A Predictive Model of Major Postoperative Respiratory Adverse Events in Pediatric Patients Undergoing Rigid Bronchoscopy for Exploration and Foreign Body Removal
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Anesthetic Protocol
- 1.
- After entering the operating room, routine monitoring was performed for Electrocardiograph (ECG), blood pressure, and SpO2, followed by the introduction of inhaled anesthetic gas.
- 2.
- When the patient was slightly sedated and relaxed, peripheral venipuncture was performed, and the intravenous drug was injected to pursue anesthetic induction.
- 3.
- The ventilatory approach was based on the different conditions of children and the judgment of several senior anesthesiologists before surgery. If oxygenation were poor, the ventilatory approach would be changed accordingly.
- The first was spontaneous respiration (Spont). Only sedatives and analgesics were used during the operation, not muscle relaxants. The anaesthesia was maintained by total intravenous anesthesia (TIVA) of propofol and remifentanil, depending on the individual’s respiratory rate.
- The second was bronchoscopic lateral ventilation (BV), during which the sedative, analgesic and muscle relaxants were given intraoperatively. Oxygenation was maintained by intermittent manual ventilation through the lateral aperture by squeezing the reservoir bag. The anaesthesia was also maintained by total intravenous anesthesia (TIVA) of propofol and remifentanil, depending on the individual’s blood pressure and heart rate.
- The third approach was manual jet ventilation (jet), in which sedatives, analgesics and muscle relaxants were also given intraoperatively, and children were given manual intermittent jet ventilation with the jet catheter tip placed 2 cm below the glottis. The anesthesia maintenance protocol was the same as above.
- The fourth method was endotracheal tube ventilation (ETT), in which sedative, analgesic and muscle relaxant drugs were given intraoperatively, and children were ventilator-assisted through the endotracheal tube. The anesthesia maintenance protocol was the same as above.
- 4.
- After AFB was removed, the jet catheter would be replaced by the laryngeal mask airway immediately for recovery in the bronchoscopic lateral ventilation and manual jet ventilation groups, and the child would be placed in the lateral decubitus position simultaneously on the operating table. The child would be resuscitated in the operating room.
- 5.
- The children in spontaneous respiration and endotracheal tube ventilation groups would be resuscitated with mask ventilation and the original endotracheal tube without laryngeal mask airway replacement.
- 6.
- After removing the laryngeal mask or endotracheal tube, the children would be sent back to the ward when sufficient oxygenation could be maintained in the supine state with air inhalation.
- 7.
- Postoperative oxygen inhalation and nebulization therapy were given according to the patient’s condition.
2.3. Predictor Variables
2.4. Primary Outcome
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
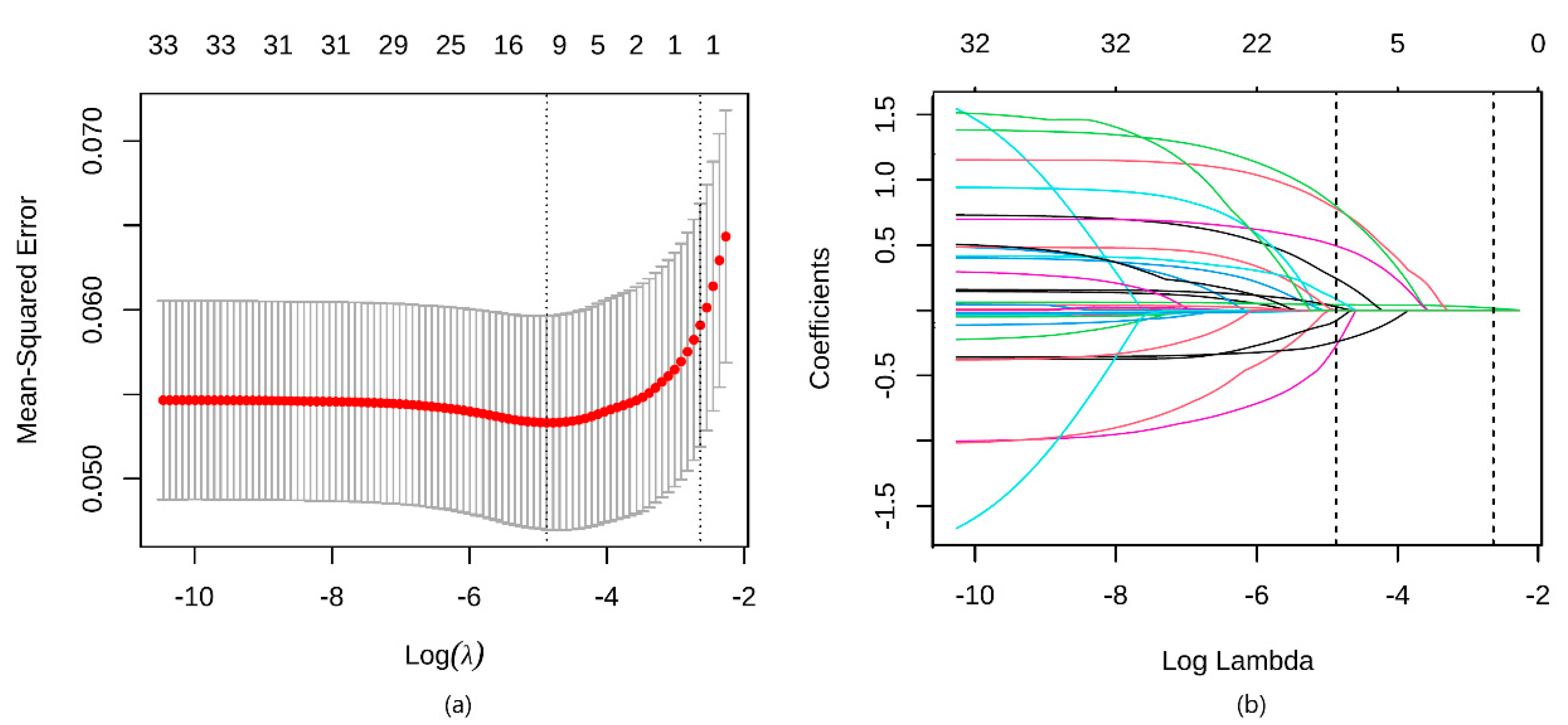
3.2. Development of the Model
3.3. Validation of the Model
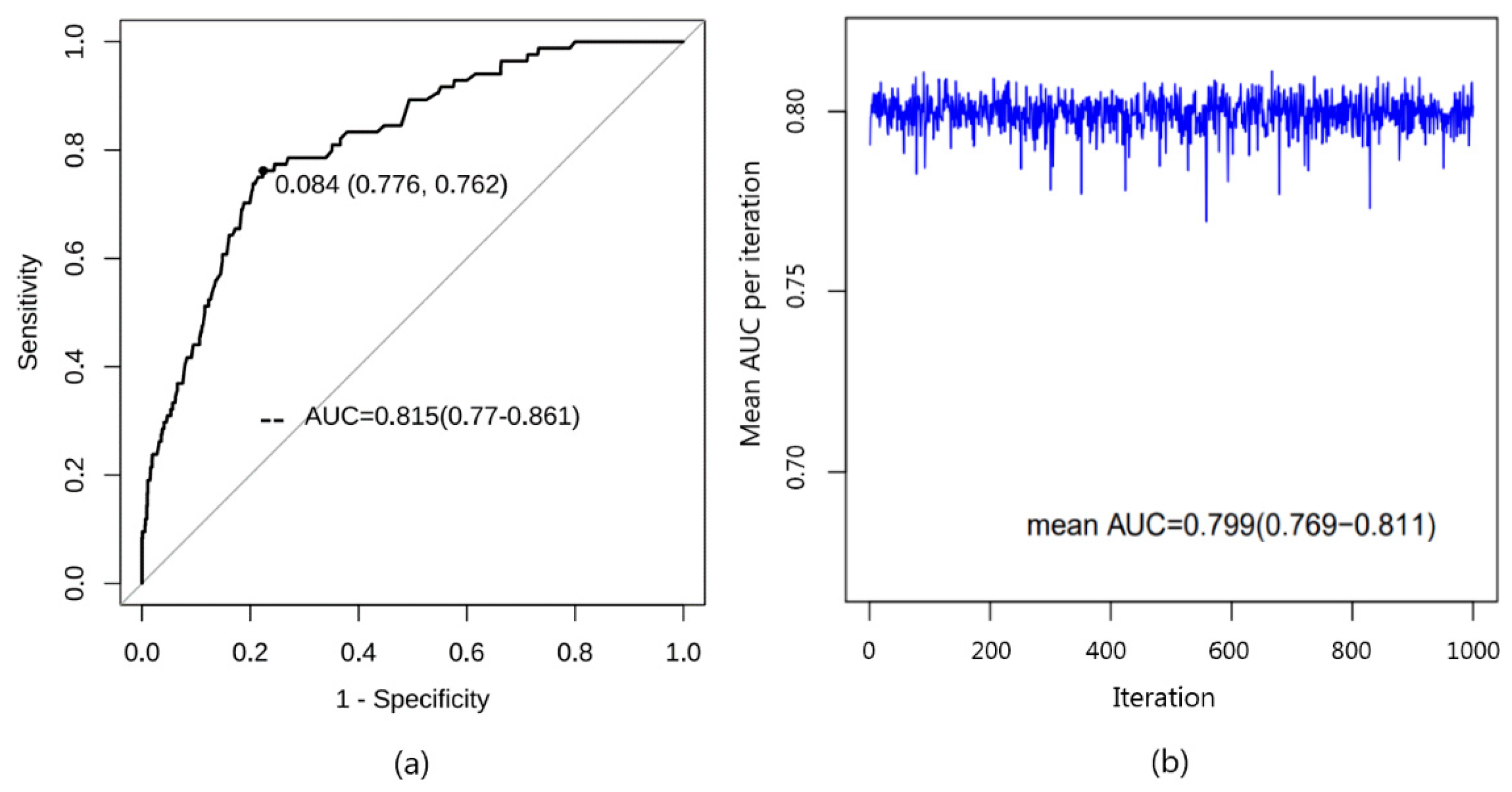
3.3.1. Discrimination
3.3.2. Calibration
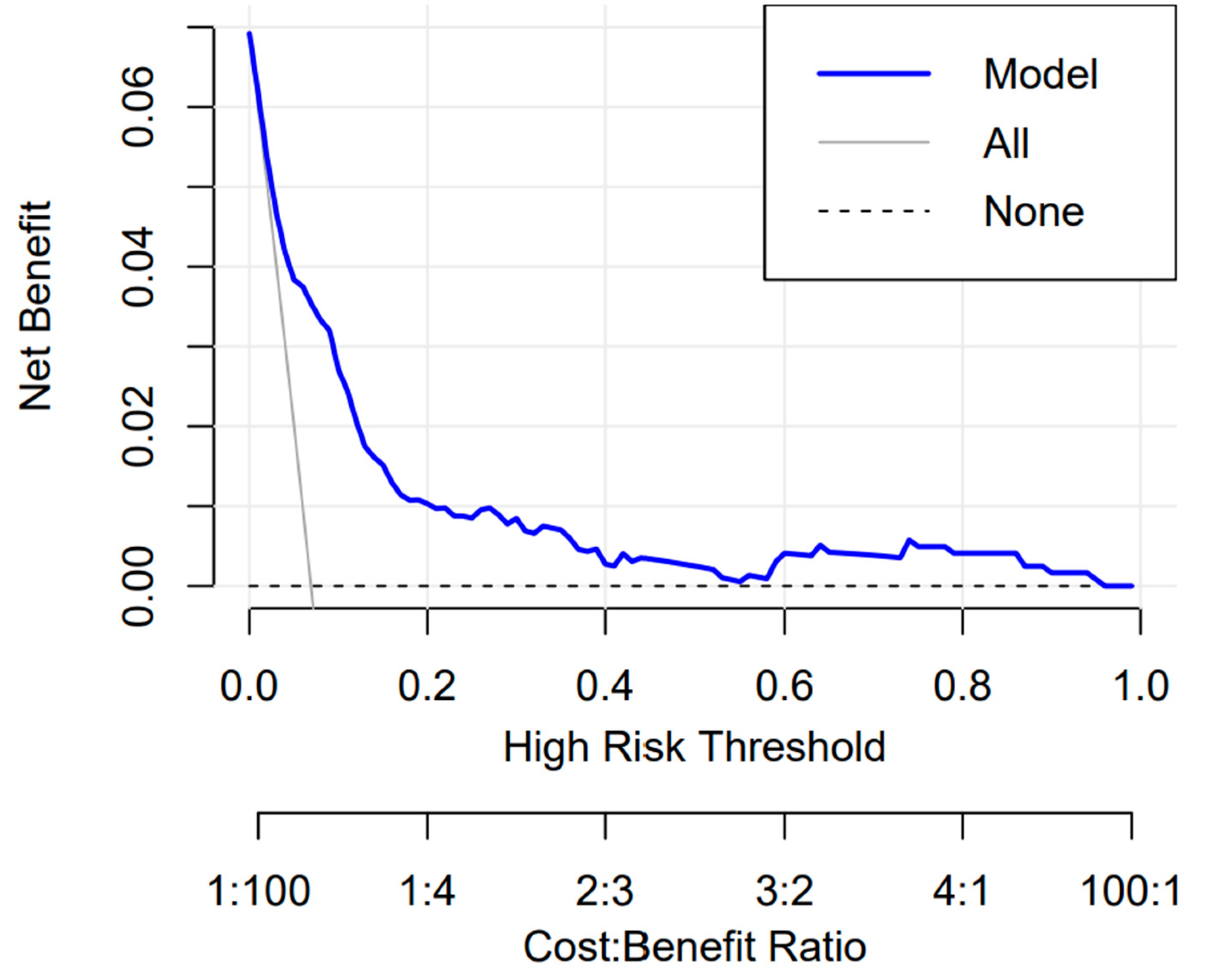
3.3.3. Clinical Use
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guazzo, E.; Burns, H. Paediatric inhaled airway foreign bodies: An update. Aust. J. Gen. Pract. 2019, 48, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Wu, X.-Y.; Han, N.; Wang, L.-Y.; Chen, W.-M. A retrospective study of anesthesia during rigid bronchoscopy for airway foreign body removal in children: Propofol and sevoflurane with spontaneous ventilation. Paediatr. Anaesth. 2014, 24, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Drake-Brockman, T.F.; Ramgolam, A.; Zhang, G.; Hall, G.L.; von Ungern-Sternberg, B.S. The effect of endotracheal tubes versus laryngeal mask airways on perioperative respiratory adverse events in infants: A randomised controlled trial. Lancet 2017, 389, 701–708. [Google Scholar] [CrossRef]
- Wudineh, D.M.; Berhe, Y.W.; Chekol, W.B.; Adane, H.; Workie, M.M. Perioperative Respiratory Adverse Events Among Pediatric Surgical Patients in University Hospitals in Northwest Ethiopia; A Prospective Observational Study. Front. Pediatr. 2022, 10, 827663. [Google Scholar] [CrossRef] [PubMed]
- Huerta, J.; Taleu, H.; Norton, R.; Gries, H.; Yun, P.; Lam, D. Use of the Snoring, Trouble Breathing, Un-Refreshed questionnaire to predict perioperative respiratory adverse events in children. J. Clin. Sleep Med. 2022, 18, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Wei, Q.; Liu, H.; Li, B.; Zhang, Q.; Zhang, Y. Development and Validation of a Risk Nomogram Model for Perioperative Respiratory Adverse Events in Children Undergoing Airway Surgery: An Observational Prospective Cohort Study. Risk Manag. Healthc. Policy 2022, 15, 1–12. [Google Scholar] [CrossRef]
- Antón-Pacheco, J.L.; Martín-Alelú, R.; López, M.; Morante, R.; Merino-Mateo, L.; Barrero, S.; Castilla, R.; Cano, I.; García, A.; Gómez, A.; et al. Foreign body aspiration in children: Treatment timing and related complications. Int. J. Pediatr. Otorhinolaryngol. 2021, 144, 110690. [Google Scholar] [CrossRef] [PubMed]
- Fidkowski, C.W.; Zheng, H.; Firth, P.G. The anesthetic considerations of tracheobronchial foreign bodies in children: A literature review of 12, 979 cases. Anesth. Analg. 2010, 111, 1016–1025. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Li, S. Controlled ventilation or spontaneous respiration in anesthesia for tracheobronchial foreign body removal: A meta-analysis. Paediatr. Anaesth. 2014, 24, 1023–1030. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 2015, 350, g7594. [Google Scholar] [CrossRef]
- van Smeden, M.; Moons, K.G.; de Groot, J.A.; Collins, G.S.; Altman, D.G.; Eijkemans, M.J.; Reitsma, J.B. Sample size for binary logistic prediction models: Beyond events per variable criteria. Stat. Methods Med. Res. 2019, 28, 2455–2474. [Google Scholar] [CrossRef] [PubMed]
- Samad, M.D.; Abrar, S.; Diawara, N. Missing Value Estimation using Clustering and Deep Learning within Multiple Imputation Framework. Knowl. Based Syst. 2022, 249, 108968. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Shao, J.; Sun, H.; Wang, X.; Zhu, Z. Risk factor analysis of bronchospasm after tracheobronchial foreign body removal: Cases report and literature review (STROBE). Medicine 2020, 99, e23170. [Google Scholar] [CrossRef]
- Mishra, P.; Bhakta, P.; Kumar, S.; Al Abri, R.; Burad, J. Sudden near-fatal tracheal aspiration of an undiagnosed nasal foreign body in a small child. Emerg. Med. Australas. 2011, 23, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, S.; Mink, R. Aspiration of fruit gel snacks. Pediatrics 2003, 111, 687–689. [Google Scholar] [CrossRef]
- Brown, K.L.; Shefler, A.; Cohen, G.; DeMunter, C.; Pigott, N.; Goldman, A.P. Near-fatal grape aspiration with complicating acute lung injury successfully treated with extracorporeal membrane oxygenation. Pediatr. Crit. Care Med. 2003, 4, 243–245. [Google Scholar] [CrossRef]
- Karaaslan, E.; Yildiz, T. Management of anesthesia and complications in children with Tracheobronchial Foreign Body Aspiration. Pak. J. Med. Sci. 2019, 35, 1592–1597. [Google Scholar] [CrossRef]
- Kendigelen, P. The anaesthetic consideration of tracheobronchial foreign body aspiration in children. J. Thorac. Dis. 2016, 8, 3803–3807. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.X.; Cai, Y.R. A time series observation of Chinese children undergoing rigid bronchoscopy for an inhaled foreign body: 3, 149 cases in 1991–2010. Chin. Med. J. 2015, 128, 504–509. [Google Scholar] [CrossRef]
- Heard, C.; Creighton, P.; Lerman, J. Intranasal flumazenil and naloxone to reverse over-sedation in a child undergoing dental restorations. Paediatr. Anaesth. 2009, 19, 795–797; discussion 798–799. [Google Scholar] [CrossRef]
- Cosgrove, P.; Krauss, B.S.; Cravero, J.P.; Fleegler, E.W. Predictors of Laryngospasm During 276, 832 Episodes of Pediatric Procedural Sedation. Ann. Emerg. Med. 2022, 80, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Blum, R.H.; McGowan, F.X., Jr. Chronic upper airway obstruction and cardiac dysfunction: Anatomy, pathophysiology and anesthetic implications. Paediatr. Anaesth. 2004, 14, 75–83. [Google Scholar] [CrossRef]
- Flick, R.P.; Wilder, R.T.; Pieper, S.F.; Vankoeverden, K.; Ellison, K.M.; Marienau, M.E.; Hanson, A.C.; Schroeder, D.R.; Sprung, J. Risk factors for laryngospasm in children during general anesthesia. Paediatr. Anaesth. 2008, 18, 289–296. [Google Scholar] [CrossRef] [PubMed]
- von Ungern-Sternberg, B.S.; Boda, K.; A Chambers, N.; Rebmann, C.; Johnson, C.; Sly, P.D.; Habre, W. Risk assessment for respiratory complications in paediatric anaesthesia: A prospective cohort study. Lancet 2010, 376, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.B.; Thung, A.; Tobias, J.D.; Jatana, K.R.; Raman, V.T. Adenotonsillectomy and postoperative respiratory adverse events: A retrospective study. Laryngoscope Investig. Otolaryngol. 2020, 5, 168–174. [Google Scholar] [CrossRef]
- Maddali, M.M.; Mathew, M.; Chandwani, J.; Alsajwani, M.J.; Ganguly, S.S. Outcomes after rigid bronchoscopy in children with suspected or confirmed foreign body aspiration: A retrospective study. J. Cardiothorac. Vasc. Anesth. 2011, 25, 1005–1008. [Google Scholar] [CrossRef]
- Farrell, P.T. Rigid bronchoscopy for foreign body removal: Anaesthesia and ventilation. Paediatr. Anaesth. 2004, 14, 84–89. [Google Scholar] [CrossRef]
- Van Calster, B.; Wynants, L.; Verbeek, J.F.; Verbakel, J.Y.; Christodoulou, E.; Vickers, A.J.; Roobol, M.J.; Steyerberg, E.W. Reporting and Interpreting Decision Curve Analysis: A Guide for Investigators. Eur. Urol. 2018, 74, 796–804. [Google Scholar] [CrossRef]
- Vickers, A.J.; Holland, F. Decision curve analysis to evaluate the clinical benefit of prediction models. Spine J. 2021, 21, 1643–1648. [Google Scholar] [CrossRef]



| Variables | Non-Major PRAEs Group (n = 1130) | Major PRAEs Group (n = 84) | Z Value | p Value |
|---|---|---|---|---|
| Gender, n (%) | 0.161 | 0.688 | ||
| Male | 771 (68.2) | 55 (65.5) | ||
| Female | 359 (31.8) | 29 (34.5) | ||
| Age, Median (IQR) (months) | 18 (14, 23) | 17.5 (14, 21) | 0.929 | 0.335 |
| Duration of anesthesia, Median (IQR) (min) | 24 (18, 33) | 46 (32.5, 68) | 105.28 | <0.001 |
| AFB type, n (%) | 1.557 | 0.459 | ||
| No AFB | 77 (6.8) | 3 (3.6) | ||
| Inorganic | 8 (0.7) | 1 (1.2) | ||
| Organic | 1045 (92.5) | 80 (95.2) | ||
| Cough, n (%) | 0.546 | 0.289 | ||
| No | 30 (2.7) | 4 (4.8) | ||
| Yes | 1100 (97.3) | 80 (95.2) | ||
| Asthma, n (%) | 5.722 | 0.017 | ||
| No | 720 (63.7) | 42 (50) | ||
| Yes | 410 (36.3) | 42 (50) | ||
| Dyspnea, n (%) | 0.239 | 0.625 | ||
| No | 995 (88.1) | 76 (90.5) | ||
| Yes | 135 (11.9) | 8 (9.5) | ||
| Wheeze, n (%) | 1.812 | 0.331 | ||
| No | 1115 (98.7) | 82 (97.6) | ||
| Yes | 15 (1.3) | 2 (2.4) | ||
| Cyanosis, n (%) | 0.235 | 0.628 | ||
| No | 1046 (92.6) | 76 (90.5) | ||
| Yes | 84 (7.4) | 8 (9.5) | ||
| Stridor, n (%) | 1.124 | 0.7 | ||
| No | 1106 (97.9) | 82 (97.6) | ||
| Yes | 24 (2.1) | 2 (2.4) | ||
| Fever, n (%) | 0.041 | 0.84 | ||
| No | 945 (83.6) | 69 (82.1) | ||
| Yes | 185 (16.4) | 15 (17.9) | ||
| Location of AFB, n (%) | 8.609 | 0.072 | ||
| No AFB | 137 (12.1) | 5 (6) | ||
| Bilateral bronchus | 10 (0.9) | 2 (2.4) | ||
| Right bronchus | 419 (37.1) | 31 (36.9) | ||
| Left bronchus | 466 (41.2) | 43 (51.2) | ||
| Main trachea | 98 (8.7) | 3 (3.6) | ||
| Emphysema, n (%) | 0.155 | 0.694 | ||
| No | 408 (36.1) | 28 (33.3) | ||
| Yes | 722 (63.9) | 56 (66.7) | ||
| Atelectasis, n (%) | 0.024 | 0.877 | ||
| No | 1022 (90.4) | 75 (89.3) | ||
| Yes | 108 (9.6) | 9 (10.7) | ||
| Pneumonia, n (%) | 5.802 | 0.016 | ||
| No | 854 (75.6) | 53 (63.1) | ||
| Yes | 276 (24.4) | 31 (36.9) | ||
| Pneumothorax, n (%) | 3.386 | 0.302 | ||
| No | 1126 (99.6) | 83 (98.8) | ||
| Yes | 4 (0.4) | 1 (1.2) | ||
| Weight, Median (IQR) (kg) | 12 (10, 13) | 11.3 (10, 12.6) | 1.94 | 0.164 |
| Height, Median (IQR) (cm) | 83 (80, 90) | 80 (77.5, 86) | 5.425 | 0.02 |
| BMI, Median (IQR) (kg/m2) | 16.9 (15.4, 18.8) | 17.4 (16.0, 18.8) | 2.913 | 0.088 |
| Ventilatory approach, n (%) | 27.217 | <0.001 | ||
| Spontaneous respiration | 175 (15.5) | 30 (35.7) | ||
| Bronchoscopy ventilation | 125 (11.1) | 4 (4.8) | ||
| Manual jet ventilation | 752 (66.5) | 41 (48.8) | ||
| Controlled ventilation by ETT | 78 (6.9) | 9 (10.7) | ||
| Change of ventilatory approach, n (%) | 87.231 | <0.001 | ||
| No | 1111 (98.3) | 67 (79.8) | ||
| Yes | 19 (1.7) | 17 (20.2) | ||
| ASA-PS, n (%) | 27.66 | <0.001 | ||
| 1 | 484 (42.8) | 12 (14.3) | ||
| 2 | 580 (51.3) | 62 (73.8) | ||
| 3 | 66 (5.8) | 10 (11.9) | ||
| Procedural duration, Median (IQR) (min) | 14 (9, 21) | 20 (14, 35.5) | 40.428 | <0.001 |
| Retention time of AFB, n (%) | 0.875 | 0.646 | ||
| ≤24 h | 307 (27.2) | 22 (26.2) | ||
| 24 h~3 days | 381 (33.7) | 25 (29.8) | ||
| >3 days | 442 (39.1) | 37 (44) | ||
| Intraoperative desaturation, n (%) | 42.083 | <0.001 | ||
| No | 876 (77.5) | 38 (45.2) | ||
| Yes | 254 (22.5) | 46 (54.8) |
| Predictors | Estimate | p Value | OR (95% CI) | Wald |
|---|---|---|---|---|
| (Intercept) | −4.079 | <0.001 | 0.017 (0.007–0.036) | 0 |
| Procedural duration | 0.041 | <0.001 | 1.042 (1.028–1.057) | 1.086 |
| Bronchoscopy ventilation | −1.439 | 0.021 | 0.237 (0.059–0.717) | 0.056 |
| Manual jet ventilation | −1.086 | <0.001 | 0.338 (0.199–0.578) | 0.114 |
| Controlled ventilation by ETT | −0.85 | 0.106 | 0.427 (0.141–1.133) | 0.183 |
| ASA-PS 2-3 | 1.34 | <0.001 | 3.821 (2.011–7.909) | 14.598 |
| Intraoperative desaturation | 1.105 | <0.001 | 3.019 (1.829–4.979) | 9.114 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, X.; Ni, W.; Han, Y.; Li, W. A Predictive Model of Major Postoperative Respiratory Adverse Events in Pediatric Patients Undergoing Rigid Bronchoscopy for Exploration and Foreign Body Removal. J. Clin. Med. 2023, 12, 5552. https://doi.org/10.3390/jcm12175552
Yi X, Ni W, Han Y, Li W. A Predictive Model of Major Postoperative Respiratory Adverse Events in Pediatric Patients Undergoing Rigid Bronchoscopy for Exploration and Foreign Body Removal. Journal of Clinical Medicine. 2023; 12(17):5552. https://doi.org/10.3390/jcm12175552
Chicago/Turabian StyleYi, Xiuwen, Wenwen Ni, Yuan Han, and Wenxian Li. 2023. "A Predictive Model of Major Postoperative Respiratory Adverse Events in Pediatric Patients Undergoing Rigid Bronchoscopy for Exploration and Foreign Body Removal" Journal of Clinical Medicine 12, no. 17: 5552. https://doi.org/10.3390/jcm12175552
APA StyleYi, X., Ni, W., Han, Y., & Li, W. (2023). A Predictive Model of Major Postoperative Respiratory Adverse Events in Pediatric Patients Undergoing Rigid Bronchoscopy for Exploration and Foreign Body Removal. Journal of Clinical Medicine, 12(17), 5552. https://doi.org/10.3390/jcm12175552









