Identifying Premature Ventricular Complexes from Outflow Tracts Based on PVC Configuration: A Machine Learning Approach
Abstract
:1. Background
2. Methods
2.1. Case Selection
2.2. Electrocardiogram Measurements
2.3. Determination of the Site of Origin of PVC
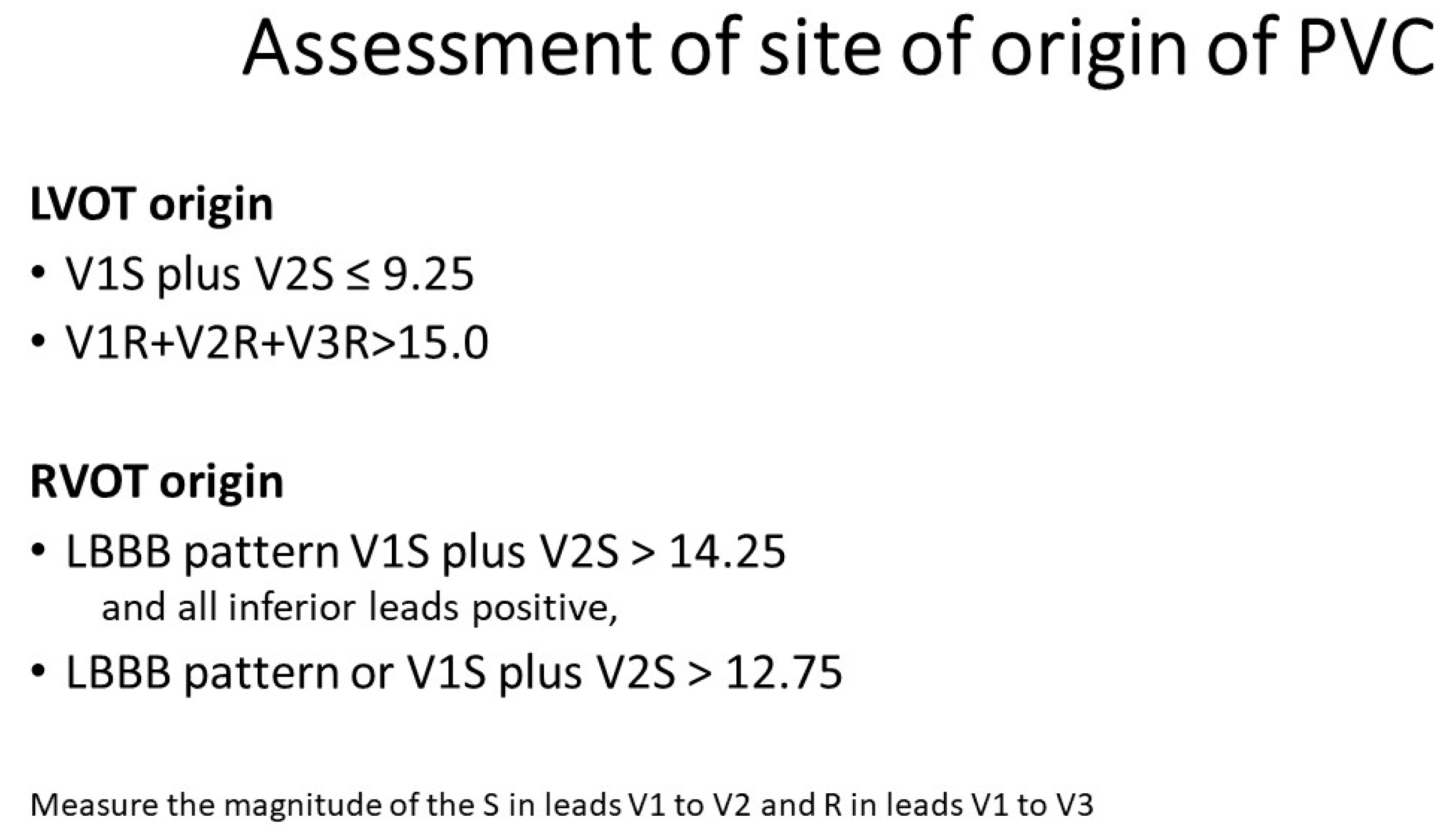
- (i)
- (a) RVOT origin (V1S – V2S) − (V1R + V2R) > 1.625(b) LVOT origin (V1S − V2S) – (V1R + V2R) ≤ 1.625 [14].
- (ii)
- (a) RVOT origin: V2 transition ≤ 0.6 (LBBB with transition in lead V3)(b) LVOT origin: V2 transition ratio ≥ 0.6 [15]
- (iii)
- (a) RVOT origin: V2S/V3R > 1.5(b) LVOT origin: V2S/V3R index ≤ 1.5 [16];
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Future Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Huizar, J.F.; Ellenbogen, K.A.; Tan, A.Y.; Kaszala, K. Arrhythmia-Induced Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2328–2344. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, S.W.; Mathewson, F.A.L.; Tate, R.B. Relationship of ventricular ectopy in men without apparent heart disease to occurrence of ischemic heart disease and sudden death. Am. Heart J. 1981, 101, 135–142. [Google Scholar] [CrossRef]
- Cheriyath, P.; He, F.; Peters, I.; Li, X.; Alagona, P.J.; Wu, C.; Pu, M.; Cascio, W.E.; Liao, D. Relation of atrial and/or ventricular premature complexes on a two-minute rhythm strip to the risk of sudden cardiac death (the Atherosclerosis Risk in Communities [ARIC] study). Am. J. Cardiol. 2011, 107, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Ataklte, F.; Erqou, S.; Laukkanen, J.; Kaptoge, S. Meta-analysis of ventricular premature complexes and their relation to cardiac mortality in general populations. Am. J. Cardiol. 2013, 112, 1263–1270. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Heinzel, F.R.; Gaita, F.; Juanatey, J.R.G.; Le Heuzey, J.Y.; Potpara, T.; Svendsen, J.H.; Vos, M.A.; Anker, S.D.; Coats, A.J.; et al. European Heart Rhythm Association/Heart Failure Association joint consensus document on arrhythmias in heart failure, endorsed by the Heart Rhythm Society and the Asia Pacific Heart Rhythm Society. EP Eur. 2016, 18, 12–36. [Google Scholar] [CrossRef]
- Rabkin, S.W.; Ohmae, M. A classification of ventricular ectopic beats. J. Electrocardiol 1982, 15, 55–60. [Google Scholar] [CrossRef]
- van Dam, P.M.; Tung, R.; Shivkumar, K.; Laks, M. Quantitative localization of premature ventricular contractions using myocardial activation ECGI from the standard 12-lead electrocardiogram. J. Electrocardiol. 2013, 46, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Tanner, H.; Wolber, T.; Schwick, N.; Fuhrer, J.; Delacretaz, E. Electrocardiographic pattern as a guide for management and radiofrequency ablation of idiopathic ventricular tachycardia. Cardiology. 2005, 103, 30–36. [Google Scholar] [CrossRef]
- Enriquez, A.; Baranchuk, A.; Briceno, D.; Saenz, L.; Garcia, F. How to use the 12-lead ECG to predict the site of origin of idiopathic ventricular arrhythmias. Heart. Rhythm. 2019, 16, 1538–1544. [Google Scholar] [CrossRef]
- Yang, Y.; Saenz, L.C.; Varosy, P.D.; Badhwar, N.; Tan, J.H.; Kilicaslan, F.; Keung, E.C.; Natale, A.; Marrouche, N.F.; Scheinman, M.M. Using the initial vector from surface electrocardiogram to distinguish the site of outflow tract tachycardia. Pacing Clin. Electrophysiol. 2007, 30, 891–898. [Google Scholar] [CrossRef]
- Movsowitz, C.; Schwartzman, D.; Callans, D.J.; Preminger, M.; Zado, E.; Gottlieb, C.D.; Marchlinski, F.E. Idiopathic right ventricular outflow tract tachycardia: Narrowing the anatomic location for successful ablation. Am. Heart J. 1996, 131, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Gerstenfeld, E.P.; Callans, D.J.; Marchlinski, F.E. Electrocardiographic patterns of superior right ventricular outflow tract tachycardias: Distinguishing septal and free-wall sites of origin. J. Cardiovasc. Electrophysiol. 2003, 14, 1–7. [Google Scholar] [CrossRef]
- Anderson, R.D.; Kumar, S.; Parameswaran, R.; Wong, G.; Voskoboinik, A.; Sugumar, H.; Watts, T.; Sparks, P.B.; Morton, J.B.; McLellan, A.; et al. Differentiating Right- and Left-Sided Outflow Tract Ventricular Arrhythmias. Circ. Arrhythm. Electrophysiol. 2019, 12, e007392. [Google Scholar] [CrossRef] [PubMed]
- Kaypakli, O.; Koca, H.; Sahin, D.Y.; Karatas, F.; Ozbicer, S.; Koc, M. S-R difference in V1-V2 is a novel criterion for differentiating the left from right ventricular outflow tract arrhythmias. Ann. Noninvasive Electrocardiol. 2018, 23, e12516. [Google Scholar] [CrossRef]
- Betensky, B.P.; Park, R.E.; Marchlinski, F.E.; Hutchinson, M.D.; Garcia, F.C.; Dixit, S.; Callans, D.J.; Cooper, J.M.; Bala, R.; Lin, D.; et al. The V(2) transition ratio: A new electrocardiographic criterion for distinguishing left from right ventricular outflow tract tachycardia origin. J. Am. Coll. Cardiol. 2011, 57, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Yamada, T.; McElderry, H.T.; Inden, Y.; Shimano, M.; Murohara, T.; Kumar, V.; Doppalapudi, H.; Plumb, V.J.; Kay, G.N. A novel electrocardiographic criterion for differentiating a left from right ventricular outflow tract tachycardia origin: The V2S/V3R index. J. Cardiovasc. Electrophysiol. 2014, 25, 747–753. [Google Scholar] [CrossRef]
- Johnson, K.W.; Torres Soto, J.; Glicksberg, B.S.; Shameer, K.; Miotto, R.; Ali, M.; Ashley, E.; Dudley, J.T. Artificial Intelligence in Cardiology. J. Am. Coll. Cardiol. 2018, 71, 2668–2679. [Google Scholar] [CrossRef]
- Attia, Z.I.; Kapa, S.; Lopez-Jimenez, F.; McKie, P.M.; Ladewig, D.J.; Satam, G.; Pellikka, P.A.; Enriquez-Sarano, M.; Noseworthy, P.A.; Munger, T.M.; et al. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat. Med. 2019, 25, 70–74. [Google Scholar] [CrossRef]
- Rousseeuw, P. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]
- Kaufman; Rousseeuw, P. Finding Groups in Data. An Introduction to Cluster Analysis, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 1990; ISBN 0-471-87876-6. [Google Scholar]
- Witten, I.; Frank, E.; Hall, M.; Pal, C. Data Mining: Practical Machine Learning Tools and Techniques, 3rd ed.; Morgan Kaufmann: San Francisco, CA, USA, 2011; ISBN 0-12-088407-0. [Google Scholar]
- Nogami, A. Mapping and ablating ventricular premature contractions that trigger ventricular fibrillation: Trigger elimination and substrate modification. J. Cardiovasc. Electrophysiol. 2015, 26, 110–115. [Google Scholar] [CrossRef]
- Shirai, Y.; Goya, M.; Ohno, S.; Horie, M.; Doi, S.; Isobe, M.; Hirao, K. Elimination of Ventricular Arrhythmia in Catecholaminergic Polymorphic Ventricular Tachycardia by Targeting “Catecholamine-Sensitive Area”: A Dominant-Subordinate Relationship between Origin Sites of Bidirectional Ventricular Premature Contractions. Pacing Clin. Electrophysiol. 2017, 40, 600–604. [Google Scholar] [CrossRef]
- Yamada, T. Twelve-lead electrocardiographic localization of idiopathic premature ventricular contraction origins. J. Cardiovasc. Electrophysiol. 2019, 30, 2603–2617. [Google Scholar] [CrossRef]
- Chang, A.; Cadaret, L.M.; Liu, K. Machine Learning in Electrocardiography and Echocardiography: Technological Advances in Clinical Cardiology. Curr. Cardiol. Rep. 2020, 22, 161. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Tanimoto, S.; Latif, A.H.M.M.; Urayama, K.Y.; Aoki, J.; Yahagi, K.; Okuno, T.; Sato, Y.; Tanaka, T.; Koseki, K.; et al. Identifying novel phenotypes of acute heart failure using cluster analysis of clinical variables. Int. J. Cardiol. 2018, 262, 57–63. [Google Scholar] [CrossRef]
- Nouraei, H.; Rabkin, S.W. A new approach to the clinical subclassification of heart failure with preserved ejection fraction. Int. J. Cardiol. 2021, 331, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Mastoi, Q.; Memon, M.S.; Lakhan, A.; Mohammed, M.A.; Qabulio, M.; Al-Turjman, F.; Abdulkareem, K.H. Machine learning-data mining integrated approach for premature ventricular contraction prediction. Neural Comput. Appl. 2021, 33, 11703–11719. [Google Scholar] [CrossRef]
- Kwon, J.-M.; Jeon, K.-H.; Kim, H.M.; Kim, M.J.; Lim, S.M.; Kim, K.-H.; Song, P.S.; Park, J.; Choi, R.K.; Oh, B.-H. Comparing the performance of artificial intelligence and conventional diagnosis criteria for detecting left ventricular hypertrophy using electrocardiographyl. Eur. Soc. Cardiol. 2020, 22, 412–419. [Google Scholar] [CrossRef]
- Kwon, J.-M.; Kim, K.-H.; Jeon, K.-H.; Lee, S.Y.; Park, J.; Oh, B.-H. Artificial intelligence algorithm for predicting cardiac arrest using electrocardiography. Scand. J. Trauma. Resusc. Emerg. Med. 2020, 28, 98. [Google Scholar] [CrossRef]
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: A retrospective analysis of outcome prediction. Lancet 2019, 394, 861–867. [Google Scholar] [CrossRef]
- Penela, D.; Teres, C.; Fernandez-Armenta, J.; Aguinaga, L.; Tercedor, L.; Soto-Iglesias, D.; Jauregui, B.; Ordonez, A.; Acosta, J.; Bisbal, F.; et al. Premature ventricular complex site of origin and ablation outcomes in patients with prior myocardial infarction. Heart. Rhythm. 2020, 18, 27–33. [Google Scholar] [CrossRef]
- Cheng, Z.; Cheng, K.; Deng, H.; Chen, T.; Gao, P.; Zhu, K.; Fang, Q. The R-wave deflection interval in lead V3 combining with R-wave amplitude index in lead V1: A new surface ECG algorithm for distinguishing left from right ventricular outflow tract tachycardia origin in patients with transitional lead at V3. Int. J. Cardiol. 2013, 168, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Gabriels, J.K.; Abdelrahman, M.; Nambiar, L.; Kim, J.; Ip, J.E.; Thomas, G.; Liu, C.F.; Markowitz, S.M.; Lerman, B.B.; Cheung, J.W. Reappraisal of electrocardiographic criteria for localization of idiopathic outflow region ventricular arrhythmias. Heart. Rhythm. 2021, 18, 1959–1965. [Google Scholar] [CrossRef]
- Anderson, R.D.; Lee, G. Scratching beneath the surface: Revisiting the accuracy of ECG-based prediction algorithms. Heart. Rhythm. 2021, 18, 1966–1967. [Google Scholar] [CrossRef] [PubMed]

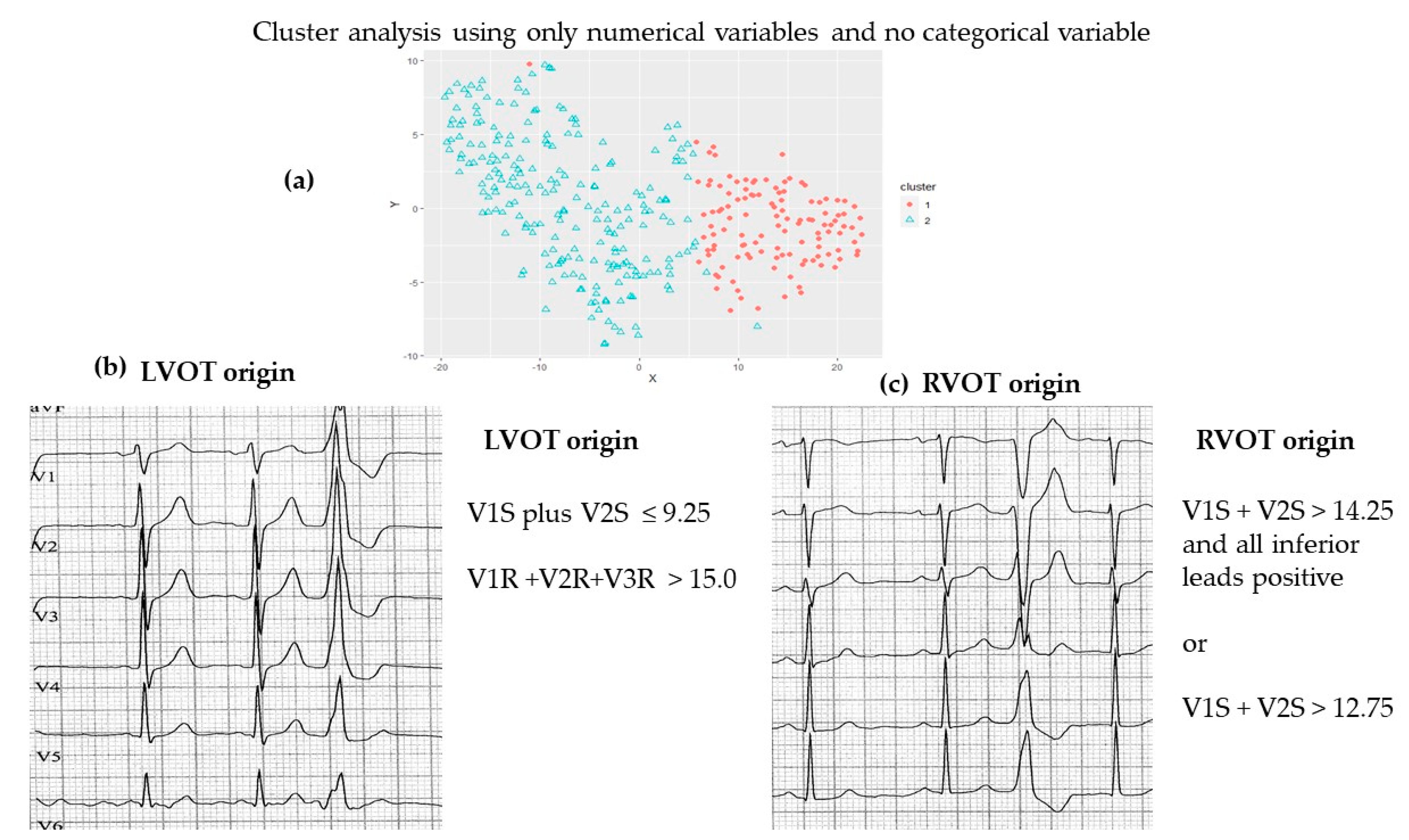
| Partitioning around Mediods | Simple K-Means | ||||
|---|---|---|---|---|---|
| Cluster 1 | Cluster 2 | p | Cluster 1 | Cluster 2 | |
| V1S | 6.50 (5.13–8.00) | 1.32 (0.5–2.00) | <2.2 × 10−16 | 6.71 | 0.98 |
| V2S | 9.00 (7.00–12.38) | 1.25 (0.75–2.50) | <2.2 × 10−16 | 9.33 | 1.73 |
| V1R | 0.50 (0.00–1.25) | 3.00 (1.5–5.25) | <2.2 × 10−16 | 0.73 | 3.78 |
| V2R | 1.25 (0.50–2.45) | 5.75 (3.50–8.50) | <2.2 × 10−16 | 1.70 | 6.48 |
| V2R Sinus | 1.75 (1.00–3.00) | 2.00 (1.00–2.26) | 0.6941 | 2.14 | 2.29 |
| V2 Total PVC | 11.00 (8.50–14.25) | 7.75 (5.00–9.80) | 5.587 × 10−13 | 11.03 | 8.16 |
| V2 Total Sinus | 8.50 (6.00–10.38) | 7.75 (5.63–10.00) | 0.1767 | 8.54 | 8.23 |
| V3R | 2.00 (1.00–3.50) | 6.50 (4.00–9.50) | <2.2 × 10−16 | 2.77 | 7.15 |
| Transition Lead | No transition lead: 1 V2.0: 2 V2.5: 21 V3.0: 5 V.35: 36 V4.0: 11 V4.5: 14 V5.0: 5 V5.5: 6 V6.0: 2 | V1.0: 5 V1.5: 31 V2.0:12 V2.5: 24 V3.0: 3 V3.5: 23 V4.0: 4 V4.5: 13 V5.0: 16 V5.5: 14 V6.0: 8 | |||
| Frontal Plane Axis | NA: 70 (58.8) RAD: 17 (14.3) LAD: 26 (21.8) +90: 5(4.2) −30: 0(0) −90: 0(0) +180: 1(0.8) | NA: 57 (26.0) RAD: 53 (24.2) LAD: 67 (30.6) EAD: 24 (11.0) +90: 10 (4.6) −30: 3 (1.4) −90: 5 (2.3) +180: 0 (0) | |||
| BBB | LBBB: 102 (85.7) RBBB: 0 (0) Posterior: 2 (1.7) Anterior: 15 (12.6) | LBBB: 61 (27.9) RBBB: 93 (42.5) Posterior: 62 (28.3) Anterior: 3 (1.4) | |||
| Average percentage of RVOT based on 3 equations | 82.3 | 7.4 | <2.2 × 10−16 | ||
| Partitioning around Mediod | Simple K-Means | ||||
|---|---|---|---|---|---|
| Cluster 1 | Cluster 2 | p | Cluster 1 | Cluster 2 | |
| V1S | 0.50 (0.00–1.50) | 0.55 (0.00–1.13) | 0.0374 | 0.50 | 1.67 |
| V2S | 1.38 (1.00–2.69) | 1.00 (0.50–1.65) | 0.133 | 1.35 | 3.07 |
| V1R | 5.38 (3.13–7.19) | 3.25 (2.25–4.63) | 0.0003287 | 3.83 | 7.56 |
| V2R | 9.00 (7.00–11.94) | 4.00 (3.00–5.00) | 2.94 × 10−14 | 5.91 | 11.04 |
| V2R Sinus | 3.38 (1.63–4.25) | 1.00 (0.68–2.50) | 3.24 × 10−06 | 1.72 | 4.94 |
| V2 Total PVC | 10.12 (8.85–13.50) | 5.50 (4.00–6.85) | 2.82 × 10−11 | 7.14 | 14.12 |
| V2 Total Sinus | 9.75 (7.60–12.50) | 7.25 (4.88–9.33) | 8.987 × 10−05 | 7.79 | 13.24 |
| V3R | 7.50 (6.00–9.94) | 2.90 (2.00–4.00) | 2.13 × 10−11 | 5.30 | 8.94 |
| Transition Lead | No transition lead: 1 V2.5: 2 V3.5: 9 V4.0: 1 V4.5: 11 V5.0: 12 V5.5: 9 V6.0: 5 | V1.5: 5 V2.0: 2 V2.5: 10 V3.0: 1 V3.5: 12 V4.0: 2 V4.5: 1 V5.0: 4 V5.5: 4 V6.0: 2 | |||
| Frontal Plane Axis | NA: 0 (0) RAD: 11 (22) LAD: 25 (50) EAD: 12 (24) +90: 0 (0) −30: 0 (0) −90: 2 (4) +180: 0 (0) | NA: 1 (2.3) RAD: 11 (25.6) LAD: 16 (37.2) EAD:12 (27.9) +90: 0 (0) −30: 0 (0) −90: 3 (7.0) +180: 0 (0) | |||
| Average percentage of RVOT based on 3 equations | 0 | 7.0 | 0.0005 | ||
| Partitioning around Mediod | Simple K-Means | ||||
|---|---|---|---|---|---|
| Cluster 1 | Cluster 2 | p | Cluster 1 | Cluster 2 | |
| V1S | 3.75 (2.19–5.31) | 7.25 (6.00–9.00) | <2.2 × 10−16 | 3.53 | 7.60 |
| V2S | 3.75 (1.69–5.56) | 10.50 (8.50–13.00) | <2.2 × 10−16 | 3.65 | 11.23 |
| V1R | 0.50 (0.00–1.56) | 0.75 (0.10–1.25) | 0.252 | 1.23 | 0.81 |
| V2R | 2.75 (1.00–4.50) | 1.5 (0.50–2.75) | 0.00037 | 3.44 | 1.60 |
| V2R Sinus | 1.50 (1.00–3.00) | 1.75 (1.00–3.00) | 0.4057 | 2.08 | 2.18 |
| V2 Total PVC | 6.375 (5.00–8.50) | 12.50 (10.50–15.00) | <2.2 × 10−16 | 7.09 | 12.84 |
| V2 Total Sinus | 7.00 (5.25–9.00) | 9.00 (5.90–10.50) | 0.001081 | 7.66 | 8.53 |
| V3R | 5.50 (3.00–7.13) | 2.25 (1.13–3.50) | 7.54 × 10−10 | 5.94 | 2.31 |
| Transition Lead | No transition lead: 1 V1.0: 4 V1.5: 26 V2.0: 10 V2.5: 23 V3.0: 3 V3.5: 6 V4.0: 4 V4.5: 5 V5.5: 1 V6.0: 1 | V2.0: 2 V2.5: 10 V3.0: 4 V3.5: 32 V4.0: 8 V4.5: 10 V5.0: 5 V5.5: 6 V6.0: 2 | |||
| Frontal Plane Axis | NA: 57 (67.9) RAD: 9 (10.7) LAD:14 (16.7) EAD: 0 (0) +90: 3 (3.6) −30: 1 (1.2) −90: 0 (0) +180: 0 (0) | NA: 49 (62.0) RAD: 15 (19.0) LAD: 10 (16.7) EAD: 0(0) +90: 5 (6.3) −30: 0 (0) −90: 0 (0) +180: 0 (0) | |||
| Average percentage of RVOT based on 3 equations | 36.4 | 82.7 | 5.39e−25 | ||
| Cluster 1 | Cluster 2 | p | |
|---|---|---|---|
| V1S | 7.25 (6.00–9.25) | 4.25 (1.50–5.950) | 8.04 × 10−13 |
| V2S | 10.5 (8.50–13.00) | 5.00 (2.50–6.30) | <2.2 × 10−16 |
| V1R | 0.80 (0.25–1.50) | 1.00 (0.00–2.00) | 0.224 |
| V2R | 1.75 (0.90–2.90) | 2.5 (1.10–4.00) | 0.035 |
| V2R Sinus | 1.75 (1.00–3.00) | 1.5 (1.00–3.00) | 0.7468 |
| V2 Total PVC | 12.75 (11.00–15.00) | 7.50 (5.63–9.13) | 2.356 × 10−15 |
| V2 Total Sinus | 9.00 (6.00–11.00) | 6.75 (4.75–8.375) | 0.002283 |
| V3R | 2.50 (1.50–4.00) | 6.00 (3.5–7.88) | <2.384 × 10−14 |
| Transition Lead | V2.0: 2 V2.5: 7 V3.0: 2 V3.5: 28 V4.0: 5 V4.5: 5 V5.0: 2 V5.5: 2 | No transition lead: 1 V1.0: 3 V1.5: 9 V2.0: 8 V2.5: 19 V3.0: 4 V3.5: 4 V4.0: 2 V6.0: 1 | |
| Frontal Plane Axis | NA: 35 () RAD: 15 () LAD: 0 (0) EAD: 0 (0) +90: 3 () −30: 0 (0) −90: 0 (0) +180: 0 (0) | NA: 37 (72.5) RAD: 9 (17.6) LAD: 0 (0) EAD: 0 (0) +90: 5 (9.8) −30: 0 (0) −90: 0 (0) +180: 0 (0) | |
| Average percentage of RVOT based on 3 equations | 81.4 | 40.5 | 1.65e−13 |
| Cluster 1 | Cluster 2 | p | |
|---|---|---|---|
| V1S | 3.75 (2.30–5.10) | 8.00 (6.81–10.88) | 2.45 × 10−8 |
| V2S | 2.50 (1.00–5.00) | 13.03 (0.03–1.00) | 1.82 × 10−15 |
| V1R | 0.10 (0.00–0.75) | 0.50 (0.03–1.00) | 0.9878 |
| V2R | 2.00 (0.50–4.50) | 0.88 (0.25–2.36) | 0.0242 |
| V2R Sinus | 1.50 (1.00–2.75) | 1.75 (1.00–3.44) | 0.07786 |
| V2 Total PVC | 6.00 (4.50–7.90) | 13.55 (11.56–15.88) | 6.91 × 10−12 |
| V2 Total Sinus | 7.50 (6.00–9.00) | 9.50 (8.25–10.50) | 0.002145 |
| V3R | 4.50 (1.75–6.25) | 1.25 (0.56–2.62) | 9.74 × 10−10 |
| Transition Lead | V1.0: 1 V1.5: 17 V2.0: 2 V2.5: 6 V3.0: 1 V3.5: 4 V4.0: 2 V4.5: 5 V5.5: 2 V6.0: 1 | V2.5: 1 V3.5: 2 V4.0: 3 V4.5: 5 V5.0: 3 V5.5: 3 V6.0: 1 | |
| Frontal Plane Axis | NA: 23 (56.1) RAD: 0 (0) LAD: 17 (41.5) EAD: 0 (0) +90:0 (0) −30: 1 (2.4) −90: 0 (0) +180: 0 (0) | NA: 11 (61.1) RAD: 0 (0) LAD: 7 (38.9) EAD: 0 (0) +90:0 (0) −30: 0 (0) −90: 0 (0) +180: 0 (0) | |
| Average percentage of RVOT based on all 3 equations | 40.5 | 86.3 | 2.28e−13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajaj, S.; Bennett, M.T.; Rabkin, S.W. Identifying Premature Ventricular Complexes from Outflow Tracts Based on PVC Configuration: A Machine Learning Approach. J. Clin. Med. 2023, 12, 5558. https://doi.org/10.3390/jcm12175558
Bajaj S, Bennett MT, Rabkin SW. Identifying Premature Ventricular Complexes from Outflow Tracts Based on PVC Configuration: A Machine Learning Approach. Journal of Clinical Medicine. 2023; 12(17):5558. https://doi.org/10.3390/jcm12175558
Chicago/Turabian StyleBajaj, Sargun, Matthew T. Bennett, and Simon W. Rabkin. 2023. "Identifying Premature Ventricular Complexes from Outflow Tracts Based on PVC Configuration: A Machine Learning Approach" Journal of Clinical Medicine 12, no. 17: 5558. https://doi.org/10.3390/jcm12175558





