Denmark. Infect. Dis. 2017, 49, 824–830. [Google Scholar] [CrossRef]
- Nickinson, R.S.; Board, T.N.; Gambhir, A.K.; Porter, M.L.; Kay, P.R. The microbiology of the infected knee arthroplasty. Int. Orthop. 2010, 34, 505–510. [Google Scholar] [CrossRef]
- Aggarwal, V.K.; Bakhshi, H.; Ecker, N.U.; Parvizi, J.; Gehrke, T.; Kendoff, D. Organism profile in periprosthetic joint infection: Pathogens differ at two arthroplasty infection referral centers in Europe and in the United States. J. Knee Surg. 2014, 27, 399–406. [Google Scholar] [CrossRef]
- Malekzadeh, D.; Osmon, D.R.; Lahr, B.D.; Hanssen, A.D.; Berbari, E.F. Prior use of antimicrobial therapy is a risk factor for culture-negative prosthetic joint infection. Clin. Orthop. Relat. Res. 2010, 468, 2039–2045. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kulkarni, S.S.; Park, J.W.; Kim, J.S.; Oh, H.K.; Rastogi, D. Comparison of infection control rates and clinical outcomes in culture-positive and culture-negative infected total-knee arthroplasty. J. Orthop. 2015, 12 (Suppl. S1), S37–S43. [Google Scholar] [CrossRef]
- Zmistowski, B.; Tetreault, M.W.; Alijanipour, P.; Chen, A.F.; Della Valle, C.J.; Parvizi, J. Recurrent periprosthetic joint infection: Persistent or new infection? J. Arthroplast. 2013, 28, 1486–1489. [Google Scholar] [CrossRef] [PubMed]
- Saleh, K.J.; Dykes, D.C.; Tweedie, R.L.; Mohamed, K.; Ravichandran, A.; Saleh, R.M.; Gioe, T.J.; Heck, D.A. Functional outcome after total knee arthroplasty revision: A meta-analysis. J. Arthroplast. 2002, 17, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, A.D.; Rand, J.A. A comparison of primary and revision total knee arthroplasty using the kinematic stabilizer prosthesis. J. Bone Jt. Surg. Am. Vol. 1988, 70, 491–499. [Google Scholar] [CrossRef]
- Baker, P.; Cowling, P.; Kurtz, S.; Jameson, S.; Gregg, P.; Deehan, D. Reason for revision influences early patient outcomes after aseptic knee revision. Clin. Orthop. Relat. Res. 2012, 470, 2244–2252. [Google Scholar] [CrossRef]
- Sharkey, P.F.; Hozack, W.J.; Rothman, R.H.; Shastri, S.; Jacoby, S.M. Insall Award paper. Why are total knee arthroplasties failing today? Clin. Orthop. Relat. Res. 2002, 7–13. [Google Scholar] [CrossRef]
- Patil, N.; Lee, K.; Huddleston, J.I.; Harris, A.H.; Goodman, S.B. Aseptic versus septic revision total knee arthroplasty: Patient satisfaction, outcome and quality of life improvement. Knee 2010, 17, 200–203. [Google Scholar] [CrossRef]
- Rajgopal, A.; Vasdev, A.; Gupta, H.; Dahiya, V. Revision total knee arthroplasty for septic versus aseptic failure. J. Orthop. Surg. 2013, 21, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Konrads, C.; Franz, A.; Hoberg, M.; Rudert, M. Similar Outcomes of Two-Stage Revisions for Infection and One-Stage Revisions for Aseptic Revisions of Knee Endoprostheses. J. Knee Surg. 2019, 32, 897–899. [Google Scholar] [CrossRef]
- Ro, D.H.; Kim, J.K.; Kim, S.; Han, H.S.; Lee, M.C. Periprosthetic Joint Infection Does Not Preclude Good Outcomes after a Revision Total Knee Arthroplasty: A 7-Year Follow-Up Study of 144 Retrospective Cases. BioMed Res. Int. 2018, 2018, 2582140. [Google Scholar] [CrossRef]
- Van Kempen, R.W.; Schimmel, J.J.; van Hellemondt, G.G.; Vandenneucker, H.; Wymenga, A.B. Reason for revision TKA predicts clinical outcome: Prospective evaluation of 150 consecutive patients with 2-years followup. Clin. Orthop. Relat. Res. 2013, 471, 2296–2302. [Google Scholar] [CrossRef]
- Matar, H.E.; Stritch, P.; Emms, N. Higher failure rate of two-stage revision for infected knee arthroplasties in significantly compromised (host-C) patients. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2206–2210. [Google Scholar] [CrossRef] [PubMed]






| Demographics | |
| Number of knees | 109 (100%) |
| Age (mean, SD), in years | 69.61 (9.35) |
| Gender n (%) | |
| Male | 55 (50.5%) |
| Female | 54 (49.5%) |
| Body mass index (mean, SD) | 30.96 (5.62) |
| Comorbidity Scores | |
| ASA score (%) | |
| ASA I | 2 (1.8%) |
| ASA II | 27 (24.8%) |
| ASA III | 75 (68.8%) |
| ASA IV | 5 (4.6%) |
| Charlson Comorbidity Index | |
| 0 | 20 (18.3%) |
| 1–2 | 36 (33.0%) |
| 3–4 | 26 (23.9%) |
| 5 | 27 (24.8%) |
| Surgical-related factors | |
| Two-stage revision | |
| Complete n (%) | 98 (90%) |
| Reimplantation n (%) | 11 (10%) |
| Pathogen isolation at index surgery | |
| Gram-positive | 70 (64%) |
| Staphylococci | 51 (47%) |
| Staphylococcus aureus | 14 (13%) |
| Methicillin-resistant Staphylococcus aureus | 1 (1%) |
| Coagulase-negative staphylococci/Staphylococcus epidermidis | 27 (25%) |
| Methicillin-resistant Staphylococcus epidermidis | 2 (2%) |
| Streptococci | 6 (6%) |
| Enterococci | 4 (4%) |
| Other gram-positive | 9 (8%) |
| Gram-negative | 8 (7%) |
| Fungus | 2 (2%) |
| Poly-microbial | 4 (4%) |
| No organism growth | 25 (23%) |
| Blood loss (mean, SD), (ml) | 1339 (915) |
| Operating time (mean, SD), (minutes) | 188 (58) |
| Previous operations (mean, SD) | 4.94 (2.92) |
| Previous revision procedure | |
| Yes | 62 (56.9%) |
| Aseptic | 23 (21.1%) |
| Septic | 39 (35.8%) |
| No | 47 (43.1%) |
| Surgical procedures between explantation and reimplantation | |
| Yes | 22 (20.2%) |
| No | 87 (79.8%) |
| Implant-related factors | |
| Generation of RHK | |
| Modular n (%) | 55 (50.5%) |
| Non-modular n (%) | 54 (49.5%) |
| Retropatellar replacement | |
| With n (%) | 19 (17.4%) |
| Without n (%) | 90 (82.6%) |
| Tibial/Femoral Augments | |
| With n (%) | 55 (50.5%) |
| Without n (%) | 54 (49.5%) |
| Comorbidity | Total n = 109 (100%) |
|---|---|
| Myocardial infarction | 13 (12%) |
| Congestive heart failure | 34 (31%) |
| Peripheral vascular disease | 5 (5%) |
| Cerebrovascular accident or transient ischemic attacks | 12 (11%) |
| Dementia | 0 (0%) |
| Chronic obstructive disease | 26 (24%) |
| Connective tissue disease | 11 (10%) |
| Peptic ulcer disease | 55 (50%) |
| Liver disease | 7 (6%) |
| mild | 6 (5%) |
| moderate to severe | 1 (1%) |
| Diabetes mellitus | 34 (31%) |
| uncomplicated | 26 (24%) |
| End-organ damage | 8 (7%) |
| Hemiplegia | 3 (3%) |
| Moderate to severe chronic kidney disease | 40 (37%) |
| Solid tumour | 4 (4%) |
| Leukemia | 0 (0%) |
| Lymphoma | 0 (0%) |
| Metastasic tumour | 1 (1%) |
| AIDS | 0 (0%) |
| Number of Knees | |
|---|---|
| Re-Revisions | 26 |
| Recurrent deep infection | 18 |
| Aseptic loosening | 3 |
| Femoral component | 2 |
| Tibial component | 1 |
| Implant failure | 1 |
| Periprosthetic fracture | 1 |
| Revision for unknown reason | 3 |
| Reoperations (excluding reasons leading to revision) | 9 |
| Open reduction and internal fixation (ORIF) | 3 |
| Wound healing/postoperative haematoma | 4 |
| Patellar maltracking | 2 |
| Risk Factor | Hazard Ratio (95% CI) | p-Value |
|---|---|---|
| Age at operation | 1.010 (0.953–1.070) | 0.733 |
| BMI | 0.993 (0.917–1.076) | 0.869 |
| Charlson Comorbidity Index | 1.200 (1.001–1.437) | 0.049 |
| Previous operations | 1.146 (1.012–1.298) | 0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, K.-A.; Spranz, D.M.; Westhauser, F.; Bruckner, T.; Lehner, B.; Alvand, A.; Merle, C.; Walker, T. Impact of Comorbidities and Previous Surgery on Mid-Term Results of Revision Total Knee Arthroplasty for Periprosthetic Joint Infection. J. Clin. Med. 2023, 12, 5542. https://doi.org/10.3390/jcm12175542
Koch K-A, Spranz DM, Westhauser F, Bruckner T, Lehner B, Alvand A, Merle C, Walker T. Impact of Comorbidities and Previous Surgery on Mid-Term Results of Revision Total Knee Arthroplasty for Periprosthetic Joint Infection. Journal of Clinical Medicine. 2023; 12(17):5542. https://doi.org/10.3390/jcm12175542
Chicago/Turabian StyleKoch, Kevin-Arno, David M. Spranz, Fabian Westhauser, Tom Bruckner, Burkhard Lehner, Abtin Alvand, Christian Merle, and Tilman Walker. 2023. "Impact of Comorbidities and Previous Surgery on Mid-Term Results of Revision Total Knee Arthroplasty for Periprosthetic Joint Infection" Journal of Clinical Medicine 12, no. 17: 5542. https://doi.org/10.3390/jcm12175542
APA StyleKoch, K.-A., Spranz, D. M., Westhauser, F., Bruckner, T., Lehner, B., Alvand, A., Merle, C., & Walker, T. (2023). Impact of Comorbidities and Previous Surgery on Mid-Term Results of Revision Total Knee Arthroplasty for Periprosthetic Joint Infection. Journal of Clinical Medicine, 12(17), 5542. https://doi.org/10.3390/jcm12175542







