Extracorporeal Membrane Oxygenation (VA-ECMO) in Management of Cardiogenic Shock
Abstract
:1. Introduction
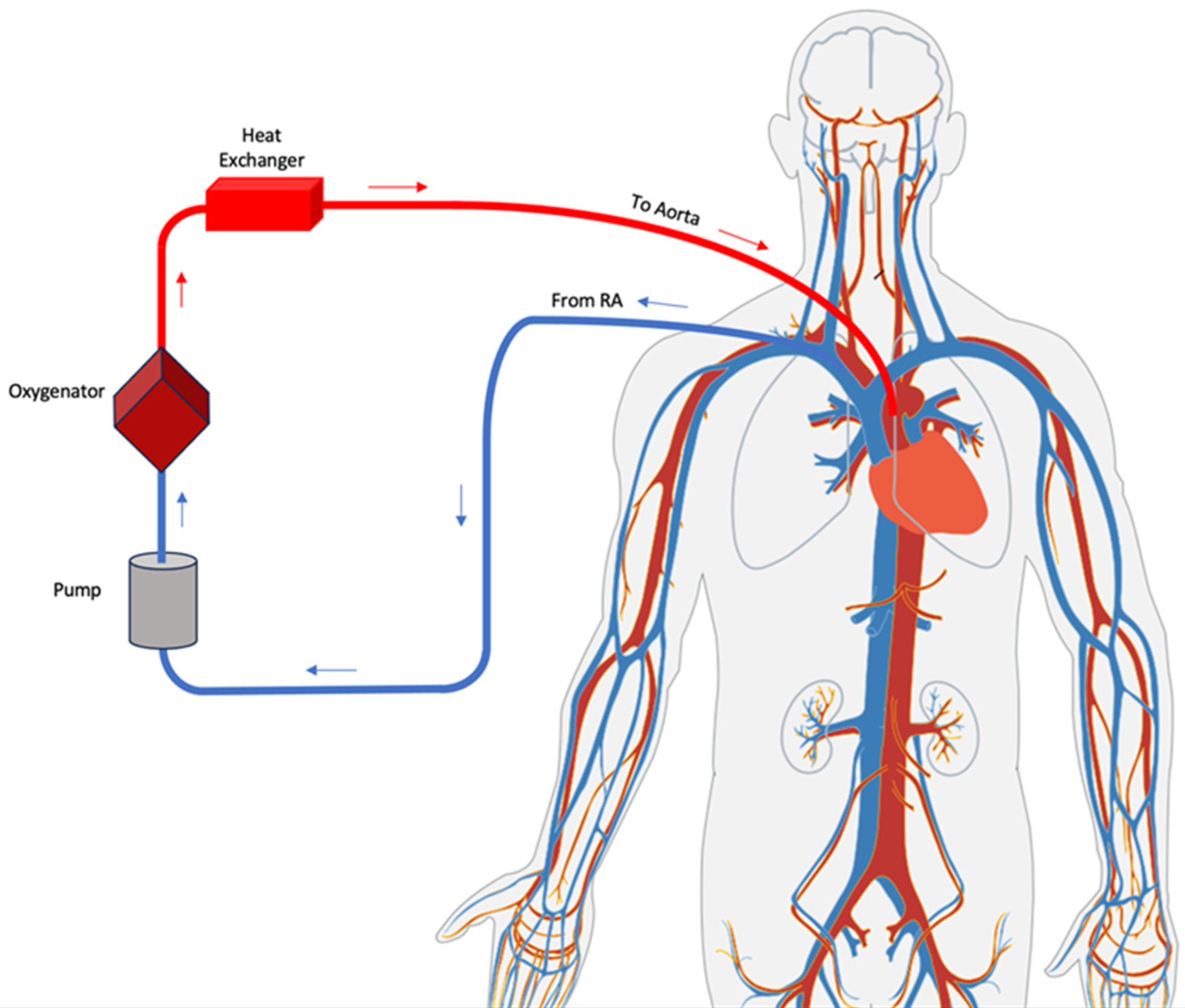
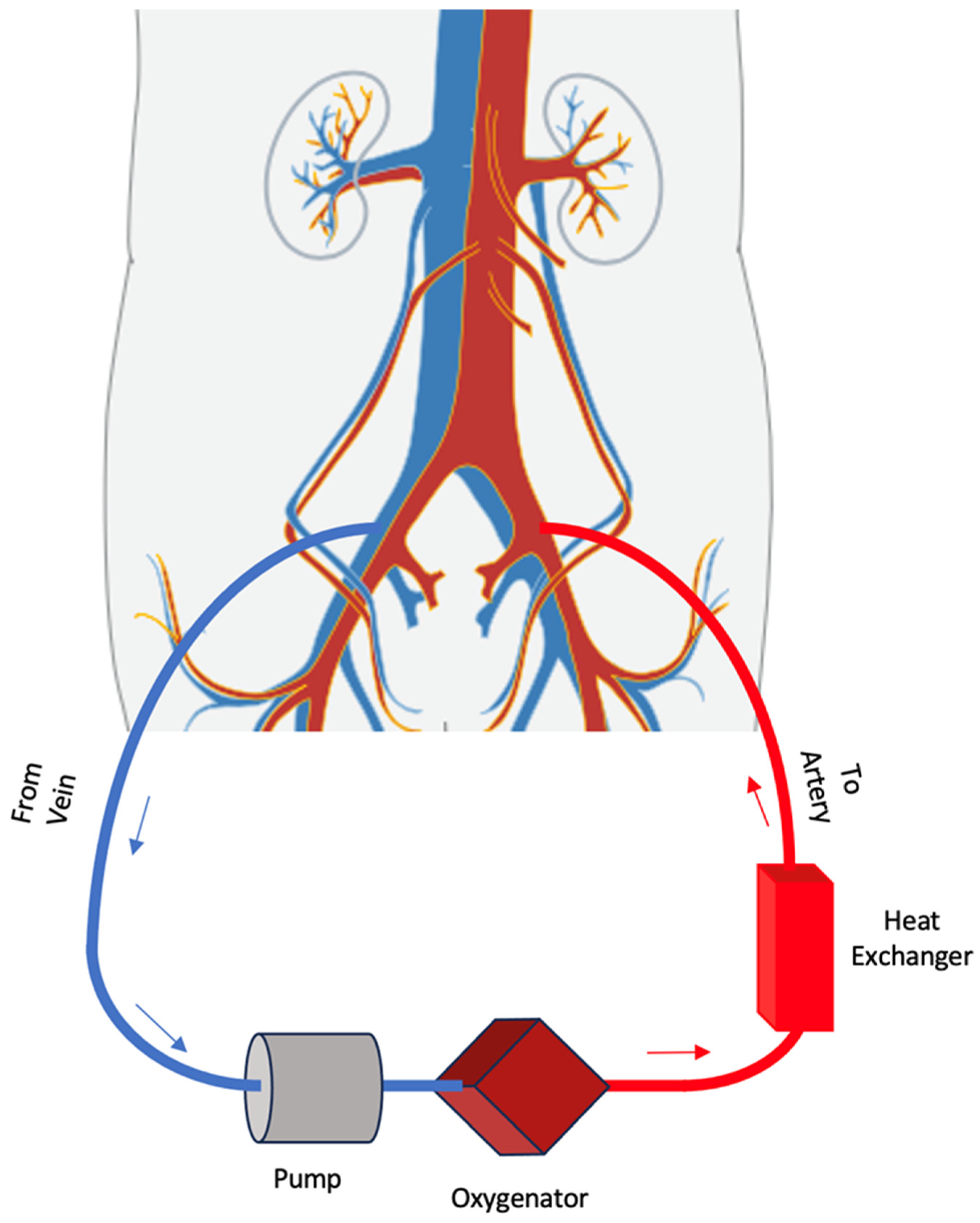
2. VA-ECMO
3. Indications and Contraindications of VA-ECMO
4. Hemodynamic Findings
5. Echocardiographic Findings
6. Complications
7. Weaning
8. ECMO Outcomes in Cardiogenic Shock
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brugts, J.J.; Caliskan, K. Short-term mechanical circulatory support by veno-arterial extracorporeal membrane oxygenation in the management of cardiogenic shock and end-stage heart failure. Expert Rev. Cardiovasc. Ther. 2014, 12, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Tsangaris, A.; Alexy, T.; Kalra, R.; Kosmopoulos, M.; Elliott, A.; Bartos, J.A.; Yannopoulos, D. Overview of Veno-Arterial Extracorporeal Membrane Oxygenation (VA-ECMO) Support for the Management of Cardiogenic Shock. Front. Cardiovasc. Med. 2021, 8, 686558. [Google Scholar] [CrossRef] [PubMed]
- Telukuntla, K.S.; Estep, J.D. Acute Mechanical Circulatory Support for Cardiogenic Shock. Methodist Debakey Cardiovasc. J. 2020, 16, 27–35. [Google Scholar] [CrossRef]
- Mehta, H.; Eisen, H.J.; Cleveland, J.C., Jr. Indications and Complications for VA-ECMO for Cardiac Failure. American College of Cardiology, 2015. Available online: https://www.acc.org/latest-in-cardiology/articles/2015/07/14/09/27/indications-and-complications-for-va-ecmo-for-cardiac-failure (accessed on 9 March 2021).
- Van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation 2017, 136, e232–e268. [Google Scholar] [CrossRef] [PubMed]
- Vora, N.; Chaudhary, R.; Upadhyay, H.V.; Konat, A.; Zalavadia, P.; Padaniya, A.; Patel, P.; Patel, N.; Prajjwal, P.; Sharma, K. Mechanical Assist Device-Assisted Percutaneous Coronary Intervention: The Use of Impella Versus Extracorporeal Membrane Oxygenation as an Emerging Frontier in Revascularization in Cardiogenic Shock. Cureus 2023, 15, e33372. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, V.K.; Sinha, S.S.; Hernandez-Montfort, J. An Evolving Roadmap for Cardiogenic Shock Requiring Temporary Mechanical Circulatory Support. JACC Asia 2023, 3, 135–137. [Google Scholar] [CrossRef]
- Koerner, M.M.; Harper, M.D.; Gordon, C.K.; Horstmanshof, D.; Long, J.W.; Sasevich, M.J.; Neel, J.D.; El Banayosy, A. Adult cardiac veno-arterial extracorporeal life support (VA-ECMO): Prevention and management of acute complications. Ann. Cardiothorac. Surg. 2019, 8, 66–75. [Google Scholar] [CrossRef]
- Ivanov, B.; Krasivskyi, I.; Gerfer, S.; Sabashnikov, A.; Doss, M.; Holzhey, D.; Eghbalzadeh, K.; Rustenbach, C.; Kuhn, E.; Rahmanian, P.B.; et al. Impact of Initial Operative Urgency on Short-Term Outcomes in Patients Treated with ECMO Due to Postcardiotomy Cardiogenic Shock. Life 2022, 12, 1872. [Google Scholar] [CrossRef]
- Keebler, M.E.; Haddad, E.V.; Choi, C.W.; McGrane, S.; Zalawadiya, S.; Schlendorf, K.H.; Brinkley, D.M.; Danter, M.R.; Wigger, M.; Menachem, J.N.; et al. Venoarterial Extracorporeal Membrane Oxygenation in Cardiogenic Shock. JACC Heart Fail. 2018, 6, 503–516. [Google Scholar] [CrossRef]
- Rao, P.; Khalpey, Z.; Smith, R.; Burkhoff, D.; Kociol, R.D. Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock and Cardiac Arrest. Circ. Heart Fail. 2018, 11, e004905. [Google Scholar] [CrossRef]
- Ghodsizad, A.; Singbartl, K.; Loebe, M.; Zeriouh, M.; Ruhparwar, A.; Grant, A.; El-Banayosy, A.; Koerner, M.M. Extracorporeal Membrane Oxygenation (ECMO): An Option for Cardiac Reccovery from Advanced Cardiogenic Shock. Heart. Surg. Forum 2017, 20, E274–E277. [Google Scholar] [CrossRef] [PubMed]
- Medical Advisory Secretariat. Extracorporeal lung support technologies—Bridge to recovery and bridge to lung transplantation in adult patients: An evidence-based analysis. Ont. Health Technol. Assess. Ser. 2010, 10, 1–47. [Google Scholar]
- Burkhoff, D.; Sayer, G.; Doshi, D.; Uriel, N. Hemodynamics of Mechanical Circulatory Support. J. Am. Coll. Cardiol. 2015, 66, 2663–2674. [Google Scholar] [CrossRef]
- Aissaoui, N.; Guerot, E.; Combes, A.; Delouche, A.; Chastre, J.; Leprince, P.; Leger, P.; Diehl, J.L.; Fagon, J.Y.; Diebold, B. Two-dimensional strain rate and Doppler tissue myocardial velocities: Analysis by echocardiography of hemodynamic and functional changes of the failed left ventricle during different degrees of extracorporeal life support. J. Am. Soc. Echocardiogr. 2012, 25, 632–640. [Google Scholar] [CrossRef]
- Shen, J.; Tse, J.R.; Chan, F.; Fleischmann, D. CT Angiography of Venoarterial Extracorporeal Membrane Oxygenation. Radiographics 2022, 42, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Ghodsizad, A.; Koerner, M.M.; Brehm, C.E.; El-Banayosy, A. The role of extracorporeal membrane oxygenation circulatory support in the ‘crash and burn’ patient: From implantation to weaning. Curr. Opin. Cardiol. 2014, 29, 275–280. [Google Scholar] [CrossRef]
- Piechura, L.M.; Coppolino, A.; Mody, G.N.; Rinewalt, D.E.; Keshk, M.; Ogawa, M.; Seethala, R.; Bohula, E.A.; Morrow, D.A.; Singh, S.K.; et al. Left ventricle unloading strategies in ECMO: A single-center experience. J. Card. Surg. 2020, 35, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.M.; Vuylsteke, A.; Fowles, J.A.; Pettit, S.; Salaunkey, K.; Bhagra, S.; Lewis, C.; Parameshwar, J.; Kydd, A.; Patvardhan, C.; et al. Transfer of Patients With Cardiogenic Shock Using Veno-Arterial Extracorporeal Membrane Oxygenation. J. Cardiothorac. Vasc. Anesth. 2020, 34, 374–382. [Google Scholar] [CrossRef]
- Pitsis, A.A.; Visouli, A.N. Mechanical assistance of the circulation during cardiogenic shock. Curr. Opin. Crit. Care 2011, 17, 425–438. [Google Scholar] [CrossRef]
- Lindholm, J.A. Ambulatory veno-venous extracorporeal membrane oxygenation. J. Thorac. Dis. 2018, 10 (Suppl. 5), S670–S673. [Google Scholar] [CrossRef]
- Rao, P.; Alouidor, B.; Smith, R.; Khalpey, Z. Ambulatory central VA-ECMO with biventricular decompression for acute cardiogenic shock. Catheter. Cardiovasc. Interv. 2018, 92, 1002–1004. [Google Scholar] [CrossRef]
- Burkhart, H.M.; Thompson, J.L.; Mascio, C.E. Ambulatory extracorporeal membrane oxygenation as a bridge to cardiac transplant: A step in the right direction? J. Thorac. Cardiovasc. Surg. 2018, 156, e11–e12. [Google Scholar] [CrossRef] [PubMed]
- Hess, N.R.; Hickey, G.W.; Murray, H.N.; Fowler, J.A.; Kaczorowski, D.J. Ambulatory simultaneous venoarterial extracorporeal membrane oxygenation and temporary percutaneous left ventricular assist device bridge to heart transplantation. JTCVS Tech. 2022, 13, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.P.; Kon, Z.N.; Evans, C.; Wu, Z.; Iacono, A.T.; McCormick, B.; Griffith, B.P. Ambulatory veno-venous extracorporeal membrane oxygenation: Innovation and pitfalls. J. Thorac. Cardiovasc. Surg. 2011, 142, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Pasrija, C.; Mackowick, K.M.; Raithel, M.; Tran, D.; Boulos, F.M.; Deatrick, K.B.; Mazzeffi, M.A.; Rector, R.; Pham, S.M.; Griffith, B.P.; et al. Ambulation With Femoral Arterial Cannulation Can Be Safely Performed on Venoarterial Extracorporeal Membrane Oxygenation. Ann. Thorac. Surg. 2019, 107, 1389–1394. [Google Scholar] [CrossRef]
- Richardson, A.S.C.; Tonna, J.E.; Nanjayya, V.; Nixon, P.; Abrams, D.C.; Raman, L.; Bernard, S.; Finney, S.J.; Grunau, B.; Youngquist, S.T.; et al. Extracorporeal Cardiopulmonary Resuscitation in Adults. Interim Guideline Consensus Statement From the Extracorporeal Life Support Organization. ASAIO J. 2021, 67, 221–228. [Google Scholar] [CrossRef]
- Keller, S.P. Management of Peripheral Venoarterial Extracorporeal Membrane Oxygenation in Cardiogenic Shock. Crit. Care Med. 2019, 47, 1235–1242. [Google Scholar] [CrossRef]
- Lafc, G.; Budak, A.B.; Yener, A.U.; Cicek, O.F. Use of extracorporeal membrane oxygenation in adults. Heart Lung Circ. 2014, 23, 10–23. [Google Scholar] [CrossRef]
- Winiszewski, H.; Guinot, P.-G.; Schmidt, M.; Besch, G.; Piton, G.; Perrotti, A.; Lorusso, R.; Kimmoun, A.; Capellier, G. Optimizing PO2 during peripheral veno-arterial ECMO: A narrative review. Crit. Care 2022, 26, 226. [Google Scholar] [CrossRef]
- Tariq, S.; Gass, A. Use of Extracorporeal Membrane Oxygenation in Refractory Cardiogenic Shock. Cardiol. Rev. 2016, 24, 26–29. [Google Scholar] [CrossRef]
- Guglin, M.; Zucker, M.J.; Bazan, V.M.; Bozkurt, B.; El Banayosy, A.; Estep, J.D.; Gurley, J.; Nelson, K.; Malyala, R.; Panjrath, G.S.; et al. Venoarterial ECMO for Adults: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 73, 698–716. [Google Scholar] [CrossRef] [PubMed]
- Mastoris, I.; Tonna, J.E.; Hu, J.; Sauer, A.J.; Haglund, N.A.; Rycus, P.; Wang, Y.; Wallisch, W.J.; Abicht, T.O.; Danter, M.R.; et al. Use of Extracorporeal Membrane Oxygenation as Bridge to Replacement Therapies in Cardiogenic Shock: Insights From the Extracorporeal Life Support Organization. Circ. Heart Fail. 2022, 15, e008777. [Google Scholar] [CrossRef] [PubMed]
- Krasivskyi, I.; Grossmann, C.; Dechow, M.; Djordjevic, I.; Ivanov, B.; Gerfer, S.; Bennour, W.; Kuhn, E.; Sabashnikov, A.; Mader, N.; et al. ECMO Retrieval Program: What Have We Learned So Far. Life 2023, 13, 157. [Google Scholar] [CrossRef]
- Hou, J.Y.; Li, X.; Yang, S.G.; Zheng, J.L.; Ma, J.F.; Su, Y.; Zhang, Y.J.; Guo, K.F.; Tu, G.W.; Luo, Z. Veno-Arterial Extracorporeal Membrane Oxygenation for Patients Undergoing Heart Transplantation: A 7-Year Experience. Front. Med. 2021, 8, 774644. [Google Scholar] [CrossRef]
- Hou, J.Y.; Wang, C.S.; Lai, H.; Sun, Y.X.; Li, X.; Zheng, J.L.; Wang, H.; Luo, J.C.; Tu, G.W.; Luo, Z. Veno-Arterial Extracorporeal Membrane Oxygenation for Patients Undergoing Acute Type A Aortic Dissection Surgery: A Six-Year Experience. Front. Cardiovasc. Med. 2021, 8, 652527. [Google Scholar] [CrossRef] [PubMed]
- Rob, D.; Spunda, R.; Lindner, J.; Rohn, V.; Kunstyr, J.; Balik, M.; Rulisek, J.; Kopecky, P.; Lips, M.; Smid, O.; et al. A rationale for early extracorporeal membrane oxygenation in patients with postinfarction ventricular septal rupture complicated by cardiogenic shock. Eur. J. Heart Fail. 2017, 19 (Suppl. 2), 97–103. [Google Scholar] [CrossRef]
- Santise, G.; Panarello, G.; Ruperto, C.; Turrisi, M.; Pilato, G.; Giunta, A.; Sciacca, S.; Pilato, M. Extracorporeal membrane oxygenation for graft failure after heart transplantation: A multidisciplinary approach to maximize weaning rate. Int. J. Artif. Organs 2014, 37, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Flagiello, M.; Al Harthy, A.; Boccalini, S.; Jacquemet, L.; Obadia, J.F.; Baudry, G.; Pozzi, M. Veno-arterial extracorporeal membrane oxygenation for COVID-19-associated acute myocardial injury complicated by refractory cardiogenic shock. J. Card. Surg. 2021, 36, 4396–4399. [Google Scholar] [CrossRef]
- Popov, A.F.; Berger, R.; Schlensak, C.; Bongers, M.N.; Haeberle, H.; Acharya, M.; Lausberg, H.F. Mechanical circulatory support for cardiovascular complications in a young COVID-19 patient. J. Card. Surg. 2020, 35, 3173–3175. [Google Scholar] [CrossRef]
- Ling, R.R.; Ramanathan, K.; Poon, W.H.; Tan, C.S.; Brechot, N.; Brodie, D.; Combes, A.; MacLaren, G. Venoarterial extracorporeal membrane oxygenation as mechanical circulatory support in adult septic shock: A systematic review and meta-analysis with individual participant data meta-regression analysis. Crit. Care 2021, 25, 246. [Google Scholar] [CrossRef]
- Chouairi, F.; Vallabhajosyula, S.; Mullan, C.; Mori, M.; Geirsson, A.; Desai, N.R.; Ahmad, T.; Miller, P.E. Transition to Advanced Therapies in Elderly Patients Supported by Extracorporeal Membrane Oxygenation Therapy. J. Card. Fail. 2020, 26, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, R.; Gelsomino, S.; Parise, O.; Mendiratta, P.; Prodhan, P.; Rycus, P.; MacLaren, G.; Brogan, T.V.; Chen, Y.S.; Maessen, J.; et al. Venoarterial Extracorporeal Membrane Oxygenation for Refractory Cardiogenic Shock in Elderly Patients: Trends in Application and Outcome From the Extracorporeal Life Support Organization (ELSO) Registry. Ann. Thorac. Surg. 2017, 104, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Peigh, G.; Cavarocchi, N.; Keith, S.W.; Hirose, H. Simple new risk score model for adult cardiac extracorporeal membrane oxygenation: Simple cardiac ECMO score. J. Surg. Res. 2015, 198, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Worku, B.; Khin, S.; Gaudino, M.; Avgerinos, D.; Gambardella, I.; D’Ayala, M.; Ramasubbu, K.; Gulkarov, I.; Salemi, A. A Simple Scoring System to Predict Survival after Venoarterial Extracorporeal Membrane Oxygenation. J. Extra Corpor. Technol. 2019, 51, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Brener, M.I.; Rosenblum, H.R.; Burkhoff, D. Pathophysiology and Advanced Hemodynamic Assessment of Cardiogenic Shock. Methodist Debakey Cardiovasc. J. 2020, 16, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.R.; Hochman, J.S. Cardiogenic shock: Current concepts and improving outcomes. Circulation 2008, 117, 686–697. [Google Scholar] [CrossRef]
- Standl, T.; Annecke, T.; Cascorbi, I.; Heller, A.R.; Sabashnikov, A.; Teske, W. The Nomenclature, Definition and Distinction of Types of Shock. Dtsch. Arztebl. Int. 2018, 115, 757–768. [Google Scholar] [CrossRef]
- Graf, T.; Desch, S.; Eitel, I.; Thiele, H. Acute myocardial infarction and cardiogenic shock: Pharmacologic and mechanical hemodynamic support pathways. Coron. Artery Dis. 2015, 26, 535–544. [Google Scholar] [CrossRef]
- Tehrani, B.N.; Truesdell, A.G.; Psotka, M.A.; Rosner, C.; Singh, R.; Sinha, S.S.; Damluji, A.A.; Batchelor, W.B. A Standardized and Comprehensive Approach to the Management of Cardiogenic Shock. JACC Heart Fail. 2020, 8, 879–891. [Google Scholar] [CrossRef]
- Berg, D.D.; Bohula, E.A.; van Diepen, S.; Katz, J.N.; Alviar, C.L.; Baird-Zars, V.M.; Barnett, C.F.; Barsness, G.W.; Burke, J.A.; Cremer, P.C.; et al. Epidemiology of Shock in Contemporary Cardiac Intensive Care Units. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005618. [Google Scholar] [CrossRef]
- Hussey, P.T.; von Mering, G.; Nanda, N.C.; Ahmed, M.I.; Addis, D.R. Echocardiography for extracorporeal membrane oxygenation. Echocardiography 2022, 39, 339–370. [Google Scholar] [CrossRef]
- Doufle, G.; Roscoe, A.; Billia, F.; Fan, E. Echocardiography for adult patients supported with extracorporeal membrane oxygenation. Crit. Care 2015, 19, 326. [Google Scholar] [CrossRef]
- Extracorporeal Life Support Organization. Guidelines for Adult Respiratory Failure. 2017. Available online: https://www.elso.org/ecmo-resources/elso-ecmo-guidelines.aspx (accessed on 9 March 2021).
- Distelmaier, K.; Wiedemann, D.; Lampichler, K.; Toth, D.; Galli, L.; Haberl, T.; Steinlechner, B.; Heinz, G.; Laufer, G.; Lang, I.M.; et al. Interdependence of VA-ECMO output, pulmonary congestion and outcome after cardiac surgery. Eur. J. Intern. Med. 2020, 81, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Grandin, E.W.; Nunez, J.I.; Willar, B.; Kennedy, K.; Rycus, P.; Tonna, J.E.; Kapur, N.K.; Shaefi, S.; Garan, A.R. Mechanical Left Ventricular Unloading in Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation. J. Am. Coll. Cardiol. 2022, 79, 1239–1250. [Google Scholar] [CrossRef]
- Nuding, S.; Werdan, K. IABP plus ECMO-Is one and one more than two? J. Thorac. Dis. 2017, 9, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Parissis, H.; Graham, V.; Lampridis, S.; Lau, M.; Hooks, G.; Mhandu, P.C. IABP: History-evolution-pathophysiology-indications: What we need to know. J. Cardiothorac. Surg. 2016, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Yang, C.; Chen, J.; Fan, Z.; Cai, W.; Huang, Y.; Xiang, Z.; Yang, J.; Zhang, J.; Yang, J. Comparison of the Efficacy of ECMO With or Without IABP in Patients With Cardiogenic Shock: A Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 917610. [Google Scholar] [CrossRef] [PubMed]
- Glazier, J.J.; Kaki, A. The Impella Device: Historical Background, Clinical Applications and Future Directions. Int. J. Angiol. 2019, 28, 118–123. [Google Scholar] [CrossRef]
- Schrage, B.; Ibrahim, K.; Loehn, T.; Werner, N.; Sinning, J.M.; Pappalardo, F.; Pieri, M.; Skurk, C.; Lauten, A.; Landmesser, U.; et al. Impella Support for Acute Myocardial Infarction Complicated by Cardiogenic Shock. Circulation 2019, 139, 1249–1258. [Google Scholar] [CrossRef]
- Fiorelli, F.; Panoulas, V. Impella as unloading strategy during VA-ECMO: Systematic review and meta-analysis. Rev. Cardiovasc. Med. 2021, 22, 1503–1511. [Google Scholar] [CrossRef]
- Mlcek, M.; Meani, P.; Cotza, M.; Kowalewski, M.; Raffa, G.M.; Kuriscak, E.; Popkova, M.; Pilato, M.; Arcadipane, A.; Ranucci, M.; et al. Atrial Septostomy for Left Ventricular Unloading During Extracorporeal Membrane Oxygenation for Cardiogenic Shock: Animal Model. JACC Cardiovasc. Interv. 2021, 14, 2698–2707. [Google Scholar] [CrossRef]
- Deshpande, S.R.; Kennedy, K.F.; Vincent, R.N.; Maher, K.O. Atrial septostomy in patients supported with venoarterial extracorporeal membrane oxygenation: Analysis of the IMPACT registry data. Int. J. Artif. Organs 2021, 44, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Loforte, A.; Baiocchi, M.; Dal Checco, E.; Gliozzi, G.; Fiorentino, M.; Lo Coco, V.; Martin Suarez, S.; Marrozzini, C.; Biffi, M.; Marinelli, G.; et al. Percutaneous Pulmonary Artery Venting via Jugular Vein While on Peripheral Extracorporeal Life Support. ASAIO J. 2020, 66, e50–e54. [Google Scholar] [CrossRef] [PubMed]
- Loforte, A.; Baiocchi, M.; Gliozzi, G.; Coppola, G.; Di Bartolomeo, R.; Lorusso, R. Percutaneous pulmonary artery venting via jugular vein while on peripheral extracorporeal membrane oxygenation running: A less invasive approach to provide full biventricular unloading. Ann. Cardiothorac. Surg. 2019, 8, 163–166. [Google Scholar] [CrossRef]
- Phillip, R.; Howard, J.; Hawamdeh, H.; Tribble, T.; Gurley, J.; Saha, S. Left Atrial Veno-Arterial Extracorporeal Membrane Oxygenation Case Series: A Single-Center Experience. J. Surg. Res. 2023, 281, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kofidis, T.; Kamiya, H.; Shrestha, M.; Tessmann, R.; Haverich, A.; Klima, U. Creatine kinase isoenzyme MB relative index as predictor of mortality on extracorporeal membrane oxygenation support for postcardiotomy cardiogenic shock in adult patients. Eur. J. Cardiothorac. Surg. 2006, 30, 617–620. [Google Scholar] [CrossRef]
- Ohira, S.; Malekan, R.; Goldberg, J.B.; Lansman, S.L.; Spielvogel, D.; Kai, M.; Spencer, P.J.; Levine, A.; Pan, S.; Aggarwal-Gupta, C.; et al. Axillary artery cannulation for veno-arterial extracorporeal membrane oxygenation support in cardiogenic shock. JTCVS Tech 2021, 5, 62–71. [Google Scholar] [CrossRef]
- Takayama, H.; Landes, E.; Truby, L.; Fujita, K.; Kirtane, A.J.; Mongero, L.; Yuzefpolskaya, M.; Colombo, P.C.; Jorde, U.P.; Kurlansky, P.A.; et al. Feasibility of smaller arterial cannulas in venoarterial extracorporeal membrane oxygenation. J. Thorac. Cardiovasc. Surg. 2015, 149, 1428–1433. [Google Scholar] [CrossRef]
- Kowalewski, M.; Malvindi, P.G.; Zielinski, K.; Martucci, G.; Slomka, A.; Suwalski, P.; Lorusso, R.; Meani, P.; Arcadipane, A.; Pilato, M.; et al. Left Ventricle Unloading with Veno-Arterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock. Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 1039. [Google Scholar] [CrossRef]
- Russo, J.J.; Aleksova, N.; Pitcher, I.; Couture, E.; Parlow, S.; Faraz, M.; Visintini, S.; Simard, T.; Di Santo, P.; Mathew, R.; et al. Left Ventricular Unloading During Extracorporeal Membrane Oxygenation in Patients With Cardiogenic Shock. J. Am. Coll. Cardiol. 2019, 73, 654–662. [Google Scholar] [CrossRef]
- Rupprecht, L.; Lunz, D.; Philipp, A.; Lubnow, M.; Schmid, C. Pitfalls in percutaneous ECMO cannulation. Heart Lung Vessel 2015, 7, 320–326. [Google Scholar] [PubMed]
- Wilson, J.; Fisher, R.; Caetano, F.; Soliman-Aboumarie, H.; Patel, B.; Ledot, S.; Price, S.; Vandenbriele, C. Managing Harlequin Syndrome in VA-ECMO—Do not forget the right ventricle. Perfusion 2022, 37, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Grant, C., Jr.; Richards, J.B.; Frakes, M.; Cohen, J.; Wilcox, S.R. ECMO and Right Ventricular Failure: Review of the Literature. J. Intensive Care Med. 2021, 36, 352–360. [Google Scholar] [CrossRef]
- Contento, C.; Battisti, A.; Agro, B.; De Marco, M.; Iaiza, A.; Pietraforte, L.; Pisani, P.; Proietti, A.; Vitalini, E.; Montalto, A.; et al. A novel veno-arteriovenous extracorporeal membrane oxygenation with double pump for the treatment of Harlequin syndrome. Perfusion 2020, 35 (Suppl. 1), 65–72. [Google Scholar] [CrossRef]
- Platts, D.G.; Sedgwick, J.F.; Burstow, D.J.; Mullany, D.V.; Fraser, J.F. The role of echocardiography in the management of patients supported by extracorporeal membrane oxygenation. J. Am. Soc. Echocardiogr. 2012, 25, 131–141. [Google Scholar] [CrossRef]
- Geller, B.J.; Sinha, S.S.; Kapur, N.K.; Bakitas, M.; Balsam, L.B.; Chikwe, J.; Klein, D.G.; Kochar, A.; Masri, S.C.; Sims, D.B.; et al. Escalating and De-escalating Temporary Mechanical Circulatory Support in Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation 2022, 146, e50–e68. [Google Scholar] [CrossRef]
- Bhat, A.G.; Golchin, A.; Pasupula, D.K.; Hernandez-Montfort, J.A. Right Sided Intracardiac Thrombosis during Veno-Arterial Extracorporeal Membrane Oxygenation: A Case Report and Literature Review. Case Rep. Crit. Care 2019, 2019, 8594681. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Bernstein, W. Review of Venoarterial Extracorporeal Membrane Oxygenation and Development of Intracardiac Thrombosis in Adult Cardiothoracic Patients. J. Extra Corpor. Technol. 2016, 48, 162–167. [Google Scholar] [CrossRef]
- Tan, C.; Rubenson, D.; Srivastava, A.; Mohan, R.; Smith, M.R.; Billick, K.; Bardarian, S.; Thomas Heywood, J. Left ventricular outflow tract velocity time integral outperforms ejection fraction and Doppler-derived cardiac output for predicting outcomes in a select advanced heart failure cohort. Cardiovasc. Ultrasound 2017, 15, 18. [Google Scholar] [CrossRef]
- Vakil, D.; Soto, C.; D’Costa, Z.; Volk, L.; Kandasamy, S.; Iyer, D.; Ikegami, H.; Russo, M.J.; Lee, L.Y.; Lemaire, A. Short-term and intermediate outcomes of cardiogenic shock and cardiac arrest patients supported by venoarterial extracorporeal membrane oxygenation. J. Cardiothorac. Surg. 2021, 16, 290. [Google Scholar] [CrossRef]
- Rajsic, S.; Breitkopf, R.; Bukumiric, Z.; Treml, B. ECMO Support in Refractory Cardiogenic Shock: Risk Factors for Mortality. J. Clin. Med. 2022, 11, 6821. [Google Scholar] [CrossRef]
- Rajsic, S.; Treml, B.; Jadzic, D.; Breitkopf, R.; Oberleitner, C.; Popovic Krneta, M.; Bukumiric, Z. Extracorporeal membrane oxygenation for cardiogenic shock: A meta-analysis of mortality and complications. Ann. Intensive Care 2022, 12, 93. [Google Scholar] [CrossRef]
- Alhussein, M.; Moayedi, Y.; Posada, J.D.; Ross, H.; Hickey, E.; Rao, V.; Billia, F. Ventricular Thrombosis Post-Venoarterial Extracorporeal Membrane Oxygenation. Circ. Heart Fail. 2017, 10, e003757. [Google Scholar] [CrossRef]
- Olson, S.R.; Murphree, C.R.; Zonies, D.; Meyer, A.D.; McCarty, O.J.T.; Deloughery, T.G.; Shatzel, J.J. Thrombosis and Bleeding in Extracorporeal Membrane Oxygenation (ECMO) Without Anticoagulation: A Systematic Review. ASAIO J. 2021, 67, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Rajsic, S.; Breitkopf, R.; Rugg, C.; Bukumiric, Z.; Reitbauer, J.; Treml, B. Thrombotic Events Develop in 1 Out of 5 Patients Receiving ECMO Support: An 11-Year Referral Centre Experience. J. Clin. Med. 2023, 12, 1082. [Google Scholar] [CrossRef] [PubMed]
- Colman, E.; Yin, E.B.; Laine, G.; Chatterjee, S.; Saatee, S.; Herlihy, J.P.; Reyes, M.A.; Bracey, A.W. Evaluation of a heparin monitoring protocol for extracorporeal membrane oxygenation and review of the literature. J. Thorac. Dis. 2019, 11, 3325–3335. [Google Scholar] [CrossRef] [PubMed]
- Rajsic, S.; Breitkopf, R.; Jadzic, D.; Popovic Krneta, M.; Tauber, H.; Treml, B. Anticoagulation Strategies during Extracorporeal Membrane Oxygenation: A Narrative Review. J. Clin. Med. 2022, 11, 5147. [Google Scholar] [CrossRef] [PubMed]
- Rajsic, S.; Treml, B.; Jadzic, D.; Breitkopf, R.; Oberleitner, C.; Bachler, M.; Bosch, J.; Bukumiric, Z. aPTT-guided anticoagulation monitoring during ECMO support: A systematic review and meta-analysis. J. Crit. Care 2023, 77, 154332. [Google Scholar] [CrossRef]
- Rajsic, S.; Breitkopf, R.; Treml, B.; Jadzic, D.; Oberleitner, C.; Oezpeker, U.C.; Innerhofer, N.; Bukumiric, Z. Association of aPTT-Guided Anticoagulation Monitoring with Thromboembolic Events in Patients Receiving V-A ECMO Support: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 3224. [Google Scholar] [CrossRef]
- Wood, K.L.; Ayers, B.; Gosev, I.; Kumar, N.; Melvin, A.L.; Barrus, B.; Prasad, S. Venoarterial-Extracorporeal Membrane Oxygenation Without Routine Systemic Anticoagulation Decreases Adverse Events. Ann. Thorac. Surg. 2020, 109, 1458–1466. [Google Scholar] [CrossRef]
- Fang, Z.A.; Navaei, A.H.; Hensch, L.; Hui, S.R.; Teruya, J. Hemostatic Management of Extracorporeal Circuits Including Cardiopulmonary Bypass and Extracorporeal Membrane Oxygenation. Semin. Thromb. Hemost. 2020, 46, 62–72. [Google Scholar] [CrossRef]
- Thomas, J.; Kostousov, V.; Teruya, J. Bleeding and Thrombotic Complications in the Use of Extracorporeal Membrane Oxygenation. Semin. Thromb. Hemost. 2018, 44, 20–29. [Google Scholar] [CrossRef]
- Ohira, S.; Kawamura, M.; Ahern, K.; Cavarocchi, N.; Hirose, H. Aggressive placement of distal limb perfusion catheter in venoarterial extracorporeal membrane oxygenation. Int. J. Artif. Organs 2020, 43, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Ohira, S.; Pan, S.; Levine, A.; Aggarwal-Gupta, C.; Lanier, G.M.; Gass, A.L.; Spielvogel, D.; Kai, M. Simple technique of distal leg perfusion during heart transplant in patients with preoperative veno-arterial extracorporeal membrane oxygenation support. Perfusion 2023, 38, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Patton-Rivera, K.; Beck, J.; Fung, K.; Chan, C.; Beck, M.; Takayama, H.; Takeda, K. Using near-infrared reflectance spectroscopy (NIRS) to assess distal-limb perfusion on venoarterial (V-A) extracorporeal membrane oxygenation (ECMO) patients with femoral cannulation. Perfusion 2018, 33, 618–623. [Google Scholar] [CrossRef]
- Tanaka, D.; Hirose, H.; Cavarocchi, N.; Entwistle, J.W. The Impact of Vascular Complications on Survival of Patients on Venoarterial Extracorporeal Membrane Oxygenation. Ann. Thorac. Surg. 2016, 101, 1729–1734. [Google Scholar] [CrossRef]
- Wong, J.K.; Smith, T.N.; Pitcher, H.T.; Hirose, H.; Cavarocchi, N.C. Cerebral and lower limb near-infrared spectroscopy in adults on extracorporeal membrane oxygenation. Artif. Organs 2012, 36, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Askenazi, D.J.; Selewski, D.T.; Paden, M.L.; Cooper, D.S.; Bridges, B.C.; Zappitelli, M.; Fleming, G.M. Renal replacement therapy in critically ill patients receiving extracorporeal membrane oxygenation. Clin. J. Am. Soc. Nephrol. 2012, 7, 1328–1336. [Google Scholar] [CrossRef]
- Selewski, D.T.; Wille, K.M. Continuous renal replacement therapy in patients treated with extracorporeal membrane oxygenation. Semin. Dial. 2021, 34, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Kilburn, D.J.; Shekar, K.; Fraser, J.F. The Complex Relationship of Extracorporeal Membrane Oxygenation and Acute Kidney Injury: Causation or Association? BioMed Res. Int. 2016, 2016, 1094296. [Google Scholar] [CrossRef]
- Ostermann, M.; Connor, M., Jr.; Kashani, K. Continuous renal replacement therapy during extracorporeal membrane oxygenation: Why, when and how? Curr. Opin. Crit. Care 2018, 24, 493–503. [Google Scholar] [CrossRef]
- Aboul Nour, H.; Poyiadji, N.; Mohamed, G.; Alsrouji, O.K.; Ramadan, A.R.; Griffith, B.; Marin, H.; Chebl, A.B. Challenges of acute phase neuroimaging in VA-ECMO, pitfalls and alternative imaging options. Interv. Neuroradiol. 2021, 27, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Al-Kawaz, M.; Shou, B.; Prokupets, R.; Whitman, G.; Geocadin, R.; Cho, S.M. Mild hypothermia and neurologic outcomes in patients undergoing venoarterial extracorporeal membrane oxygenation. J. Card. Surg. 2022, 37, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.K.; Bhatti, Z.; Bosserman, A.J.; Mathew, M.C.; Vaidehi, K.; Kalva, S.P. Management of vascular complications of extra-corporeal membrane oxygenation. Cardiovasc. Diagn. Ther. 2018, 8, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Burgos, L.M.; Seoane, L.; Diez, M.; Baro Vila, R.C.; Furmento, J.F.; Vrancic, M.; Aissaoui, N. Multiparameters associated to successful weaning from VA ECMO in adult patients with cardiogenic shock or cardiac arrest: Systematic review and meta-analysis. Ann. Card. Anaesth. 2023, 26, 4–11. [Google Scholar] [CrossRef]
- Cavarocchi, N.C.; Pitcher, H.T.; Yang, Q.; Karbowski, P.; Miessau, J.; Hastings, H.M.; Hirose, H. Weaning of extracorporeal membrane oxygenation using continuous hemodynamic transesophageal echocardiography. J. Thorac. Cardiovasc. Surg. 2013, 146, 1474–1479. [Google Scholar] [CrossRef]
- Aissaoui, N.; El-Banayosy, A.; Combes, A. How to wean a patient from veno-arterial extracorporeal membrane oxygenation. Intensive Care Med. 2015, 41, 902–905. [Google Scholar] [CrossRef]
- Lusebrink, E.; Stremmel, C.; Stark, K.; Joskowiak, D.; Czermak, T.; Born, F.; Kupka, D.; Scherer, C.; Orban, M.; Petzold, T.; et al. Update on Weaning from Veno-Arterial Extracorporeal Membrane Oxygenation. J. Clin. Med. 2020, 9, 992. [Google Scholar] [CrossRef]
- Pappalardo, F.; Pieri, M.; Arnaez Corada, B.; Ajello, S.; Melisurgo, G.; De Bonis, M.; Zangrillo, A. Timing and Strategy for Weaning From Venoarterial ECMO are Complex Issues. J. Cardiothorac. Vasc. Anesth. 2015, 29, 906–911. [Google Scholar] [CrossRef]
- Ortuno, S.; Delmas, C.; Diehl, J.L.; Bailleul, C.; Lancelot, A.; Naili, M.; Cholley, B.; Pirracchio, R.; Aissaoui, N. Weaning from veno-arterial extra-corporeal membrane oxygenation: Which strategy to use? Ann. Cardiothorac. Surg. 2019, 8, E1–E8. [Google Scholar] [CrossRef]
- Firstenberg, M.S.; Orsinelli, D.A. ECMO and ECHO: The evolving role of quantitative echocardiography in the management of patients requiring extracorporeal membrane oxygenation. J. Am. Soc. Echocardiogr. 2012, 25, 641–643. [Google Scholar] [CrossRef] [PubMed]
- Loforte, A.; Marinelli, G.; Musumeci, F.; Folesani, G.; Pilato, E.; Martin Suarez, S.; Montalto, A.; Lilla Della Monica, P.; Grigioni, F.; Frascaroli, G.; et al. Extracorporeal membrane oxygenation support in refractory cardiogenic shock: Treatment strategies and analysis of risk factors. Artif. Organs 2014, 38, E129–E141. [Google Scholar] [CrossRef]
- Negi, S.I.; Sokolovic, M.; Koifman, E.; Kiramijyan, S.; Torguson, R.; Lindsay, J.; Ben-Dor, I.; Suddath, W.; Pichard, A.; Satler, L.; et al. Contemporary Use of Veno-Arterial Extracorporeal Membrane Oxygenation for Refractory Cardiogenic Shock in Acute Coronary Syndrome. J. Invasive Cardiol. 2016, 28, 52–57. [Google Scholar]
- Khorsandi, M.; Dougherty, S.; Bouamra, O.; Pai, V.; Curry, P.; Tsui, S.; Clark, S.; Westaby, S.; Al-Attar, N.; Zamvar, V. Extra-corporeal membrane oxygenation for refractory cardiogenic shock after adult cardiac surgery: A systematic review and meta-analysis. J. Cardiothorac. Surg. 2017, 12, 55. [Google Scholar] [CrossRef]
- Garcia-Gigorro, R.; Renes-Carreno, E.; Perez-Vela, J.L.; Marin-Mateos, H.; Gutierrez, J.; Corres-Peiretti, M.A.; Delgado, J.F.; Perez-de la Sota, E.; Cortina-Romero, J.M.; Montejo-Gonzalez, J.C. Mechanical support with venoarterial extracorporeal membrane oxygenation (ECMO-VA): Short-term and long-term prognosis after a successful weaning. Med. Intensiv. 2017, 41, 513–522. [Google Scholar] [CrossRef]
- Fux, T.; Holm, M.; Corbascio, M.; Lund, L.H.; van der Linden, J. VA-ECMO Support in Nonsurgical Patients With Refractory Cardiogenic Shock: Pre-Implant Outcome Predictors. Artif. Organs 2019, 43, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Krasivskyi, I.; Ivanov, B.; Vehrenberg, J.; Eghbalzadeh, K.; Gerfer, S.; Gaisendrees, C.; Kuhn, E.; Sabashnikov, A.; Mader, N.; Djordjevic, I.; et al. Sex-Related Differences in Short-Term Outcomes after Mobile VA-ECMO Implantation: Five-Year Experience of an ECMO Retrieval Program. Life 2022, 12, 1746. [Google Scholar] [CrossRef] [PubMed]
- Ostadal, P.; Rokyta, R.; Karasek, J.; Kruger, A.; Vondrakova, D.; Janotka, M.; Naar, J.; Smalcova, J.; Hubatova, M.; Hromadka, M.; et al. Extracorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock: Results of the ECMO-CS Randomized Clinical Trial. Circulation 2023, 147, 454–464. [Google Scholar] [CrossRef]
- Banning, A.S.; Sabate, M.; Orban, M.; Gracey, J.; López-Sobrino, T.; Massberg, S.; Kastrati, A.; Bogaerts, K.; Adriaenssens, T.; Berry, C.; et al. Venoarterial extracorporeal membrane oxygenation or standard care in patients with cardiogenic shock complicating acute myocardial infarction: The multicentre, randomised EURO SHOCK trial. EuroIntervention 2023, 19, 482–492. [Google Scholar] [CrossRef]
- Abrams, D.; MacLaren, G.; Lorusso, R.; Price, S.; Yannopoulos, D.; Vercaemst, L.; Belohlavek, J.; Taccone, F.S.; Aissaoui, N.; Shekar, K.; et al. Extracorporeal cardiopulmonary resuscitation in adults: Evidence and implications. Intensive Care Med. 2022, 48, 1–15. [Google Scholar] [CrossRef]
- Rob, D.; Smalcova, J.; Smid, O.; Kral, A.; Kovarnik, T.; Zemanek, D.; Kavalkova, P.; Huptych, M.; Komarek, A.; Franek, O.; et al. Extracorporeal versus conventional cardiopulmonary resuscitation for refractory out-of-hospital cardiac arrest: A secondary analysis of the Prague OHCA trial. Crit. Care 2022, 26, 330. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.J.; Lee, H.Y.; Ahn, H.S.; Lee, S.W. Comparing extracorporeal cardiopulmonary resuscitation with conventional cardiopulmonary resuscitation: A meta-analysis. Resuscitation 2016, 103, 106–116. [Google Scholar] [CrossRef]
- Tonna, J.E.; Johnson, N.J.; Greenwood, J.; Gaieski, D.F.; Shinar, Z.; Bellezo, J.M.; Becker, L.; Shah, A.P.; Youngquist, S.T.; Mallin, M.P.; et al. Practice characteristics of Emergency Department extracorporeal cardiopulmonary resuscitation (eCPR) programs in the United States: The current state of the art of Emergency Department extracorporeal membrane oxygenation (ED ECMO). Resuscitation 2016, 107, 38–46. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lin, J.W.; Yu, H.Y.; Ko, W.J.; Jerng, J.S.; Chang, W.T.; Chen, W.J.; Huang, S.C.; Chi, N.H.; Wang, C.H.; et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: An observational study and propensity analysis. Lancet 2008, 372, 554–561. [Google Scholar] [CrossRef]
- Belohlavek, J.; Smalcova, J.; Rob, D.; Franek, O.; Smid, O.; Pokorna, M.; Horak, J.; Mrazek, V.; Kovarnik, T.; Zemanek, D.; et al. Effect of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment on Functional Neurologic Outcome in Refractory Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA 2022, 327, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.G.; Jo, I.J.; Sim, M.S.; Song, Y.B.; Yang, J.H.; Hahn, J.Y.; Choi, S.H.; Gwon, H.C.; Jeon, E.S.; Sung, K.; et al. Two-year survival and neurological outcome of in-hospital cardiac arrest patients rescued by extracorporeal cardiopulmonary resuscitation. Int. J. Cardiol. 2013, 168, 3424–3430. [Google Scholar] [CrossRef]
- Tonna, J.E.; Selzman, C.H.; Girotra, S.; Presson, A.P.; Thiagarajan, R.R.; Becker, L.B.; Zhang, C.; Rycus, P.; Keenan, H.T.; American Heart Association Get With the Guidelines-Resuscitation, I. Resuscitation Using ECPR During In-Hospital Cardiac Arrest (RESCUE-IHCA) Mortality Prediction Score and External Validation. JACC Cardiovasc. Interv. 2022, 15, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Ostadal, P.; Rokyta, R.; Kruger, A.; Vondrakova, D.; Janotka, M.; Smid, O.; Smalcova, J.; Hromadka, M.; Linhart, A.; Belohlavek, J. Extra corporeal membrane oxygenation in the therapy of cardiogenic shock (ECMO-CS): Rationale and design of the multicenter randomized trial. Eur. J. Heart Fail. 2017, 19 (Suppl. 2), 124–127. [Google Scholar] [CrossRef] [PubMed]
| Patient Selection | |
|---|---|
| Patient-Specific Risk Factors, Potential Benefit, Patient Prognosis, Comorbidities, and Weaning Approaches Need to Be Considered in Each Individual Patient Prior to VA-ECMO Initiation | |
| Indications | Contraindications |
| Cardiogenic Shock Refractory to Conventional Medical and Device-Based Therapy | Patients with an Overall Poor Prognosis and at High Risk of Morbidity and Mortality |
| Absolute
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koziol, K.J.; Isath, A.; Rao, S.; Gregory, V.; Ohira, S.; Van Diepen, S.; Lorusso, R.; Krittanawong, C. Extracorporeal Membrane Oxygenation (VA-ECMO) in Management of Cardiogenic Shock. J. Clin. Med. 2023, 12, 5576. https://doi.org/10.3390/jcm12175576
Koziol KJ, Isath A, Rao S, Gregory V, Ohira S, Van Diepen S, Lorusso R, Krittanawong C. Extracorporeal Membrane Oxygenation (VA-ECMO) in Management of Cardiogenic Shock. Journal of Clinical Medicine. 2023; 12(17):5576. https://doi.org/10.3390/jcm12175576
Chicago/Turabian StyleKoziol, Klaudia J., Ameesh Isath, Shiavax Rao, Vasiliki Gregory, Suguru Ohira, Sean Van Diepen, Roberto Lorusso, and Chayakrit Krittanawong. 2023. "Extracorporeal Membrane Oxygenation (VA-ECMO) in Management of Cardiogenic Shock" Journal of Clinical Medicine 12, no. 17: 5576. https://doi.org/10.3390/jcm12175576
APA StyleKoziol, K. J., Isath, A., Rao, S., Gregory, V., Ohira, S., Van Diepen, S., Lorusso, R., & Krittanawong, C. (2023). Extracorporeal Membrane Oxygenation (VA-ECMO) in Management of Cardiogenic Shock. Journal of Clinical Medicine, 12(17), 5576. https://doi.org/10.3390/jcm12175576







