Coronary No-Reflow after Primary Percutaneous Coronary Intervention—Current Knowledge on Pathophysiology, Diagnosis, Clinical Impact and Therapy
Abstract
1. Historical Perspective
2. Pathophysiology of CNR
2.1. A Short Description of Myocardial Microcirculation
2.2. Myocardial Ischemia
2.3. Distal Embolization
2.4. Reperfusion-Related Injury
3. Diagnosis and Frequency of CNR
4. Individual Susceptibility to CNR
5. Impact of CNR on Clinical Outcome
6. Therapy of CNR
6.1. Therapy against Distal Embolization
6.2. Pharmacological Therapy
7. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Majno, G.; Ames, A.; Chiang, J.; Wright, R.L. No Reflow after Cerebral Ischaemia. Lancet 1967, 2, 569–570. [Google Scholar] [CrossRef]
- Ames, A., 3rd; Wright, R.L.; Kowada, M.; Thurston, J.M.; Majno, G. Cerebral ischemia. II. The no-reflow phenomenon. Am. J. Pathol. 1968, 52, 437–453. [Google Scholar]
- Chiang, J.; Kowada, M.; Ames, A., 3rd; Wright, R.L.; Majno, G. Cerebral ischemia. III. Vascular changes. Am. J. Pathol. 1968, 52, 455–476. [Google Scholar] [PubMed]
- Harman, J.W. The Significance of Local Vascular Phenomena in the Production of Ischemic Necrosis in Skeletal Muscle. Am. J. Pathol. 1948, 24, 625–641. [Google Scholar] [PubMed]
- Sheehan, H.L.; Davis, J.C. Renal Ischaemia with Failed Reflow. J. Pathol. Bacteriol. 1959, 78, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Flores, J.; DiBona, D.R.; Beck, C.H.; Leaf, A. The role of cell swelling in ischemic renal damage and the protective effect of hypertonic solute. J. Clin. Investig. 1972, 51, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, K.; Carroll, R.; Tapp, E. Temporary Ischaemia of Rat Adrenal Gland. J. Pathol. Bacteriol. 1966, 91, 235–240. [Google Scholar] [CrossRef]
- Krug, A.; De Rochemont, W.D.M.; Korb, G. Blood supply of the myocardium after temporary coronary occlusion. Circ. Res. 1966, 19, 57–62. [Google Scholar] [CrossRef]
- Willms-Kretschmer, K.; Majno, G. Ischemia of the skin. Electron microscopic study of vascular injury. Am. J. Pathol. 1969, 54, 327–353. [Google Scholar]
- Kloner, R.A.; Ganote, C.E.; Jennings, R.B. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J. Clin. Investig. 1974, 54, 1496–1508. [Google Scholar] [CrossRef]
- Kloner, R.A.; Ganote, C.E.; Whalen, D.A., Jr.; Jennings, R.B. Effect of a transient period of ischemia on myocardial cells. II. Fine structure during the first few minutes of reflow. Am. J. Pathol. 1974, 74, 399–422. [Google Scholar] [PubMed]
- Jefferson, G. “Arterial Embolectomy”. Br. Med. J. 1934, 2, 1090–1094. [Google Scholar] [CrossRef]
- Griffiths, D.L. Volkmann’s ischaemic contracture. Br. J. Surg. 1940, 28, 239–260. [Google Scholar] [CrossRef]
- Schofer, J.; Montz, R.; Mathey, D.G. Scintigraphic evidence of the “no reflow” phenomenon in human beings after coronary thrombolysis. J. Am. Coll. Cardiol. 1985, 5, 593–598. [Google Scholar] [CrossRef]
- Bates, E.R.; Krell, M.J.; Dean, E.N.; O’Neill, W.W.; Vogel, R.A. Demonstration of the “no-reflow” phenomenon by digital coronary arteriography. Am. J. Cardiol. 1986, 57, 177–178. [Google Scholar] [CrossRef]
- Pomerantz, R.M.; Kuntz, R.E.; Diver, D.J.; Safian, R.D.; Baim, D.S. Intracoronary verapamil for the treatment of distal microvascular coronary artery spasm following PTCA. Cathet Cardiovasc. Diagn. 1991, 24, 283–285. [Google Scholar] [CrossRef]
- Feld, H.; Lichstein, E.; Schachter, J.; Shani, J. Early and late angiographic findings of the “no-reflow” phenomenon following direct angioplasty as primary treatment for acute myocardial infarction. Am. Heart J. 1992, 123, 782–784. [Google Scholar] [CrossRef]
- Wilson, R.F.; Laxson, D.D.; Lesser, J.R.; White, C.W. Intense microvascular constriction after angioplasty of acute thrombotic coronary arterial lesions. Lancet 1989, 1, 807–811. [Google Scholar] [CrossRef]
- Ito, H.; Tomooka, T.; Sakai, N.; Yu, H.; Higashino, Y.; Fujii, K.; Masuyama, T.; Kitabatake, A.; Minamino, T. Lack of myocardial perfusion immediately after successful thrombolysis. A predictor of poor recovery of left ventricular function in anterior myocardial infarction. Circulation 1992, 85, 1699–1705. [Google Scholar] [CrossRef]
- Piana, R.N.; Paik, G.Y.; Moscucci, M.; Cohen, D.J.; Gibson, C.M.; Kugelmass, A.D.; Carrozza, J.P., Jr.; Kuntz, R.E.; Baim, D.S. Incidence and treatment of ‘no-reflow’ after percutaneous coronary intervention. Circulation 1994, 89, 2514–2518. [Google Scholar] [CrossRef]
- Morishima, I.; Sone, T.; Mokuno, S.; Taga, S.; Shimauchi, A.; Oki, Y.; Kondo, J.; Tsuboi, H.; Sassa, H. Clinical significance of no-reflow phenomenon observed on angiography after successful treatment of acute myocardial infarction with percutaneous transluminal coronary angioplasty. Am. Heart J. 1995, 130, 239–243. [Google Scholar] [CrossRef]
- Morishima, I.; Sone, T.; Okumura, K.; Tsuboi, H.; Kondo, J.; Mukawa, H.; Matsui, H.; Toki, Y.; Ito, T.; Hayakawa, T. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J. Am. Coll. Cardiol. 2000, 36, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Rakusan, K.; Flanagan, M.F.; Geva, T.; Southern, J.; Van Praagh, R. Morphometry of human coronary capillaries during normal growth and the effect of age in left ventricular pressure-overload hypertrophy. Circulation 1992, 86, 38–46. [Google Scholar] [CrossRef]
- Kaul, S.; Jayaweera, A.R. Coronary and myocardial blood volumes: Noninvasive tools to assess the coronary microcirculation? Circulation 1997, 96, 719–724. [Google Scholar]
- Kaul, S.; Ito, H. Microvasculature in acute myocardial ischemia: Part I: Evolving concepts in pathophysiology, diagnosis, and treatment. Circulation 2004, 109, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Kurose, I.; Anderson, D.C.; Miyasaka, M.; Tamatani, T.; Paulson, J.C.; Todd, R.F.; Rusche, J.R.; Granger, D.N. Molecular determinants of reperfusion-induced leukocyte adhesion and vascular protein leakage. Circ. Res. 1994, 74, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. The Coronary Circulation as a Target of Cardioprotection. Circ. Res. 2016, 118, 1643–1658. [Google Scholar] [CrossRef]
- Stempien-Otero, A.; Karsan, A.; Cornejo, C.J.; Xiang, H.; Eunson, T.; Morrison, R.S.; Kay, M.; Winn, R.; Harlan, J. Mechanisms of hypoxia-induced endothelial cell death. Role of p53 in apoptosis. J. Biol. Chem. 1999, 274, 8039–8045. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Ischemia/Reperfusion. Compr. Physiol. 2016, 7, 113–170. [Google Scholar] [CrossRef]
- Baldea, I.; Teacoe, I.; Olteanu, D.E.; Vaida-Voievod, C.; Clichici, A.; Sirbu, A.; Filip, G.A.; Clichici, S. Effects of different hypoxia degrees on endothelial cell cultures-Time course study. Mech. Ageing Dev. 2018, 172, 45–50. [Google Scholar] [CrossRef]
- Jennings, R.B.; Reimer, K.A. The cell biology of acute myocardial ischemia. Annu. Rev. Med. 1991, 42, 225–246. [Google Scholar] [CrossRef]
- Steenbergen, C.; Hill, M.L.; Jennings, R.B. Volume regulation and plasma membrane injury in aerobic, anaerobic, and ischemic myocardium in vitro. Effects of osmotic cell swelling on plasma membrane integrity. Circ. Res. 1985, 57, 864–875. [Google Scholar] [CrossRef] [PubMed]
- Kasseckert, S.A.; Schafer, C.; Kluger, A.; Gligorievski, D.; Tillmann, J.; Schluter, K.D.; Noll, T.; Sauer, H.; Piper, H.M.; Abdallah, Y. Stimulation of cGMP signalling protects coronary endothelium against reperfusion-induced intercellular gap formation. Cardiovasc. Res. 2009, 83, 381–387. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patterson, C.E.; Lum, H. Update on pulmonary edema: The role and regulation of endothelial barrier function. Endothelium 2001, 8, 75–105. [Google Scholar] [CrossRef]
- Waschke, J.; Curry, F.E.; Adamson, R.H.; Drenckhahn, D. Regulation of actin dynamics is critical for endothelial barrier functions. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1296–H1305. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G.; Colleran, R.; Kastrati, A. Reperfusion injury in ST-segment elevation myocardial infarction: The final frontier. Coron. Artery Dis. 2017, 28, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, L.; Gavin, J.B. The role of post-ischaemic reperfusion in the development of microvascular incompetence and ultrastructural damage in the myocardium. Basic Res. Cardiol. 1991, 86, 544–553. [Google Scholar] [CrossRef]
- Dvorak, H.F. Discovery of vascular permeability factor (VPF). Exp. Cell Res. 2006, 312, 522–526. [Google Scholar] [CrossRef]
- Weis, S.M.; Cheresh, D.A. Pathophysiological consequences of VEGF-induced vascular permeability. Nature 2005, 437, 497–504. [Google Scholar] [CrossRef]
- Orsenigo, F.; Giampietro, C.; Ferrari, A.; Corada, M.; Galaup, A.; Sigismund, S.; Ristagno, G.; Maddaluno, L.; Koh, G.Y.; Franco, D.; et al. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat. Commun. 2012, 3, 1208. [Google Scholar] [CrossRef]
- Bouleti, C.; Mewton, N.; Germain, S. The no-reflow phenomenon: State of the art. Arch. Cardiovasc. Dis. 2015, 108, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Venema, V.J.; Venema, R.C.; Tsai, N.; Behzadian, M.A.; Caldwell, R.B. VEGF-induced permeability increase is mediated by caveolae. Investig. Ophthalmol. Vis. Sci. 1999, 40, 157–167. [Google Scholar]
- Scotland, R.S.; Cohen, M.; Foster, P.; Lovell, M.; Mathur, A.; Ahluwalia, A.; Hobbs, A.J. C-type natriuretic peptide inhibits leukocyte recruitment and platelet-leukocyte interactions via suppression of P-selectin expression. Proc. Natl. Acad. Sci. USA 2005, 102, 14452–14457. [Google Scholar] [CrossRef]
- Kerner, T.; Ahlers, O.; Reschreiter, H.; Buhrer, C.; Mockel, M.; Gerlach, H. Adhesion molecules in different treatments of acute myocardial infarction. Crit. Care 2001, 5, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Vink, H.; Duling, B.R. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ. Res. 1996, 79, 581–589. [Google Scholar] [CrossRef]
- Kolarova, H.; Ambruzova, B.; Sindlerova, L.S.; Klinke, A.; Kubala, L. Modulation of Endothelial Glycocalyx Structure under Inflammatory Conditions. Mediat. Inflamm. 2014, 2014, 694312. [Google Scholar] [CrossRef]
- Ostergaard, L.; Kristiansen, S.B.; Angleys, H.; Frokiaer, J.; Hasenkam, J.M.; Jespersen, S.N.; Botker, H.E. The role of capillary transit time heterogeneity in myocardial oxygenation and ischemic heart disease. Basic Res. Cardiol. 2014, 109, 409. [Google Scholar] [CrossRef] [PubMed]
- Ishiharajima, S.; Aida, T.; Nakagawa, R.; Kameyama, K.; Sugano, K.; Oguro, T.; Asano, G. Early membrane damage during ischemia in rat heart. Exp. Mol. Pathol. 1986, 44, 1–6. [Google Scholar] [CrossRef]
- Czarnowska, E.; Karwatowskaprokopczuk, E. Ultrastructural Demonstration of Endothelial Glycocalyx Disruption in the Reperfused Rat-Heart—Involvement of Oxygen-Free Radicals. Basic Res. Cardiol. 1995, 90, 357–364. [Google Scholar] [CrossRef]
- Rubio-Gayosso, I.; Platts, S.H.; Duling, B.R. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2247–H2256. [Google Scholar] [CrossRef]
- Vink, H.; Constantinescu, A.A.; Spaan, J.A. Oxidized lipoproteins degrade the endothelial surface layer: Implications for platelet-endothelial cell adhesion. Circulation 2000, 101, 1500–1502. [Google Scholar] [CrossRef]
- Nieuwdorp, M.; Mooij, H.L.; Kroon, J.; Atasever, B.; Spaan, J.A.; Ince, C.; Holleman, F.; Diamant, M.; Heine, R.J.; Hoekstra, J.B.; et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 2006, 55, 1127–1132. [Google Scholar] [CrossRef]
- Chappell, D.; Hofmann-Kiefer, K.; Jacob, M.; Rehm, M.; Briegel, J.; Welsch, U.; Conzen, P.; Becker, B.F. TNF-alpha induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic Res. Cardiol. 2009, 104, 78–89. [Google Scholar] [CrossRef]
- Granger, D.N.; Kvietys, P.R. Reperfusion therapy-What’s with the obstructed, leaky and broken capillaries? Pathophysiology 2017, 24, 213–228. [Google Scholar] [CrossRef]
- Hahn, R.G.; Patel, V.; Dull, R.O. Human glycocalyx shedding: Systematic review and critical appraisal. Acta Anaesthesiol. Scand. 2021, 65, 590–606. [Google Scholar] [CrossRef]
- Bruegger, D.; Rehm, M.; Jacob, M.; Chappell, D.; Stoeckelhuber, M.; Welsch, U.; Conzen, P.; Becker, B.F. Exogenous nitric oxide requires an endothelial glycocalyx to prevent postischemic coronary vascular leak in guinea pig hearts. Crit. Care 2008, 12, R73. [Google Scholar] [CrossRef]
- van den Berg, B.M.; Vink, H.; Spaan, J.A. The endothelial glycocalyx protects against myocardial edema. Circ. Res. 2003, 92, 592–594. [Google Scholar] [CrossRef]
- Chappell, D.; Dorfler, N.; Jacob, M.; Rehm, M.; Welsch, U.; Conzen, P.; Becker, B.F. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock 2010, 34, 133–139. [Google Scholar] [CrossRef]
- Chappell, D.; Brettner, F.; Doerfler, N.; Jacob, M.; Rehm, M.; Bruegger, D.; Conzen, P.; Jacob, B.; Becker, B.F. Protection of glycocalyx decreases platelet adhesion after ischaemia/reperfusion: An animal study. Eur. J. Anaesthesiol. 2014, 31, 474–481. [Google Scholar] [CrossRef]
- Bonaventura, A.; Montecucco, F.; Dallegri, F. Cellular recruitment in myocardial ischaemia/reperfusion injury. Eur. J. Clin. Investig. 2016, 46, 590–601. [Google Scholar] [CrossRef]
- Charron, T.; Jaffe, R.; Segev, A.; Bang, K.W.; Qiang, B.; Sparkes, J.D.; Butany, J.; Dick, A.J.; Freedman, J.; Strauss, B.H. Effects of distal embolization on the timing of platelet and inflammatory cell activation in interventional coronary no-reflow. Thromb. Res. 2010, 126, 50–55. [Google Scholar] [CrossRef]
- Konijnenberg, L.S.F.; Damman, P.; Duncker, D.J.; Kloner, R.A.; Nijveldt, R.; van Geuns, R.M.; Berry, C.; Riksen, N.P.; Escaned, J.; van Royen, N. Pathophysiology and diagnosis of coronary microvascular dysfunction in ST-elevation myocardial infarction. Cardiovasc. Res. 2020, 116, 787–805. [Google Scholar] [CrossRef]
- Battinelli, E.M.; Markens, B.A.; Italiano, J.E., Jr. Release of angiogenesis regulatory proteins from platelet alpha granules: Modulation of physiologic and pathologic angiogenesis. Blood 2011, 118, 1359–1369. [Google Scholar] [CrossRef]
- Ziegler, M.; Wang, X.; Peter, K. Platelets in cardiac ischaemia/reperfusion injury: A promising therapeutic target. Cardiovasc. Res. 2019, 115, 1178–1188. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Chilian, W.; Crea, F.; Davidson, S.M.; Ferdinandy, P.; Garcia-Dorado, D.; van Royen, N.; Schulz, R.; Heusch, G.; Action, E.-C.C. The coronary circulation in acute myocardial ischaemia/reperfusion injury: A target for cardioprotection. Cardiovasc. Res. 2019, 115, 1143–1155. [Google Scholar] [CrossRef]
- Schanze, N.; Bode, C.; Duerschmied, D. Platelet Contributions to Myocardial Ischemia/Reperfusion Injury. Front. Immunol. 2019, 10, 1260. [Google Scholar] [CrossRef]
- Yang, B.C.; Virmani, R.; Nichols, W.W.; Mehta, J.L. Platelets protect against myocardial dysfunction and injury induced by ischemia and reperfusion in isolated rat hearts. Circ. Res. 1993, 72, 1181–1190. [Google Scholar] [CrossRef]
- Heindl, B.; Zahler, S.; Welsch, U.; Becker, B.F. Disparate effects of adhesion and degranulation of platelets on myocardial and coronary function in postischaemic hearts. Cardiovasc. Res. 1998, 38, 383–394. [Google Scholar] [CrossRef]
- Choudhri, T.F.; Hoh, B.L.; Zerwes, H.G.; Prestigiacomo, C.J.; Kim, S.C.; Connolly, E.S., Jr.; Kottirsch, G.; Pinsky, D.J. Reduced microvascular thrombosis and improved outcome in acute murine stroke by inhibiting GP IIb/IIIa receptor-mediated platelet aggregation. J. Clin. Investig. 1998, 102, 1301–1310. [Google Scholar] [CrossRef]
- Golino, P.; Maroko, P.R.; Carew, T.E. Efficacy of platelet depletion in counteracting the detrimental effect of acute hypercholesterolemia on infarct size and the no-reflow phenomenon in rabbits undergoing coronary artery occlusion-reperfusion. Circulation 1987, 76, 173–180. [Google Scholar] [CrossRef]
- Neumann, F.J.; Blasini, R.; Schmitt, C.; Alt, E.; Dirschinger, J.; Gawaz, M.; Kastrati, A.; Schomig, A. Effect of glycoprotein IIb/IIIa receptor blockade on recovery of coronary flow and left ventricular function after the placement of coronary-artery stents in acute myocardial infarction. Circulation 1998, 98, 2695–2701. [Google Scholar] [CrossRef]
- Ong, S.B.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Mukhametshina, R.T.; Kwek, X.Y.; Cabrera-Fuentes, H.A.; Hausenloy, D.J. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol. Ther. 2018, 186, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Gotz, A.K.; Zahler, S.; Stumpf, P.; Welsch, U.; Becker, B.F. Intracoronary formation and retention of micro aggregates of leukocytes and platelets contribute to postischemic myocardial dysfunction. Basic Res. Cardiol. 2005, 100, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Zhou, X.; Ji, W.J.; Lu, R.Y.; Zhang, Y.; Zhang, Y.D.; Ma, Y.Q.; Zhao, J.H.; Li, Y.M. Neutrophil extracellular traps in ischemia-reperfusion injury-induced myocardial no-reflow: Therapeutic potential of DNase-based reperfusion strategy. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H500–H509. [Google Scholar] [CrossRef]
- Jaeschke, H.; Smith, C.W. Mechanisms of neutrophil-induced parenchymal cell injury. J. Leukoc. Biol. 1997, 61, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G. Myeloperoxidase—A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin. Chim. Acta 2019, 493, 36–51. [Google Scholar] [CrossRef]
- Romanic, A.M.; Harrison, S.M.; Bao, W.; Burns-Kurtis, C.L.; Pickering, S.; Gu, J.; Grau, E.; Mao, J.; Sathe, G.M.; Ohlstein, E.H.; et al. Myocardial protection from ischemia/reperfusion injury by targeted deletion of matrix metalloproteinase-9. Cardiovasc. Res. 2002, 54, 549–558. [Google Scholar] [CrossRef]
- Lindsey, M.; Wedin, K.; Brown, M.D.; Keller, C.; Evans, A.J.; Smolen, J.; Burns, A.R.; Rossen, R.D.; Michael, L.; Entman, M. Matrix-dependent mechanism of neutrophil-mediated release and activation of matrix metalloproteinase 9 in myocardial ischemia/reperfusion. Circulation 2001, 103, 2181–2187. [Google Scholar] [CrossRef]
- Nees, S.; Weiss, D.R.; Senftl, A.; Knott, M.; Forch, S.; Schnurr, M.; Weyrich, P.; Juchem, G. Isolation, bulk cultivation, and characterization of coronary microvascular pericytes: The second most frequent myocardial cell type in vitro. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H69–H84. [Google Scholar] [CrossRef]
- O’Farrell, F.M.; Mastitskaya, S.; Hammond-Haley, M.; Freitas, F.; Wah, W.R.; Attwell, D. Capillary pericytes mediate coronary no-reflow after myocardial ischaemia. Elife 2017, 6, e29280. [Google Scholar] [CrossRef]
- Hall, C.N.; Reynell, C.; Gesslein, B.; Hamilton, N.B.; Mishra, A.; Sutherland, B.A.; O’Farrell, F.M.; Buchan, A.M.; Lauritzen, M.; Attwell, D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014, 508, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Yemisci, M.; Gursoy-Ozdemir, Y.; Vural, A.; Can, A.; Topalkara, K.; Dalkara, T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat. Med. 2009, 15, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A.; Paiva, A.E.; Andreotti, J.P.; Cardoso, M.V.; Cardoso, C.D.; Mintz, A.; Birbrair, A. Pericytes constrict blood vessels after myocardial ischemia. J. Mol. Cell Cardiol. 2018, 116, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Methner, C.; Cao, Z.; Mishra, A.; Kaul, S. Mechanism and potential treatment of the “no reflow” phenomenon after acute myocardial infarction: Role of pericytes and GPR39. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H1030–H1041. [Google Scholar] [CrossRef]
- Li, Q.; Guo, Z.; Wu, C.; Tu, Y.; Wu, Y.; Xie, E.; Yu, C.; Sun, W.; Li, X.; Zheng, J.; et al. Ischemia preconditioning alleviates ischemia/reperfusion injury-induced coronary no-reflow and contraction of microvascular pericytes in rats. Microvasc. Res. 2022, 142, 104349. [Google Scholar] [CrossRef] [PubMed]
- Canty, J.M., Jr.; Klocke, F.J. Reduced regional myocardial perfusion in the presence of pharmacologic vasodilator reserve. Circulation 1985, 71, 370–377. [Google Scholar] [CrossRef]
- VanBenthuysen, K.M.; McMurtry, I.F.; Horwitz, L.D. Reperfusion after acute coronary occlusion in dogs impairs endothelium-dependent relaxation to acetylcholine and augments contractile reactivity in vitro. J. Clin. Investig. 1987, 79, 265–274. [Google Scholar] [CrossRef]
- Quillen, J.E.; Sellke, F.W.; Brooks, L.A.; Harrison, D.G. Ischemia-reperfusion impairs endothelium-dependent relaxation of coronary microvessels but does not affect large arteries. Circulation 1990, 82, 586–594. [Google Scholar] [CrossRef]
- Malliani, A.; Schwartz, P.J.; Zanchetti, A. A sympathetic reflex elicited by experimental coronary occlusion. Am. J. Physiol. 1969, 217, 703–709. [Google Scholar] [CrossRef]
- Gregorini, L.; Marco, J.; Kozakova, M.; Palombo, C.; Anguissola, G.B.; Marco, I.; Bernies, M.; Cassagneau, B.; Distante, A.; Bossi, I.M.; et al. Alpha-adrenergic blockade improves recovery of myocardial perfusion and function after coronary stenting in patients with acute myocardial infarction. Circulation 1999, 99, 482–490. [Google Scholar] [CrossRef]
- Shimizu, M.; Wang, Q.D.; Sjoquist, P.O.; Ryden, L. The angiotensin II AT1-receptor antagonist candesartan improves functional recovery and reduces the no-reflow area in reperfused ischemic rat hearts. J. Cardiovasc. Pharmacol. 1999, 34, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Ryckwaert, F.; Colson, P.; Guillon, G.; Foex, P. Cumulative effects of AT1 and AT2 receptor blockade on ischaemia-reperfusion recovery in rat hearts. Pharmacol. Res. 2005, 51, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, H.; Belmadani, S.; Wu, J.; Xu, X.; Elford, H.; Potter, B.J.; Zhang, C. Role of TNF-alpha-induced reactive oxygen species in endothelial dysfunction during reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2242–H2249. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P.; Bose, D.; Baars, T.; Mohlenkamp, S.; Konorza, T.; Schoner, S.; Elter-Schulz, M.; Eggebrecht, H.; Degen, H.; Haude, M.; et al. Vasoconstrictor potential of coronary aspirate from patients undergoing stenting of saphenous vein aortocoronary bypass grafts and its pharmacological attenuation. Circ. Res. 2011, 108, 344–352. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Baars, T.; Mohlenkamp, S.; Kahlert, P.; Erbel, R.; Heusch, G. Aspirate from human stented native coronary arteries vs. saphenous vein grafts: More endothelin but less particulate debris. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1222–H1229. [Google Scholar] [CrossRef]
- Herring, N.; Tapoulal, N.; Kalla, M.; Ye, X.; Borysova, L.; Lee, R.; Dall’Armellina, E.; Stanley, C.; Ascione, R.; Lu, C.J.; et al. Neuropeptide-Y causes coronary microvascular constriction and is associated with reduced ejection fraction following ST-elevation myocardial infarction. Eur. Heart J. 2019, 40, 1920–1929. [Google Scholar] [CrossRef]
- Xue, H.M.; He, G.W.; Huang, J.H.; Yang, Q. New strategy of endothelial protection in cardiac surgery: Use of enhancer of endothelial nitric oxide synthase. World J. Surg. 2010, 34, 1461–1469. [Google Scholar] [CrossRef]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef]
- Hein, T.W.; Zhang, C.; Wang, W.; Chang, C.I.; Thengchaisri, N.; Kuo, L. Ischemia-reperfusion selectively impairs nitric oxide-mediated dilation in coronary arterioles: Counteracting role of arginase. FASEB J. 2003, 17, 2328–2330. [Google Scholar] [CrossRef]
- Schreckenberg, R.; Weber, P.; Cabrera-Fuentes, H.A.; Steinert, I.; Preissner, K.T.; Bencsik, P.; Sarkozy, M.; Csonka, C.; Ferdinandy, P.; Schulz, R.; et al. Mechanism and consequences of the shift in cardiac arginine metabolism following ischaemia and reperfusion in rats. Thromb. Haemost. 2015, 113, 482–493. [Google Scholar] [CrossRef]
- Zhou, T.; Chuang, C.C.; Zuo, L. Molecular Characterization of Reactive Oxygen Species in Myocardial Ischemia-Reperfusion Injury. Biomed. Res. Int. 2015, 2015, 864946. [Google Scholar] [CrossRef] [PubMed]
- Viehman, G.E.; Ma, X.L.; Lefer, D.J.; Lefer, A.M. Time course of endothelial dysfunction and myocardial injury during coronary arterial occlusion. Am. J. Physiol. 1991, 261, H874–H881. [Google Scholar] [CrossRef] [PubMed]
- Hollander, M.R.; de Waard, G.A.; Konijnenberg, L.S.; Meijer-van Putten, R.M.; van den Brom, C.E.; Paauw, N.; de Vries, H.E.; van de Ven, P.M.; Aman, J.; Van Nieuw-Amerongen, G.P.; et al. Dissecting the Effects of Ischemia and Reperfusion on the Coronary Microcirculation in a Rat Model of Acute Myocardial Infarction. PLoS ONE 2016, 11, e0157233. [Google Scholar] [CrossRef]
- Gavin, J.B.; Seelye, R.N.; Nevalainen, T.J.; Armiger, L.C. The effect of ischaemia on the function and fine structure of the microvasculature of myocardium. Pathology 1978, 10, 103–111. [Google Scholar] [CrossRef]
- Lonborg, J.; Kelbaek, H.; Helqvist, S.; Holmvang, L.; Jorgensen, E.; Saunamaki, K.; Klovgaard, L.; Kaltoft, A.; Botker, H.E.; Lassen, J.F.; et al. The impact of distal embolization and distal protection on long-term outcome in patients with ST elevation myocardial infarction randomized to primary percutaneous coronary intervention--results from a randomized study. Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Napodano, M.; Peluso, D.; Marra, M.P.; Frigo, A.C.; Tarantini, G.; Buja, P.; Gasparetto, V.; Fraccaro, C.; Isabella, G.; Razzolini, R.; et al. Time-dependent detrimental effects of distal embolization on myocardium and microvasculature during primary percutaneous coronary intervention. JACC Cardiovasc. Interv. 2012, 5, 1170–1177. [Google Scholar] [CrossRef]
- Henriques, J.P.; Zijlstra, F.; Ottervanger, J.P.; de Boer, M.J.; van ‘t Hof, A.W.; Hoorntje, J.C.; Suryapranata, H. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction. Eur. Heart J. 2002, 23, 1112–1117. [Google Scholar] [CrossRef]
- Yameogo, N.V.; Guenancia, C.; Porot, G.; Stamboul, K.; Richard, C.; Gudjoncik, A.; Hamblin, J.; Buffet, P.; Lorgis, L.; Cottin, Y. Predictors of angiographically visible distal embolization in STEMI. Herz 2020, 45, 288–292. [Google Scholar] [CrossRef]
- Stone, G.W.; Webb, J.; Cox, D.A.; Brodie, B.R.; Qureshi, M.; Kalynych, A.; Turco, M.; Schultheiss, H.P.; Dulas, D.; Rutherford, B.D.; et al. Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction: A randomized controlled trial. JAMA 2005, 293, 1063–1072. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Heusch, G. A fresh look at coronary microembolization. Nat. Rev. Cardiol. 2022, 19, 265–280. [Google Scholar] [CrossRef]
- Yunoki, K.; Naruko, T.; Inoue, T.; Sugioka, K.; Inaba, M.; Iwasa, Y.; Komatsu, R.; Itoh, A.; Haze, K.; Yoshiyama, M.; et al. Relationship of thrombus characteristics to the incidence of angiographically visible distal embolization in patients with ST-segment elevation myocardial infarction treated with thrombus aspiration. JACC Cardiovasc. Interv. 2013, 6, 377–385. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abela, G.S.; Kalavakunta, J.K.; Janoudi, A.; Leffler, D.; Dhar, G.; Salehi, N.; Cohn, J.; Shah, I.; Karve, M.; Kotaru, V.P.K.; et al. Frequency of Cholesterol Crystals in Culprit Coronary Artery Aspirate During Acute Myocardial Infarction and Their Relation to Inflammation and Myocardial Injury. Am. J. Cardiol. 2017, 120, 1699–1707. [Google Scholar] [CrossRef]
- Katayama, Y.; Taruya, A.; Kashiwagi, M.; Ozaki, Y.; Shiono, Y.; Tanimoto, T.; Yoshikawa, T.; Kondo, T.; Tanaka, A. No-reflow phenomenon and in vivo cholesterol crystals combined with lipid core in acute myocardial infarction. Int. J. Cardiol. Heart Vasc. 2022, 38, 100953. [Google Scholar] [CrossRef]
- Kawaguchi, R.; Oshima, S.; Jingu, M.; Tsurugaya, H.; Toyama, T.; Hoshizaki, H.; Taniguchi, K. Usefulness of virtual histology intravascular ultrasound to predict distal embolization for ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2007, 50, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Napodano, M.; Ramondo, A.; Tarantini, G.; Peluso, D.; Compagno, S.; Fraccaro, C.; Frigo, A.C.; Razzolini, R.; Iliceto, S. Predictors and time-related impact of distal embolization during primary angioplasty. Eur. Heart J. 2009, 30, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Fokkema, M.L.; Vlaar, P.J.; Svilaas, T.; Vogelzang, M.; Amo, D.; Diercks, G.F.; Suurmeijer, A.J.; Zijlstra, F. Incidence and clinical consequences of distal embolization on the coronary angiogram after percutaneous coronary intervention for ST-elevation myocardial infarction. Eur. Heart J. 2009, 30, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Falk, E.; Thuesen, L. Pathology of coronary microembolisation and no reflow. Heart 2003, 89, 983–985. [Google Scholar] [CrossRef]
- Skyschally, A.; Walter, B.; Heusch, G. Coronary microembolization during early reperfusion: Infarct extension, but protection by ischaemic postconditioning. Eur. Heart J. 2013, 34, 3314–3321. [Google Scholar] [CrossRef]
- Carlsson, M.; Martin, A.J.; Ursell, P.C.; Saloner, D.; Saeed, M. Magnetic resonance imaging quantification of left ventricular dysfunction following coronary microembolization. Magn. Reson. Med. 2009, 61, 595–602. [Google Scholar] [CrossRef]
- Tucker, B.; Vaidya, K.; Cochran, B.J.; Patel, S. Inflammation during Percutaneous Coronary Intervention-Prognostic Value, Mechanisms and Therapeutic Targets. Cells 2021, 10, 1391. [Google Scholar] [CrossRef]
- Quinones, A.; Saric, M. The cholesterol emboli syndrome in atherosclerosis. Curr. Atheroscler. Rep. 2013, 15, 315. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.M.; Lim, S.Y.; Qiang, B.; Osherov, A.B.; Ghugre, N.R.; Noyan, H.; Qi, X.; Wolff, R.; Ladouceur-Wodzak, M.; Berk, T.A.; et al. Distal coronary embolization following acute myocardial infarction increases early infarct size and late left ventricular wall thinning in a porcine model. J. Cardiovasc. Magn. Reson. 2015, 17, 106. [Google Scholar] [CrossRef] [PubMed]
- Jennings, R.B.; Sommers, H.M.; Smyth, G.A.; Flack, H.A.; Linn, H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch. Pathol. 1960, 70, 68–78. [Google Scholar] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef]
- Frohlich, G.M.; Meier, P.; White, S.K.; Yellon, D.M.; Hausenloy, D.J. Myocardial reperfusion injury: Looking beyond primary PCI. Eur. Heart J. 2013, 34, 1714–1722. [Google Scholar] [CrossRef]
- Kloner, R.A.; Alker, K.J. The Effect of Streptokinase on Intramyocardial Hemorrhage, Infarct Size, and the No-Reflow Phenomenon during Coronary Reperfusion. Circulation 1984, 70, 513–521. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Tiroch, K.; Keta, D.; Fusaro, M.; Seyfarth, M.; Pache, J.; Mehilli, J.; Schoemig, A.; Kastrati, A. Predictive Factors and Impact of No Reflow after Primary Percutaneous Coronary Intervention in Patients with Acute Myocardial Infarction. Circ. Cardiovasc. Interv. 2010, 3, 27–33. [Google Scholar] [CrossRef]
- Kloner, R.A.; Rude, R.E.; Carlson, N.; Maroko, P.R.; DeBoer, L.W.; Braunwald, E. Ultrastructural evidence of microvascular damage and myocardial cell injury after coronary artery occlusion: Which comes first? Circulation 1980, 62, 945–952. [Google Scholar] [CrossRef]
- Kloner, R.A.; King, K.S.; Harrington, M.G. No-reflow phenomenon in the heart and brain. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H550–H562. [Google Scholar] [CrossRef]
- Ambrosio, G.; Weisman, H.F.; Mannisi, J.A.; Becker, L.C. Progressive impairment of regional myocardial perfusion after initial restoration of postischemic blood flow. Circulation 1989, 80, 1846–1861. [Google Scholar] [CrossRef]
- Rochitte, C.E.; Lima, J.A.; Bluemke, D.A.; Reeder, S.B.; McVeigh, E.R.; Furuta, T.; Becker, L.C.; Melin, J.A. Magnitude and time course of microvascular obstruction and tissue injury after acute myocardial infarction. Circulation 1998, 98, 1006–1014. [Google Scholar] [CrossRef]
- Reffelmann, T.; Kloner, R.A. Microvascular reperfusion injury: Rapid expansion of anatomic no reflow during reperfusion in the rabbit. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1099–H1107. [Google Scholar] [CrossRef]
- Galiuto, L.; Lombardo, A.; Maseri, A.; Santoro, L.; Porto, I.; Cianflone, D.; Rebuzzi, A.G.; Crea, F. Temporal evolution and functional outcome of no reflow: Sustained and spontaneously reversible patterns following successful coronary recanalisation. Heart 2003, 89, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Turschner, O.; D’Hooge, J.; Dommke, C.; Claus, P.; Verbeken, E.; De Scheerder, I.; Bijnens, B.; Sutherland, G.R. The sequential changes in myocardial thickness and thickening which occur during acute transmural infarction, infarct reperfusion and the resultant expression of reperfusion injury. Eur. Heart J. 2004, 25, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Jimenez, R.; Sanchez-Gonzalez, J.; Aguero, J.; Garcia-Prieto, J.; Lopez-Martin, G.J.; Garcia-Ruiz, J.M.; Molina-Iracheta, A.; Rossello, X.; Fernandez-Friera, L.; Pizarro, G.; et al. Myocardial edema after ischemia/reperfusion is not stable and follows a bimodal pattern: Imaging and histological tissue characterization. J. Am. Coll. Cardiol. 2015, 65, 315–323. [Google Scholar] [CrossRef]
- Alkhalil, M.; Borlotti, A.; De Maria, G.L.; Gaughran, L.; Langrish, J.; Lucking, A.; Ferreira, V.; Kharbanda, R.K.; Banning, A.P.; Channon, K.M.; et al. Dynamic changes in injured myocardium, very early after acute myocardial infarction, quantified using T1 mapping cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2018, 20, 82. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, R.; Barreiro-Perez, M.; Martin-Garcia, A.; Sanchez-Gonzalez, J.; Aguero, J.; Galan-Arriola, C.; Garcia-Prieto, J.; Diaz-Pelaez, E.; Vara, P.; Martinez, I.; et al. Dynamic Edematous Response of the Human Heart to Myocardial Infarction: Implications for Assessing Myocardial Area at Risk and Salvage. Circulation 2017, 136, 1288–1300. [Google Scholar] [CrossRef]
- Veinot, J.P.; Gattinger, D.A.; Fliss, H. Early apoptosis in human myocardial infarcts. Hum. Pathol. 1997, 28, 485–492. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Candilio, L.; Evans, R.; Ariti, C.; Jenkins, D.P.; Kolvekar, S.; Knight, R.; Kunst, G.; Laing, C.; Nicholas, J.; et al. Remote Ischemic Preconditioning and Outcomes of Cardiac Surgery. N. Engl. J. Med. 2015, 373, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Kidambi, A.; Mather, A.N.; Motwani, M.; Swoboda, P.; Uddin, A.; Greenwood, J.P.; Plein, S. The effect of microvascular obstruction and intramyocardial hemorrhage on contractile recovery in reperfused myocardial infarction: Insights from cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2013, 15, 58. [Google Scholar] [CrossRef]
- Kandler, D.; Lucke, C.; Grothoff, M.; Andres, C.; Lehmkuhl, L.; Nitzsche, S.; Riese, F.; Mende, M.; de Waha, S.; Desch, S.; et al. The relation between hypointense core, microvascular obstruction and intramyocardial haemorrhage in acute reperfused myocardial infarction assessed by cardiac magnetic resonance imaging. Eur. Radiol. 2014, 24, 3277–3288. [Google Scholar] [CrossRef]
- Higginson, L.A.; White, F.; Heggtveit, H.A.; Sanders, T.M.; Bloor, C.M.; Covell, J.W. Determinants of myocardial hemorrhage after coronary reperfusion in the anesthetized dog. Circulation 1982, 65, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Mathey, D.G.; Schofer, J.; Kuck, K.H.; Beil, U.; Kloppel, G. Transmural, haemorrhagic myocardial infarction after intracoronary streptokinase. Clinical, angiographic, and necropsy findings. Br. Heart J. 1982, 48, 546–551. [Google Scholar] [CrossRef] [PubMed]
- McNamara, J.J.; Lacro, R.V.; Yee, M.; Smith, G.T. Hemorrhagic infarction and coronary reperfusion. J. Thorac. Cardiovasc. Surg. 1981, 81, 498–501. [Google Scholar] [CrossRef]
- Lotan, C.S.; Bouchard, A.; Cranney, G.B.; Bishop, S.P.; Pohost, G.M. Assessment of postreperfusion myocardial hemorrhage using proton NMR imaging at 1.5 T. Circulation 1992, 86, 1018–1025. [Google Scholar] [CrossRef]
- Adamson, R.H.; Zeng, M.; Adamson, G.N.; Lenz, J.F.; Curry, F.E. PAF- and bradykinin-induced hyperpermeability of rat venules is independent of actin-myosin contraction. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H406–H417. [Google Scholar] [CrossRef]
- Inauen, W.; Granger, D.N.; Meininger, C.J.; Schelling, M.E.; Granger, H.J.; Kvietys, P.R. Anoxia-reoxygenation-induced, neutrophil-mediated endothelial cell injury: Role of elastase. Am. J. Physiol. 1990, 259, H925–H931. [Google Scholar] [CrossRef]
- Gupta, A.K.; Joshi, M.B.; Philippova, M.; Erne, P.; Hasler, P.; Hahn, S.; Resink, T.J. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 2010, 584, 3193–3197. [Google Scholar] [CrossRef]
- Tarikuz Zaman, A.K.; Spees, J.L.; Sobel, B.E. Attenuation of cardiac vascular rhexis: A promising therapeutic target. Coron. Artery Dis. 2013, 24, 245–252. [Google Scholar] [CrossRef]
- Zeman, A.K.M.T.; French, C.J.; Spees, J.L.; Binbrek, A.S.; Sobel, B.E. Vascular rhexis in mice subjected to non-sustained myocardial ischemia and its therapeutic implications. Exp. Biol. Med. 2011, 236, 598–603. [Google Scholar] [CrossRef]
- Robbers, L.F.; Eerenberg, E.S.; Teunissen, P.F.; Jansen, M.F.; Hollander, M.R.; Horrevoets, A.J.; Knaapen, P.; Nijveldt, R.; Heymans, M.W.; Levi, M.M.; et al. Magnetic resonance imaging-defined areas of microvascular obstruction after acute myocardial infarction represent microvascular destruction and haemorrhage. Eur. Heart J. 2013, 34, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Carrick, D.; Haig, C.; Ahmed, N.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; Hood, S.; Watkins, S.; Lindsay, M.M.; Davie, A.; et al. Myocardial Hemorrhage after Acute Reperfused ST-Segment-Elevation Myocardial Infarction: Relation to Microvascular Obstruction and Prognostic Significance. Circ. Cardiovasc. Imaging 2016, 9, e004148. [Google Scholar] [CrossRef] [PubMed]
- Bulluck, H.; Rosmini, S.; Abdel-Gadir, A.; White, S.K.; Bhuva, A.N.; Treibel, T.A.; Fontana, M.; Ramlall, M.; Hamarneh, A.; Sirker, A.; et al. Residual Myocardial Iron Following Intramyocardial Hemorrhage During the Convalescent Phase of Reperfused ST-Segment-Elevation Myocardial Infarction and Adverse Left Ventricular Remodeling. Circ. Cardiovasc. Imaging 2016, 9, e004940. [Google Scholar] [CrossRef] [PubMed]
- Kali, A.; Kumar, A.; Cokic, I.; Tang, R.L.; Tsaftaris, S.A.; Friedrich, M.G.; Dharmakumar, R. Chronic manifestation of postreperfusion intramyocardial hemorrhage as regional iron deposition: A cardiovascular magnetic resonance study with ex vivo validation. Circ. Cardiovasc. Imaging 2013, 6, 218–228. [Google Scholar] [CrossRef]
- Waller, B.F.; Rothbaum, D.A.; Pinkerton, C.A.; Cowley, M.J.; Linnemeier, T.J.; Orr, C.; Irons, M.; Helmuth, R.A.; Wills, E.R.; Aust, C. Status of the myocardium and infarct-related coronary artery in 19 necropsy patients with acute recanalization using pharmacologic (streptokinase, r-tissue plasminogen activator), mechanical (percutaneous transluminal coronary angioplasty) or combined types of reperfusion therapy. J. Am. Coll. Cardiol. 1987, 9, 785–801. [Google Scholar] [CrossRef]
- Amier, R.P.; Tijssen, R.Y.G.; Teunissen, P.F.A.; Fernandez-Jimenez, R.; Pizarro, G.; Garcia-Lunar, I.; Bastante, T.; van de Ven, P.M.; Beek, A.M.; Smulders, M.W.; et al. Predictors of Intramyocardial Hemorrhage after Reperfused ST-Segment Elevation Myocardial Infarction. J. Am. Heart Assoc. 2017, 6, e005651. [Google Scholar] [CrossRef]
- Liu, T.; Howarth, A.G.; Chen, Y.; Nair, A.R.; Yang, H.J.; Ren, D.; Tang, R.; Sykes, J.; Kovacs, M.S.; Dey, D.; et al. Intramyocardial Hemorrhage and the “Wave Front” of Reperfusion Injury Compromising Myocardial Salvage. J. Am. Coll. Cardiol. 2022, 79, 35–48. [Google Scholar] [CrossRef]
- Wu, K.C.; Zerhouni, E.A.; Judd, R.M.; Lugo-Olivieri, C.H.; Barouch, L.A.; Schulman, S.P.; Blumenthal, R.S.; Lima, J.A. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 1998, 97, 765–772. [Google Scholar] [CrossRef]
- Kloner, R.A.; Giacomelli, F.; Alker, K.J.; Hale, S.L.; Matthews, R.; Bellows, S. Influx of neutrophils into the walls of large epicardial coronary arteries in response to ischemia/reperfusion. Circulation 1991, 84, 1758–1772. [Google Scholar] [CrossRef]
- Hansen, P.R. Role of neutrophils in myocardial ischemia and reperfusion. Circulation 1995, 91, 1872–1885. [Google Scholar] [CrossRef]
- Toldo, S.; Abbate, A. The NLRP3 inflammasome in acute myocardial infarction. Nat. Rev. Cardiol. 2018, 15, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; La Vecchia, G. Interplay between inflammation and microvascular obstruction in ST-segment elevation myocardial infarction: The importance of velocity. Int. J. Cardiol. 2021, 339, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Mayr, A.; Klug, G.; Schocke, M.; Trieb, T.; Mair, J.; Pedarnig, K.; Pachinger, O.; Jaschke, W.; Metzler, B. Late microvascular obstruction after acute myocardial infarction: Relation with cardiac and inflammatory markers. Int. J. Cardiol. 2012, 157, 391–396. [Google Scholar] [CrossRef]
- Reindl, M.; Reinstadler, S.J.; Feistritzer, H.J.; Klug, G.; Tiller, C.; Mair, J.; Mayr, A.; Jaschke, W.; Metzler, B. Relation of inflammatory markers with myocardial and microvascular injury in patients with reperfused ST-elevation myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 2017, 6, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Dong, M.; Ren, F.; Zhang, C.; Li, J.; Tao, Z.; Yang, J.; Li, G. Association between local interleukin-6 levels and slow flow/microvascular dysfunction. J. Thromb. Thrombolysis 2014, 37, 475–482. [Google Scholar] [CrossRef]
- Shetelig, C.; Limalanathan, S.; Hoffmann, P.; Seljeflot, I.; Gran, J.M.; Eritsland, J.; Andersen, G.O. Association of IL-8 with Infarct Size and Clinical Outcomes in Patients with STEMI. J. Am. Coll. Cardiol. 2018, 72, 187–198. [Google Scholar] [CrossRef]
- Funayama, H.; Ishikawa, S.E.; Sugawara, Y.; Kubo, N.; Momomura, S.; Kawakami, M. Myeloperoxidase may contribute to the no-reflow phenomenon in patients with acute myocardial infarction. Int. J. Cardiol. 2010, 139, 187–192. [Google Scholar] [CrossRef]
- Stamboul, K.; Zeller, M.; Rochette, L.; Cottin, Y.; Cochet, A.; Leclercq, T.; Porot, G.; Guenancia, C.; Fichot, M.; Maillot, N.; et al. Relation between high levels of myeloperoxidase in the culprit artery and microvascular obstruction, infarct size and reverse remodeling in ST-elevation myocardial infarction. PLoS ONE 2017, 12, e0179929. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Mehilli, J.; Schulz, S.; Iijima, R.; Keta, D.; Byrne, R.A.; Pache, J.; Seyfarth, M.; Schomig, A.; Kastrati, A. Prognostic significance of epicardial blood flow before and after percutaneous coronary intervention in patients with acute coronary syndromes. J. Am. Coll. Cardiol. 2008, 52, 512–517. [Google Scholar] [CrossRef]
- Rezkalla, S.H.; Dharmashankar, K.C.; Abdalrahman, I.B.; Kloner, R.A. No-reflow phenomenon following percutaneous coronary intervention for acute myocardial infarction: Incidence, outcome, and effect of pharmacologic therapy. J. Interv. Cardiol. 2010, 23, 429–436. [Google Scholar] [CrossRef]
- Henriques, J.P.; Zijlstra, F.; van ‘t Hof, A.W.; de Boer, M.J.; Dambrink, J.H.; Gosselink, M.; Hoorntje, J.C.; Suryapranata, H. Angiographic assessment of reperfusion in acute myocardial infarction by myocardial blush grade. Circulation 2003, 107, 2115–2119. [Google Scholar] [CrossRef]
- Eitel, I.; Wohrle, J.; Suenkel, H.; Meissner, J.; Kerber, S.; Lauer, B.; Pauschinger, M.; Birkemeyer, R.; Axthelm, C.; Zimmermann, R.; et al. Intracoronary compared with intravenous bolus abciximab application during primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: Cardiac magnetic resonance substudy of the AIDA STEMI trial. J. Am. Coll. Cardiol. 2013, 61, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G.; Alger, P.; Fusaro, M.; Kufner, S.; Seyfarth, M.; Keta, D.; Mehilli, J.; Schomig, A.; Kastrati, A. Impact of perfusion restoration at epicardial and tissue levels on markers of myocardial necrosis and clinical outcome of patients with acute myocardial infarction. Eurointervention 2011, 7, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.M.; Cannon, C.P.; Daley, W.L.; Dodge, J.T., Jr.; Alexander, B., Jr.; Marble, S.J.; McCabe, C.H.; Raymond, L.; Fortin, T.; Poole, W.K.; et al. TIMI frame count: A quantitative method of assessing coronary artery flow. Circulation 1996, 93, 879–888. [Google Scholar] [CrossRef]
- Gibson, C.M.; Murphy, S.A.; Rizzo, M.J.; Ryan, K.A.; Marble, S.J.; McCabe, C.H.; Cannon, C.P.; Van de Werf, F.; Braunwald, E. Relationship between TIMI frame count and clinical outcomes after thrombolytic administration. Thrombolysis In Myocardial Infarction (TIMI) Study Group. Circulation 1999, 99, 1945–1950. [Google Scholar] [CrossRef]
- Hamada, S.; Nishiue, T.; Nakamura, S.; Sugiura, T.; Kamihata, H.; Miyoshi, H.; Imuro, Y.; Iwasaka, T. TIMI frame count immediately after primary coronary angioplasty as a predictor of functional recovery in patients with TIMI 3 reperfused acute myocardial infarction. J. Am. Coll. Cardiol. 2001, 38, 666–671. [Google Scholar] [CrossRef][Green Version]
- Ohara, Y.; Hiasa, Y.; Takahashi, T.; Yamaguchi, K.; Ogura, R.; Ogata, T.; Yuba, K.; Kusunoki, K.; Hosokawa, S.; Kishi, K.; et al. Relation between the TIMI frame count and the degree of microvascular injury after primary coronary angioplasty in patients with acute anterior myocardial infarction. Heart 2005, 91, 64–67. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van ‘t Hof, A.W.; Liem, A.; Suryapranata, H.; Hoorntje, J.C.; de Boer, M.J.; Zijlstra, F. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: Myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation 1998, 97, 2302–2306. [Google Scholar] [CrossRef]
- Allencherril, J.; Jneid, H.; Atar, D.; Alam, M.; Levine, G.; Kloner, R.A.; Birnbaum, Y. Pathophysiology, Diagnosis, and Management of the No-Reflow Phenomenon. Cardiovasc. Drugs Ther. 2019, 33, 589–597. [Google Scholar] [CrossRef]
- Vicente, J.; Mewton, N.; Croisille, P.; Staat, P.; Bonnefoy-Cudraz, E.; Ovize, M.; Revel, D. Comparison of the angiographic myocardial blush grade with delayed-enhanced cardiac magnetic resonance for the assessment of microvascular obstruction in acute myocardial infarctions. Catheter. Cardiovasc. Interv. 2009, 74, 1000–1007. [Google Scholar] [CrossRef]
- Nijveldt, R.; van der Vleuten, P.A.; Hirsch, A.; Beek, A.M.; Tio, R.A.; Tijssen, J.G.; Piek, J.J.; van Rossum, A.C.; Zijlstra, F. Early electrocardiographic findings and MR imaging-verified microvascular injury and myocardial infarct size. JACC Cardiovasc. Imaging 2009, 2, 1187–1194. [Google Scholar] [CrossRef]
- Kampinga, M.A.; Nijsten, M.W.; Gu, Y.L.; Dijk, W.A.; de Smet, B.J.; van den Heuvel, A.F.; Tan, E.S.; Zijlstra, F. Is the myocardial blush grade scored by the operator during primary percutaneous coronary intervention of prognostic value in patients with ST-elevation myocardial infarction in routine clinical practice? Circ. Cardiovasc. Interv. 2010, 3, 216–223. [Google Scholar] [CrossRef][Green Version]
- Sorajja, P.; Gersh, B.J.; Costantini, C.; McLaughlin, M.G.; Zimetbaum, P.; Cox, D.A.; Garcia, E.; Tcheng, J.E.; Mehran, R.; Lansky, A.J.; et al. Combined prognostic utility of ST-segment recovery and myocardial blush after primary percutaneous coronary intervention in acute myocardial infarction. Eur. Heart J. 2005, 26, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.; Beek, A.M.; Hirsch, A.; Stoel, M.G.; Hofman, M.B.; Umans, V.A.; Algra, P.R.; Twisk, J.W.; van Rossum, A.C. Functional recovery after acute myocardial infarction: Comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J. Am. Coll. Cardiol. 2008, 52, 181–189. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Tiroch, K.; Fusaro, M.; Keta, D.; Seyfarth, M.; Byrne, R.A.; Pache, J.; Alger, P.; Mehilli, J.; Schomig, A.; et al. 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 2010, 55, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.T.; Leung, M.C.; Richardson, J.D.; Puri, R.; Bertaso, A.G.; Williams, K.; Meredith, I.T.; Teo, K.S.; Worthley, M.I.; Worthley, S.G. Cardiac magnetic resonance derived late microvascular obstruction assessment post ST-segment elevation myocardial infarction is the best predictor of left ventricular function: A comparison of angiographic and cardiac magnetic resonance derived measurements. Int. J. Cardiovasc. Imaging 2012, 28, 1971–1981. [Google Scholar] [CrossRef]
- Wong, D.T.; Leung, M.C.; Das, R.; Liew, G.Y.; Teo, K.S.; Chew, D.P.; Meredith, I.T.; Worthley, M.I.; Worthley, S.G. Intracoronary ECG during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction predicts microvascular obstruction and infarct size. Int. J. Cardiol. 2013, 165, 61–66. [Google Scholar] [CrossRef]
- Appelbaum, E.; Abraham, J.M.; Pride, Y.B.; Harrigan, C.J.; Peters, D.C.; Biller, L.H.; Manning, W.J.; Gibson, C.M. Association of Thrombolysis in Myocardial Infarction Myocardial Perfusion Grade with cardiovascular magnetic resonance measures of infarct architecture after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Am. Heart J. 2009, 158, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.M.; Kirtane, A.J.; Morrow, D.A.; Palabrica, T.M.; Murphy, S.A.; Stone, P.H.; Scirica, B.M.; Jennings, L.K.; Herrmann, H.C.; Cohen, D.J.; et al. Association between thrombolysis in myocardial infarction myocardial perfusion grade, biomarkers, and clinical outcomes among patients with moderate- to high-risk acute coronary syndromes: Observations from the randomized trial to evaluate the relative PROTECTion against post-PCI microvascular dysfunction and post-PCI ischemia among antiplatelet and antithrombotic agents-Thrombolysis In Myocardial Infarction 30 (PROTECT-TIMI 30). Am. Heart J. 2006, 152, 756–761. [Google Scholar] [CrossRef]
- Kaul, S. Evaluating the ‘no reflow’ phenomenon with myocardial contrast echocardiography. Basic Res. Cardiol. 2006, 101, 391–399. [Google Scholar] [CrossRef]
- Lanzer, P.; Leigh, R.; Berry, C.; van de Hoef, T.; Heiss, W.D.; Senior, R.; Schwartz, A.; Rischpler, C.; Liebeskind, D. Salutary reperfusion is the ultimate target of ST-Segment Elevation Myocardial Infarction (STEMI) and Acute Ischemic Stroke (AIS) interventions and should be routinely assessed in standard clinical and research protocols. Card. Res. Med. 2017, 1, 20–34. [Google Scholar]
- Heusch, G. Coronary microvascular obstruction: The new frontier in cardioprotection. Basic Res. Cardiol. 2019, 114, 45. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Maruyama, A.; Iwakura, K.; Takiuchi, S.; Masuyama, T.; Hori, M.; Higashino, Y.; Fujii, K.; Minamino, T. Clinical implications of the ‘no reflow’ phenomenon. A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation 1996, 93, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Jayaweera, A.R.; Firoozan, S.; Linka, A.; Skyba, D.M.; Kaul, S. Basis for detection of stenosis using venous administration of microbubbles during myocardial contrast echocardiography: Bolus or continuous infusion? J. Am. Coll. Cardiol. 1998, 32, 252–260. [Google Scholar] [CrossRef][Green Version]
- Vogel, R.; Indermuhle, A.; Reinhardt, J.; Meier, P.; Siegrist, P.T.; Namdar, M.; Kaufmann, P.A.; Seiler, C. The quantification of absolute myocardial perfusion in humans by contrast echocardiography: Algorithm and validation. J. Am. Coll. Cardiol. 2005, 45, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.N.; Chahal, N.S.; Bhattacharyya, S.; Li, W.; Roussin, I.; Khattar, R.S.; Senior, R. The feasibility and clinical utility of myocardial contrast echocardiography in clinical practice: Results from the incorporation of myocardial perfusion assessment into clinical testing with stress echocardiography study. J. Am. Soc. Echocardiogr. 2014, 27, 520–530. [Google Scholar] [CrossRef]
- Bulluck, H.; Dharmakumar, R.; Arai, A.E.; Berry, C.; Hausenloy, D.J. Cardiovascular Magnetic Resonance in Acute ST-Segment-Elevation Myocardial Infarction: Recent Advances, Controversies, and Future Directions. Circulation 2018, 137, 1949–1964. [Google Scholar] [CrossRef]
- Mather, A.N.; Lockie, T.; Nagel, E.; Marber, M.; Perera, D.; Redwood, S.; Radjenovic, A.; Saha, A.; Greenwood, J.P.; Plein, S. Appearance of microvascular obstruction on high resolution first-pass perfusion, early and late gadolinium enhancement CMR in patients with acute myocardial infarction. J. Cardiovasc. Magn. Reson. 2009, 11, 33. [Google Scholar] [CrossRef]
- Nijveldt, R.; Hofman, M.B.; Hirsch, A.; Beek, A.M.; Umans, V.A.; Algra, P.R.; Piek, J.J.; van Rossum, A.C. Assessment of microvascular obstruction and prediction of short-term remodeling after acute myocardial infarction: Cardiac MR imaging study. Radiology 2009, 250, 363–370. [Google Scholar] [CrossRef]
- Weir, R.A.; Murphy, C.A.; Petrie, C.J.; Martin, T.N.; Balmain, S.; Clements, S.; Steedman, T.; Wagner, G.S.; Dargie, H.J.; McMurray, J.J. Microvascular obstruction remains a portent of adverse remodeling in optimally treated patients with left ventricular systolic dysfunction after acute myocardial infarction. Circ. Cardiovasc. Imaging 2010, 3, 360–367. [Google Scholar] [CrossRef]
- Taylor, A.J.; Al-Saadi, N.; Abdel-Aty, H.; Schulz-Menger, J.; Messroghli, D.R.; Friedrich, M.G. Detection of acutely impaired microvascular reperfusion after infarct angioplasty with magnetic resonance imaging. Circulation 2004, 109, 2080–2085. [Google Scholar] [CrossRef]
- de Waha, S.; Patel, M.R.; Granger, C.B.; Ohman, E.M.; Maehara, A.; Eitel, I.; Ben-Yehuda, O.; Jenkins, P.; Thiele, H.; Stone, G.W. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: An individual patient data pooled analysis from seven randomized trials. Eur. Heart J. 2017, 38, 3502–3510. [Google Scholar] [CrossRef]
- Guerra, E.; Hadamitzky, M.; Ndrepepa, G.; Bauer, C.; Ibrahim, T.; Ott, I.; Laugwitz, K.L.; Schunkert, H.; Kastrati, A. Microvascular obstruction in patients with non-ST-elevation myocardial infarction: A contrast-enhanced cardiac magnetic resonance study. Int. J. Cardiovasc. Imaging 2014, 30, 1087–1095. [Google Scholar] [CrossRef]
- Mileva, N.; Paolisso, P.; Gallinoro, E.; Fabbricatore, D.; Munhoz, D.; Bergamaschi, L.; Belmonte, M.; Panayotov, P.; Pizzi, C.; Barbato, E.; et al. Diagnostic and Prognostic Role of Cardiac Magnetic Resonance in MINOCA: Systematic Review and Meta-Analysis. JACC Cardiovasc. Imaging 2023, 16, 376–389. [Google Scholar] [CrossRef]
- Bergamaschi, L.; Foa, A.; Paolisso, P.; Renzulli, M.; Angeli, F.; Fabrizio, M.; Bartoli, L.; Armillotta, M.; Sansonetti, A.; Amicone, S.; et al. Prognostic Role of Early Cardiac Magnetic Resonance in Myocardial Infarction with Nonobstructive Coronary Arteries. JACC Cardiovasc. Imaging 2023, in press. [CrossRef]
- Wehrens, X.H.; Doevendans, P.A.; Ophuis, T.J.; Wellens, H.J. A comparison of electrocardiographic changes during reperfusion of acute myocardial infarction by thrombolysis or percutaneous transluminal coronary angioplasty. Am. Heart J. 2000, 139, 430–436. [Google Scholar] [CrossRef]
- Santoro, G.M.; Valenti, R.; Buonamici, P.; Bolognese, L.; Cerisano, G.; Moschi, G.; Trapani, M.; Antoniucci, D.; Fazzini, P.F. Relation between ST-segment changes and myocardial perfusion evaluated by myocardial contrast echocardiography in patients with acute myocardial infarction treated with direct angioplasty. Am. J. Cardiol. 1998, 82, 932–937. [Google Scholar] [CrossRef]
- Reffelmann, T.; Hale, S.L.; Li, G.; Kloner, R.A. Relationship between no reflow and infarct size as influenced by the duration of ischemia and reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H766–H772. [Google Scholar] [CrossRef]
- Bouleti, C.; Mathivet, T.; Serfaty, J.M.; Vignolles, N.; Berland, E.; Monnot, C.; Cluzel, P.; Steg, P.G.; Montalescot, G.; Germain, S. Angiopoietin-like 4 serum levels on admission for acute myocardial infarction are associated with no-reflow. Int. J. Cardiol. 2015, 187, 511–516. [Google Scholar] [CrossRef]
- Niccoli, G.; Lanza, G.A.; Shaw, S.; Romagnoli, E.; Gioia, D.; Burzotta, F.; Trani, C.; Mazzari, M.A.; Mongiardo, R.; De Vita, M.; et al. Endothelin-1 and acute myocardial infarction: A no-reflow mediator after successful percutaneous myocardial revascularization. Eur. Heart J. 2006, 27, 1793–1798. [Google Scholar] [CrossRef]
- Nallamothu, B.K.; Bradley, E.H.; Krumholz, H.M. Time to treatment in primary percutaneous coronary intervention. N. Engl. J. Med. 2007, 357, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Durante, A.; Camici, P.G. Novel insights into an “old” phenomenon: The no reflow. Int. J. Cardiol. 2015, 187, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, G.; Scalone, G.; Lerman, A.; Crea, F. Coronary microvascular obstruction in acute myocardial infarction. Eur. Heart J. 2016, 37, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Sorop, O.; Merkus, D.; de Beer, V.J.; Houweling, B.; Pistea, A.; McFalls, E.O.; Boomsma, F.; van Beusekom, H.M.; van der Giessen, W.J.; VanBavel, E.; et al. Functional and structural adaptations of coronary microvessels distal to a chronic coronary artery stenosis. Circ. Res. 2008, 102, 795–803. [Google Scholar] [CrossRef]
- Vaidya, K.; Tucker, B.; Patel, S.; Ng, M.K.C. Acute Coronary Syndromes (ACS)-Unravelling Biology to Identify New Therapies-The Microcirculation as a Frontier for New Therapies in ACS. Cells 2021, 10, 2188. [Google Scholar] [CrossRef]
- Lerman, A.; Holmes, D.R.; Herrmann, J.; Gersh, B.J. Microcirculatory dysfunction in ST-elevation myocardial infarction: Cause, consequence, or both? Eur. Heart J. 2007, 28, 788–797. [Google Scholar] [CrossRef]
- Ng, M.K.; Yong, A.S.; Ho, M.; Shah, M.G.; Chawantanpipat, C.; O’Connell, R.; Keech, A.; Kritharides, L.; Fearon, W.F. The index of microcirculatory resistance predicts myocardial infarction related to percutaneous coronary intervention. Circ. Cardiovasc. Interv. 2012, 5, 515–522. [Google Scholar] [CrossRef]
- Soeda, T.; Higuma, T.; Abe, N.; Yamada, M.; Yokoyama, H.; Shibutani, S.; Ong, D.S.; Vergallo, R.; Minami, Y.; Lee, H.; et al. Morphological predictors for no reflow phenomenon after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction caused by plaque rupture. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 103–110. [Google Scholar] [CrossRef]
- Aggarwal, P.; Rekwal, L.; Sinha, S.K.; Nath, R.K.; Khanra, D.; Singh, A.P. Predictors of no-reflow phenomenon following percutaneous coronary intervention for ST-segment elevation myocardial infarction. Ann. Cardiol. Angeiol. 2021, 70, 136–142. [Google Scholar] [CrossRef]
- Tanaka, A.; Kawarabayashi, T.; Nishibori, Y.; Sano, T.; Nishida, Y.; Fukuda, D.; Shimada, K.; Yoshikawa, J. No-reflow phenomenon and lesion morphology in patients with acute myocardial infarction. Circulation 2002, 105, 2148–2152. [Google Scholar] [CrossRef]
- Yip, H.K.; Chen, M.C.; Chang, H.W.; Hang, C.L.; Hsieh, Y.K.; Fang, C.Y.; Wu, C.J. Angiographic morphologic features of infarct-related arteries and timely reperfusion in acute myocardial infarction: Predictors of slow-flow and no-reflow phenomenon. Chest 2002, 122, 1322–1332. [Google Scholar] [CrossRef]
- Mehran, R.; Dangas, G.; Mintz, G.S.; Lansky, A.J.; Pichard, A.D.; Satler, L.F.; Kent, K.M.; Stone, G.W.; Leon, M.B. Atherosclerotic plaque burden and CK-MB enzyme elevation after coronary interventions: Intravascular ultrasound study of 2256 patients. Circulation 2000, 101, 604–610. [Google Scholar] [CrossRef]
- Magro, M.; Nauta, S.T.; Simsek, C.; Boersma, E.; van der Heide, E.; Regar, E.; van Domburg, R.T.; Zijlstra, F.; Serruys, P.W.; van Geuns, R.J. Usefulness of the SYNTAX score to predict “no reflow” in patients treated with primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Am. J. Cardiol. 2012, 109, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Baghdasaryan, P.; Natarajan, B.; Sethi, P.; Mukherjee, A.; Varadarajan, P.; Pai, R.G. Pathophysiology, Diagnosis, and Management of Coronary No-Reflow Phenomenon. Int. J. Angiol. 2021, 30, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.W.; Aggarwal, A.; Ou, F.S.; Klein, L.W.; Rumsfeld, J.S.; Roe, M.T.; Wang, T.Y.; American College of Cardiology National Cardiovascular Data, R. Incidence and outcomes of no-reflow phenomenon during percutaneous coronary intervention among patients with acute myocardial infarction. Am. J. Cardiol. 2013, 111, 178–184. [Google Scholar] [CrossRef]
- Galiuto, L.; Paraggio, L.; Liuzzo, G.; de Caterina, A.R.; Crea, F. Predicting the no-reflow phenomenon following successful percutaneous coronary intervention. Biomark. Med. 2010, 4, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yang, X.; Li, X.; Li, G.; Zhou, Y.; Dong, H. Elevated uric acid is related to the no-/slow-reflow phenomenon in STEMI undergoing primary PCI. Eur. J. Clin. Investig. 2022, 52, e13719. [Google Scholar] [CrossRef]
- Kai, T.; Oka, S.; Hoshino, K.; Watanabe, K.; Nakamura, J.; Abe, M.; Watanabe, A. Renal Dysfunction as a Predictor of Slow-Flow/No-Reflow Phenomenon and Impaired ST Segment Resolution After Percutaneous Coronary Intervention in ST-Elevation Myocardial Infarction with Initial Thrombolysis in Myocardial Infarction Grade 0. Circ. J. 2021, 85, 1770–1778. [Google Scholar] [CrossRef]
- Savic, L.; Mrdovic, I.; Asanin, M.; Stankovic, S.; Lasica, R.; Krljanac, G.; Rajic, D.; Simic, D. The Impact of Kidney Function on the Slow-Flow/No-Reflow Phenomenon in Patients Treated with Primary Percutaneous Coronary Intervention: Registry Analysis. J. Interv. Cardiol. 2022, 2022, 5815274. [Google Scholar] [CrossRef]
- Esenboga, K.; Kurtul, A.; Yamanturk, Y.Y.; Tan, T.S.; Tutar, D.E. Systemic immune-inflammation index predicts no-reflow phenomenon after primary percutaneous coronary intervention. Acta Cardiol. 2022, 77, 59–65. [Google Scholar] [CrossRef]
- Selcuk, M.; Cinar, T.; Saylik, F.; Demiroz, O.; Yildirim, E. The Association of a PRECISE-DAPT Score with No-Reflow in Patients with ST-Segment Elevation Myocardial Infarction. Angiology 2022, 73, 68–72. [Google Scholar] [CrossRef]
- Sen, O.; Sen, S.B.; Topuz, A.N.; Topuz, M. Vitamin D level predicts angiographic no-reflow phenomenon after percutaneous coronary intervention in patients with ST segment elevation myocardial infarction. Biomark. Med. 2021, 15, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ren, J.; Li, L.; Wang, C.S.; Yao, H.C. RDW as A Predictor for No-Reflow Phenomenon in DM Patients with ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. J. Clin. Med. 2023, 12, 807. [Google Scholar] [CrossRef]
- Sondergaard, F.T.; Beske, R.P.; Frydland, M.; Moller, J.E.; Helgestad, O.K.L.; Jensen, L.O.; Holmvang, L.; Goetze, J.P.; Engstrom, T.; Hassager, C. Soluble ST2 in plasma is associated with post-procedural no-or-slow reflow after primary percutaneous coronary intervention in ST-elevation myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 48–52. [Google Scholar] [CrossRef]
- Hadi, H.A.; Suwaidi, J.A. Endothelial dysfunction in diabetes mellitus. Vasc. Health Risk Manag. 2007, 3, 853–876. [Google Scholar]
- Murthy, V.L.; Naya, M.; Foster, C.R.; Gaber, M.; Hainer, J.; Klein, J.; Dorbala, S.; Blankstein, R.; Di Carli, M.F. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation 2012, 126, 1858–1868. [Google Scholar] [CrossRef]
- Di Carli, M.F.; Janisse, J.; Grunberger, G.; Ager, J. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J. Am. Coll. Cardiol. 2003, 41, 1387–1393. [Google Scholar] [CrossRef]
- Rawshani, A.; Rawshani, A.; Gudbjornsdottir, S. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 300–301. [Google Scholar] [CrossRef] [PubMed]
- Eitel, I.; Hintze, S.; de Waha, S.; Fuernau, G.; Lurz, P.; Desch, S.; Schuler, G.; Thiele, H. Prognostic Impact of Hyperglycemia in Nondiabetic and Diabetic Patients with ST-Elevation Myocardial Infarction Insights from Contrast-Enhanced Magnetic Resonance Imaging. Circ.-Cardiovasc. Imaging 2012, 5, 708–718. [Google Scholar] [CrossRef]
- Reinstadler, S.J.; Stiermaier, T.; Eitel, C.; Metzler, B.; de Waha, S.; Fuernau, G.; Desch, S.; Thiele, H.; Eitel, I. Relationship between diabetes and ischaemic injury among patients with revascularized ST-elevation myocardial infarction. Diabetes Obes. Metab. 2017, 19, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Iwakura, K.; Ito, H.; Ikushima, M.; Kawano, S.; Okamura, A.; Asano, K.; Kuroda, T.; Tanaka, K.; Masuyama, T.; Hori, M.; et al. Association between hyperglycemia and the no-reflow phenomenon in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 2003, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.; Tanimoto, T.; Orii, M.; Hirata, K.; Shiono, Y.; Shimamura, K.; Matsuo, Y.; Yamano, T.; Ino, Y.; Kitabata, H.; et al. Association between hyperglycemia at admission and microvascular obstruction in patients with ST-segment elevation myocardial infarction. J. Cardiol. 2015, 65, 272–277. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jensen, C.J.; Eberle, H.C.; Nassenstein, K.; Schlosser, T.; Farazandeh, M.; Naber, C.K.; Sabin, G.V.; Bruder, O. Impact of hyperglycemia at admission in patients with acute ST-segment elevation myocardial infarction as assessed by contrast-enhanced MRI. Clin. Res. Cardiol. 2011, 100, 649–659. [Google Scholar] [CrossRef]
- Barrett, E.J.; Liu, Z.Q.; Khamaisi, M.; King, G.L.; Klein, R.; Klein, B.E.K.; Hughes, T.M.; Craft, S.; Freedman, B.I.; Bowden, D.W.; et al. Diabetic Microvascular Disease: An Endocrine Society Scientific Statement. J. Clin. Endocr. Metab. 2017, 102, 4343–4410. [Google Scholar] [CrossRef] [PubMed]
- Panza, J.A.; Casino, P.R.; Kilcoyne, C.M.; Quyyumi, A.A. Role of Endothelium-Derived Nitric-Oxide in the Abnormal Endothelium-Dependent Vascular Relaxation of Patients with Essential-Hypertension. Circulation 1993, 87, 1468–1474. [Google Scholar] [CrossRef]
- Carrick, D.; Haig, C.; Maznyczka, A.M.; Carberry, J.; Mangion, K.; Ahmed, N.; May, V.T.Y.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; et al. Hypertension, Microvascular Pathology, and Prognosis after an Acute Myocardial Infarction. Hypertension 2018, 72, 720–730. [Google Scholar] [CrossRef]
- Reinstadler, S.J.; Stiermaier, T.; Eitel, C.; Saad, M.; Metzler, B.; de Waha, S.; Fuernau, G.; Desch, S.; Thiele, H.; Eitel, I. Antecedent hypertension and myocardial injury in patients with reperfused ST-elevation myocardial infarction. J. Cardiovasc. Magn. R. 2016, 18, 80. [Google Scholar] [CrossRef]
- Quyyumi, A.A.; Mulcahy, D.; Andrews, N.P.; Husain, S.; Panza, J.A.; Cannon, R.O., 3rd. Coronary vascular nitric oxide activity in hypertension and hypercholesterolemia. Comparison of acetylcholine and substance P. Circulation 1997, 95, 104–110. [Google Scholar] [CrossRef]
- Golino, P.; Maroko, P.R.; Carew, T.E. The effect of acute hypercholesterolemia on myocardial infarct size and the no-reflow phenomenon during coronary occlusion-reperfusion. Circulation 1987, 75, 292–298. [Google Scholar] [CrossRef]
- Iwakura, K.; Ito, H.; Kawano, S.; Okamura, A.; Kurotobi, T.; Date, M.; Inoue, K.; Fujii, K. Chronic pre-treatment of statins is associated with the reduction of the no-reflow phenomenon in the patients with reperfused acute myocardial infarction. Eur. Heart J. 2006, 27, 534–539. [Google Scholar] [CrossRef]
- Reindl, M.; Reinstadler, S.J.; Feistritzer, H.J.; Theurl, M.; Basic, D.; Eigler, C.; Holzknecht, M.; Mair, J.; Mayr, A.; Klug, G.; et al. Relation of Low-Density Lipoprotein Cholesterol with Microvascular Injury and Clinical Outcome in Revascularized ST-Elevation Myocardial Infarction. J. Am. Heart Assoc. 2017, 6, e006957. [Google Scholar] [CrossRef]
- Surendran, A.; Ismail, U.; Atefi, N.; Bagchi, A.K.; Singal, P.K.; Shah, A.; Aliani, M.; Ravandi, A. Lipidomic Predictors of Coronary No-Reflow. Metabolites 2023, 13, 79. [Google Scholar] [CrossRef]
- Messner, B.; Bernhard, D. Smoking and cardiovascular disease: Mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 509–515. [Google Scholar] [CrossRef]
- Symons, R.; Masci, P.G.; Francone, M.; Claus, P.; Barison, A.; Carbone, I.; Agati, L.; Galea, N.; Janssens, S.; Bogaert, J. Impact of active smoking on myocardial infarction severity in reperfused ST-segment elevation myocardial infarction patients: The smoker’s paradox revisited. Eur. Heart J. 2016, 37, 2756–2764. [Google Scholar] [CrossRef]
- Reinstadler, S.J.; Eitel, C.; Fuernau, G.; de Waha, S.; Desch, S.; Mende, M.; Metzler, B.; Schuler, G.; Thiele, H.; Eitel, I. Association of smoking with myocardial injury and clinical outcome in patients undergoing mechanical reperfusion for ST-elevation myocardial infarction. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 39–45. [Google Scholar] [CrossRef]
- Haig, C.; Carrick, D.; Carberry, J.; Mangion, K.; Maznyczka, A.; Wetherall, K.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; Lindsay, M.; et al. Current Smoking and Prognosis after Acute ST-Segment Elevation Myocardial Infarction: New Pathophysiological Insights. JACC Cardiovasc. Imaging 2019, 12, 993–1003. [Google Scholar] [CrossRef]
- Ipek, G.; Onuk, T.; Karatas, M.B.; Gungor, B.; Osken, A.; Keskin, M.; Oz, A.; Tanik, O.; Hayiroglu, M.I.; Yaka, H.Y.; et al. CHA2DS2-VASc Score is a Predictor of No-Reflow in Patients with ST-Segment Elevation Myocardial Infarction Who Underwent Primary Percutaneous Intervention. Angiology 2016, 67, 840–845. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, M.; Ma, S.; Niu, T. New R(2)-CHA(2)DS(2)-VASc score predicts no-reflow phenomenon and long-term prognosis in patients with ST-segment elevation myocardial infarction after primary percutaneous coronary intervention. Front. Cardiovasc. Med. 2022, 9, 899739. [Google Scholar] [CrossRef]
- Karila-Cohen, D.; Czitrom, D.; Brochet, E.; Faraggi, M.; Seknadji, P.; Himbert, D.; Juliard, J.M.; Assayag, P.; Steg, P.G. Decreased no-reflow in patients with anterior myocardial infarction and pre-infarction angina. Eur. Heart J. 1999, 20, 1724–1730. [Google Scholar] [CrossRef]
- Takahashi, T.; Anzai, T.; Yoshikawa, T.; Asakura, Y.; Ishikawa, S.; Mitamura, H.; Ogawa, S. Absence of preinfarction angina is associated with a risk of no-reflow phenomenon after primary coronary angioplasty for a first anterior wall acute myocardial infarction. Int. J. Cardiol. 2000, 75, 253–260. [Google Scholar] [CrossRef]
- Zalewski, J.; Undas, A.; Godlewski, J.; Stepien, E.; Zmudka, K. No-reflow phenomenon after acute myocardial infarction is associated with reduced clot permeability and susceptibility to lysis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2258–2265. [Google Scholar] [CrossRef]
- Bolayir, H.A.; Gunes, H.; Kivrak, T.; Sahin, O.; Akaslan, D.; Kurt, R.; Bolayir, A.; Imadoglu, O. The role of SCUBE1 in the pathogenesis of no-reflow phenomenon presenting with ST segment elevation myocardial infarction. Anatol. J. Cardiol. 2017, 18, 122–127. [Google Scholar] [CrossRef]
- Yoshino, S.; Cilluffo, R.; Best, P.J.; Atkinson, E.J.; Aoki, T.; Cunningham, J.M.; de Andrade, M.; Choi, B.J.; Lerman, L.O.; Lerman, A. Single nucleotide polymorphisms associated with abnormal coronary microvascular function. Coron. Artery Dis. 2014, 25, 281–289. [Google Scholar] [CrossRef]
- Niccoli, G.; Celestini, A.; Calvieri, C.; Cosentino, N.; Falcioni, E.; Carnevale, R.; Nocella, C.; Fracassi, F.; Roberto, M.; Antonazzo, R.P.; et al. Patients with microvascular obstruction after primary percutaneous coronary intervention show a gp91phox (NOX2) mediated persistent oxidative stress after reperfusion. Eur. Heart J. Acute Cardiovasc. Care 2013, 2, 379–388. [Google Scholar] [CrossRef]
- Galasso, G.; Schiekofer, S.; D’Anna, C.; Gioia, G.D.; Piccolo, R.; Niglio, T.; Rosa, R.D.; Strisciuglio, T.; Cirillo, P.; Piscione, F.; et al. No-reflow phenomenon: Pathophysiology, diagnosis, prevention, and treatment. A review of the current literature and future perspectives. Angiology 2014, 65, 180–189. [Google Scholar] [CrossRef]
- Resnic, F.S.; Wainstein, M.; Lee, M.K.; Behrendt, D.; Wainstein, R.V.; Ohno-Machado, L.; Kirshenbaum, J.M.; Rogers, C.D.; Popma, J.J.; Piana, R. No-reflow is an independent predictor of death and myocardial infarction after percutaneous coronary intervention. Am. Heart J. 2003, 145, 42–46. [Google Scholar] [CrossRef]
- Brosh, D.; Assali, A.R.; Mager, A.; Porter, A.; Hasdai, D.; Teplitsky, I.; Rechavia, E.; Fuchs, S.; Battler, A.; Kornowski, R. Effect of no-reflow during primary percutaneous coronary intervention for acute myocardial infarction on six-month mortality. Am. J. Cardiol. 2007, 99, 442–445. [Google Scholar] [CrossRef]
- Bolognese, L.; Carrabba, N.; Parodi, G.; Santoro, G.M.; Buonamici, P.; Cerisano, G.; Antoniucci, D. Impact of microvascular dysfunction on left ventricular remodeling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation 2004, 109, 1121–1126. [Google Scholar] [CrossRef]
- Mehta, R.H.; Harjai, K.J.; Boura, J.; Cox, D.; Stone, G.W.; O’Neill, W.; Grines, C.L.; Primary Angioplasty in Myocardial Infarction, I. Prognostic significance of transient no-reflow during primary percutaneous coronary intervention for ST-elevation acute myocardial infarction. Am. J. Cardiol. 2003, 92, 1445–1447. [Google Scholar] [CrossRef]
- Jinnouchi, H.; Sakakura, K.; Wada, H.; Arao, K.; Kubo, N.; Sugawara, Y.; Funayama, H.; Momomura, S.; Ako, J. Transient no reflow following primary percutaneous coronary intervention. Heart Vessel. 2014, 29, 429–436. [Google Scholar] [CrossRef]
- Fujii, T.; Masuda, N.; Nakano, M.; Nakazawa, G.; Shinozaki, N.; Matsukage, T.; Ogata, N.; Yoshimachi, F.; Ikari, Y. Impact of transient or persistent slow flow and adjunctive distal protection on mortality in ST-segment elevation myocardial infarction. Cardiovasc. Interv. Ther. 2015, 30, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.C.; Cho, J.Y.; Jeong, H.C.; Lee, K.H.; Park, K.H.; Sim, D.S.; Yoon, N.S.; Youn, H.J.; Kim, K.H.; Hong, Y.J.; et al. Long-Term Clinical Outcomes of Transient and Persistent No Reflow Phenomena following Percutaneous Coronary Intervention in Patients with Acute Myocardial Infarction. Korean Circ. J. 2016, 46, 490–498. [Google Scholar] [CrossRef]
- Nakazawa, G.; Tanabe, K.; Onuma, Y.; Yachi, S.; Aoki, J.; Yamamoto, H.; Higashikuni, Y.; Yagishita, A.; Nakajima, H.; Hara, K. Efficacy of culprit plaque assessment by 64-slice multidetector computed tomography to predict transient no-reflow phenomenon during percutaneous coronary intervention. Am. Heart J. 2008, 155, 1150–1157. [Google Scholar] [CrossRef]
- Iijima, R.; Shinji, H.; Ikeda, N.; Itaya, H.; Makino, K.; Funatsu, A.; Yokouchi, I.; Komatsu, H.; Ito, N.; Nuruki, H.; et al. Comparison of coronary arterial finding by intravascular ultrasound in patients with “transient no-reflow” versus “reflow” during percutaneous coronary intervention in acute coronary syndrome. Am. J. Cardiol. 2006, 97, 29–33. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Colleran, R.; Kastrati, A. No-reflow after percutaneous coronary intervention: A correlate of poor outcome in both persistent and transient forms. Eurointervention 2018, 14, 139–141. [Google Scholar] [CrossRef]
- Reffelmann, T.; Hale, S.L.; Dow, J.S.; Kloner, R.A. No-reflow phenomenon persists long-term after ischemia/reperfusion in the rat and predicts infarct expansion. Circulation 2003, 108, 2911–2917. [Google Scholar] [CrossRef]
- Galiuto, L.; Garramone, B.; Scara, A.; Rebuzzi, A.G.; Crea, F.; La Torre, G.; Funaro, S.; Madonna, M.; Fedele, F.; Agati, L.; et al. The extent of microvascular damage during myocardial contrast echocardiography is superior to other known indexes of post-infarct reperfusion in predicting left ventricular remodeling: Results of the multicenter AMICI study. J. Am. Coll. Cardiol. 2008, 51, 552–559. [Google Scholar] [CrossRef]
- Husser, O.; Monmeneu, J.V.; Sanchis, J.; Nunez, J.; Lopez-Lereu, M.P.; Bonanad, C.; Chaustre, F.; Gomez, C.; Bosch, M.J.; Hinarejos, R.; et al. Cardiovascular magnetic resonance-derived intramyocardial hemorrhage after STEMI: Influence on long-term prognosis, adverse left ventricular remodeling and relationship with microvascular obstruction. Int. J. Cardiol. 2013, 167, 2047–2054. [Google Scholar] [CrossRef]
- Loubeyre, C.; Morice, M.C.; Lefevre, T.; Piechaud, J.F.; Louvard, Y.; Dumas, P. A randomized comparison of direct stenting with conventional stent implantation in selected patients with acute myocardial infarction. J. Am. Coll. Cardiol. 2002, 39, 15–21. [Google Scholar] [CrossRef]
- Gasior, M.; Gierlotka, M.; Lekston, A.; Wilczek, K.; Zebik, T.; Hawranek, M.; Wojnar, R.; Szkodzinski, J.; Piegza, J.; Dyrbus, K.; et al. Comparison of outcomes of direct stenting versus stenting after balloon predilation in patients with acute myocardial infarction (DIRAMI). Am. J. Cardiol. 2007, 100, 798–805. [Google Scholar] [CrossRef]
- Azzalini, L.; Millan, X.; Ly, H.Q.; L’Allier, P.L.; Jolicoeur, E.M. Direct stenting versus pre-dilation in ST-elevation myocardial infarction: A systematic review and meta-analysis. J. Interv. Cardiol. 2015, 28, 119–131. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Li, M.; Liu, J.; Wang, L.; Liu, Y.; Wang, Z.; Wen, S. Comparing Direct Stenting with Conventional Stenting in Patients with Acute Coronary Syndromes: A Meta-Analysis of 12 Clinical Trials. Angiology 2016, 67, 317–325. [Google Scholar] [CrossRef]
- Mahmoud, K.D.; Jolly, S.S.; James, S.; Dzavik, V.; Cairns, J.A.; Olivecrona, G.K.; Renlund, H.; Gao, P.; Lagerqvist, B.; Alazzoni, A.; et al. Clinical impact of direct stenting and interaction with thrombus aspiration in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention: Thrombectomy Trialists Collaboration. Eur. Heart J. 2018, 39, 2472–2479. [Google Scholar] [CrossRef]
- Gick, M.; Jander, N.; Bestehorn, H.P.; Kienzle, R.P.; Ferenc, M.; Werner, K.; Comberg, T.; Peitz, K.; Zohlnhofer, D.; Bassignana, V.; et al. Randomized evaluation of the effects of filter-based distal protection on myocardial perfusion and infarct size after primary percutaneous catheter intervention in myocardial infarction with and without ST-segment elevation. Circulation 2005, 112, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Kelbaek, H.; Terkelsen, C.J.; Helqvist, S.; Lassen, J.F.; Clemmensen, P.; Klovgaard, L.; Kaltoft, A.; Engstrom, T.; Botker, H.E.; Saunamaki, K.; et al. Randomized comparison of distal protection versus conventional treatment in primary percutaneous coronary intervention: The drug elution and distal protection in ST-elevation myocardial infarction (DEDICATION) trial. J. Am. Coll. Cardiol. 2008, 51, 899–905. [Google Scholar] [CrossRef]
- Kumbhani, D.J.; Bavry, A.A.; Desai, M.Y.; Bangalore, S.; Bhatt, D.L. Role of aspiration and mechanical thrombectomy in patients with acute myocardial infarction undergoing primary angioplasty: An updated meta-analysis of randomized trials. J. Am. Coll. Cardiol. 2013, 62, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Frobert, O.; Lagerqvist, B.; Olivecrona, G.K.; Omerovic, E.; Gudnason, T.; Maeng, M.; Aasa, M.; Angeras, O.; Calais, F.; Danielewicz, M.; et al. Thrombus Aspiration during ST-Segment Elevation Myocardial Infarction. N. Engl. J. Med. 2013, 369, 1587–1597. [Google Scholar] [CrossRef]
- Jolly, S.S.; Cairns, J.A.; Yusuf, S.; Meeks, B.; Pogue, J.; Rokoss, M.J.; Kedev, S.; Thabane, L.; Stankovic, G.; Moreno, R.; et al. Randomized trial of primary PCI with or without routine manual thrombectomy. N. Engl. J. Med. 2015, 372, 1389–1398. [Google Scholar] [CrossRef]
- Carrick, D.; Oldroyd, K.G.; McEntegart, M.; Haig, C.; Petrie, M.C.; Eteiba, H.; Hood, S.; Owens, C.; Watkins, S.; Layland, J.; et al. A randomized trial of deferred stenting versus immediate stenting to prevent no- or slow-reflow in acute ST-segment elevation myocardial infarction (DEFER-STEMI). J. Am. Coll. Cardiol. 2014, 63, 2088–2098. [Google Scholar] [CrossRef]
- Lonborg, J.; Engstrom, T.; Ahtarovski, K.A.; Nepper-Christensen, L.; Helqvist, S.; Vejlstrup, N.; Kyhl, K.; Schoos, M.M.; Ghotbi, A.; Goransson, C.; et al. Myocardial Damage in Patients with Deferred Stenting after STEMI: A DANAMI-3-DEFER Substudy. J. Am. Coll. Cardiol. 2017, 69, 2794–2804. [Google Scholar] [CrossRef] [PubMed]
- Cassese, S.; Belle, L.; Ndrepepa, G.; Bosson, J.L.; Fusaro, M.; Lonborg, J.; Ahtarovski, K.A.; Kelbaek, H.; Fusaro, M. Deferred vs Immediate Stenting in Primary Percutaneous Coronary Intervention: A Collaborative Meta-analysis of Randomized Trials with Cardiac Magnetic Resonance Imaging Data. Can. J. Cardiol. 2018, 34, 1573–1580. [Google Scholar] [CrossRef]
- Stone, G.W.; Abizaid, A.; Silber, S.; Dizon, J.M.; Merkely, B.; Costa, R.A.; Kornowski, R.; Abizaid, A.; Wojdyla, R.; Maehara, A.; et al. Prospective, Randomized, Multicenter Evaluation of a Polyethylene Terephthalate Micronet Mesh-Covered Stent (MGuard) in ST-Segment Elevation Myocardial Infarction The MASTER Trial. J. Am. Coll. Cardiol. 2012, 60, 1975–1984. [Google Scholar] [CrossRef]
- Dudek, D.; Dziewierz, A.; Brener, S.J.; Abizaid, A.; Merkely, B.; Costa, R.A.; Bar, E.; Rakowski, T.; Kornowski, R.; Dressler, O.; et al. Mesh-Covered Embolic Protection Stent Implantation in ST-Segment-Elevation Myocardial Infarction Final 1-Year Clinical and Angiographic Results From the MGUARD for Acute ST Elevation Reperfusion Trial. Circ. Cardiovasc. Interv. 2015, 8, e001484. [Google Scholar] [CrossRef]
- Romaguera, R.; Gomez-Hospital, J.A.; Sanchez-Elvira, G.; Gomez-Lara, J.; Ferreiro, J.L.; Roura, G.; Gracida, M.; Homs, S.; Teruel, L.; Cequier, A. MGuard Mesh-Covered Stent for Treatment of ST-Segment Elevation Myocardial Infarction with High Thrombus Burden Despite Manual Aspiration. J. Interv. Cardiol. 2013, 26, 1–7. [Google Scholar] [CrossRef]
- De Maria, G.L.; Alkhalil, M.; Borlotti, A.; Wolfrum, M.; Gaughran, L.; Dall’Armellina, E.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; Kharbanda, R.K.; et al. Index of microcirculatory resistance-guided therapy with pressure-controlled intermittent coronary sinus occlusion improves coronary microvascular function and reduces infarct size in patients with ST-elevation myocardial infarction: The Oxford Acute Myocardial Infarction—Pressure-controlled Intermittent Coronary Sinus Occlusion study (OxAMI-PICSO study). Eurointervention 2018, 14, e352–e359. [Google Scholar] [CrossRef]
- Van’t Hof, A.W.; Ten Berg, J.; Heestermans, T.; Dill, T.; Funck, R.C.; van Werkum, W.; Dambrink, J.H.; Suryapranata, H.; van Houwelingen, G.; Ottervanger, J.P.; et al. Prehospital initiation of tirofiban in patients with ST-elevation myocardial infarction undergoing primary angioplasty (On-TIME 2): A multicentre, double-blind, randomised controlled trial. Lancet 2008, 372, 537–546. [Google Scholar] [CrossRef]
- Akpek, M.; Sahin, O.; Sarli, B.; Baktir, A.O.; Saglam, H.; Urkmez, S.; Ergin, A.; Oguzhan, A.; Arinc, H.; Kaya, M.G. Acute Effects of Intracoronary Tirofiban on No-Reflow Phenomena in Patients with ST-Segment Elevated Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Angiology 2015, 66, 560–567. [Google Scholar] [CrossRef]
- Ma, Q.M.; Ma, Y.; Wang, X.N.; Li, S.S.; Yu, T.T.; Duan, W.L.; Wu, J.K.; Wen, Z.Y.; Jiao, Y.D.; Sun, Z.Q.; et al. Intracoronary compared with intravenous bolus tirofiban on the microvascular obstruction in patients with STEMI undergoing PCI: A cardiac MR study. Int. J. Cardiovasc. Imaging 2020, 36, 1121–1132. [Google Scholar] [CrossRef]
- Stone, G.W.; Maehara, A.; Witzenbichler, B.; Godlewski, J.; Parise, H.; Dambrink, J.H.; Ochala, A.; Carlton, T.W.; Cristea, E.; Wolff, S.D.; et al. Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: The INFUSE-AMI randomized trial. JAMA 2012, 307, 1817–1826. [Google Scholar] [CrossRef]
- Thiele, H.; Wohrle, J.; Hambrecht, R.; Rittger, H.; Birkemeyer, R.; Lauer, B.; Neuhaus, P.; Brosteanu, O.; Sick, P.; Wiemer, M.; et al. Intracoronary versus intravenous bolus abciximab during primary percutaneous coronary intervention in patients with acute ST-elevation myocardial infarction: A randomised trial. Lancet 2012, 379, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Ndrepepa, G. Improving myocardial injury, infarct size, and myocardial salvage in the era of primary PCI for STEMI. Coron. Artery Dis. 2015, 26, 341–355. [Google Scholar] [CrossRef]
- Mahaffey, K.W.; Puma, J.A.; Barbagelata, N.A.; DiCarli, M.F.; Leesar, M.A.; Browne, K.F.; Eisenberg, P.R.; Bolli, R.; Casas, A.C.; Molina-Viamonte, V.; et al. Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: Results of a multicenter, randomized, placebo-controlled trial: The Acute Myocardial Infarction STudy of ADenosine (AMISTAD) trial. J. Am. Coll. Cardiol. 1999, 34, 1711–1720. [Google Scholar] [CrossRef]
- Ross, A.M.; Gibbons, R.J.; Stone, G.W.; Kloner, R.A.; Alexander, R.W.; Investigators, A.-I. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). J. Am. Coll. Cardiol. 2005, 45, 1775–1780. [Google Scholar] [CrossRef]
- Niccoli, G.; Rigattieri, S.; De Vita, M.R.; Valgimigli, M.; Corvo, P.; Fabbiocchi, F.; Romagnoli, E.; De Caterina, A.R.; La Torre, G.; Lo Schiavo, P.; et al. Open-label, randomized, placebo-controlled evaluation of intracoronary adenosine or nitroprusside after thrombus aspiration during primary percutaneous coronary intervention for the prevention of microvascular obstruction in acute myocardial infarction: The REOPEN-AMI study (Intracoronary Nitroprusside Versus Adenosine in Acute Myocardial Infarction). JACC Cardiovasc. Interv. 2013, 6, 580–589. [Google Scholar] [CrossRef]
- Desmet, W.; Bogaert, J.; Dubois, C.; Sinnaeve, P.; Adriaenssens, T.; Pappas, C.; Ganame, J.; Dymarkowski, S.; Janssens, S.; Belmans, A.; et al. High-dose intracoronary adenosine for myocardial salvage in patients with acute ST-segment elevation myocardial infarction. Eur. Heart J. 2011, 32, 867–877. [Google Scholar] [CrossRef]
- Nazir, S.A.; McCann, G.P.; Greenwood, J.P.; Kunadian, V.; Khan, J.N.; Mahmoud, I.Z.; Blackman, D.J.; Been, M.; Abrams, K.R.; Shipley, L.; et al. Strategies to attenuate micro-vascular obstruction during P-PCI: The randomized reperfusion facilitated by local adjunctive therapy in ST-elevation myocardial infarction trial. Eur. Heart J. 2016, 37, 1910–1919. [Google Scholar] [CrossRef]
- Laborante, R.; Bianchini, E.; Restivo, A.; Ciliberti, G.; Galli, M.; Vergallo, R.; Rodolico, D.; Zito, A.; Princi, G.; Leone, A.M.; et al. Adenosine as adjunctive therapy in acute coronary syndrome: A meta-analysis of randomized controlled trials. Eur. Heart J. Cardiovasc. Pharmacother. 2023, 9, 173–182. [Google Scholar] [CrossRef]
- Siddiqi, N.; Neil, C.; Bruce, M.; MacLennan, G.; Cotton, S.; Papadopoulou, S.; Feelisch, M.; Bunce, N.; Lim, P.O.; Hildick-Smith, D.; et al. Intravenous sodium nitrite in acute ST-elevation myocardial infarction: A randomized controlled trial (NIAMI). Eur. Heart J. 2014, 35, 1255–1262. [Google Scholar] [CrossRef]
- Annibali, G.; Scrocca, I.; Aranzulla, T.C.; Meliga, E.; Maiellaro, F.; Musumeci, G. “No-Reflow” Phenomenon: A Contemporary Review. J. Clin. Med. 2022, 11, 2233. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, Z.; Gu, Y.; Peng, D. Short-Term Effects of Verapamil and Diltiazem in the Treatment of No Reflow Phenomenon: A Meta-Analysis of Randomized Controlled Trials. Biomed. Res. Int. 2015, 2015, 382086. [Google Scholar] [CrossRef]
- Huang, R.I.; Patel, P.; Walinsky, P.; Fischman, D.L.; Ogilby, J.D.; Awar, M.; Frankil, C.; Savage, M.P. Efficacy of intracoronary nicardipine in the treatment of no-reflow during percutaneous coronary intervention. Catheter. Cardiovasc. Interv. 2006, 68, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Fischell, T.A.; Haller, S.; Pulukurthy, S.; Virk, I.S. Nicardipine and adenosine “flush cocktail” to prevent no-reflow during rotational atherectomy. Cardiovasc. Revasc Med. 2008, 9, 224–228. [Google Scholar] [CrossRef]
- Ibanez, B.; Prat-Gonzalez, S.; Speidl, W.S.; Vilahur, G.; Pinero, A.; Cimmino, G.; Garcia, M.J.; Fuster, V.; Sanz, J.; Badimon, J.J. Early metoprolol administration before coronary reperfusion results in increased myocardial salvage analysis of ischemic myocardium at risk using cardiac magnetic resonance. Circulation 2007, 115, 2909–2916. [Google Scholar] [CrossRef]
- Garcia-Prieto, J.; Villena-Gutierrez, R.; Gomez, M.; Bernardo, E.; Pun-Garcia, A.; Garcia-Lunar, I.; Crainiciuc, G.; Fernandez-Jimenez, R.; Sreeramkumar, V.; Bourio-Martinez, R.; et al. Neutrophil stunning by metoprolol reduces infarct size. Nat. Commun. 2017, 8, 14780. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.L.; Yang, Y.J.; Pei, W.D.; Sun, Y.H.; Zhai, M.; Liu, Y.X.; Gao, R.L. Carvedilol reduces myocardial no-reflow by decreasing endothelin-1 via activation of the ATP-sensitive K+ channel. Perfusion 2008, 23, 111–115. [Google Scholar] [CrossRef]
- Sorrentino, S.A.; Doerries, C.; Manes, C.; Speer, T.; Dessy, C.; Lobysheva, I.; Mohmand, W.; Akbar, R.; Bahlmann, F.; Besler, C.; et al. Nebivolol exerts beneficial effects on endothelial function, early endothelial progenitor cells, myocardial neovascularization, and left ventricular dysfunction early after myocardial infarction beyond conventional beta1-blockade. J. Am. Coll. Cardiol. 2011, 57, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; Macaya, C.; Sanchez-Brunete, V.; Pizarro, G.; Fernandez-Friera, L.; Mateos, A.; Fernandez-Ortiz, A.; Garcia-Ruiz, J.M.; Garcia-Alvarez, A.; Iniguez, A.; et al. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: The Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction (METOCARD-CNIC) trial. Circulation 2013, 128, 1495–1503. [Google Scholar] [CrossRef]
- Roolvink, V.; Ibanez, B.; Ottervanger, J.P.; Pizarro, G.; van Royen, N.; Mateos, A.; Dambrink, J.E.; Escalera, N.; Lipsic, E.; Albarran, A.; et al. Early Intravenous Beta-Blockers in Patients with ST-Segment Elevation Myocardial Infarction Before Primary Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2016, 67, 2705–2715. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, J.; Choi, D.; Lee, C.J.; Lee, S.H.; Ko, Y.G.; Hong, M.K.; Kim, B.K.; Oh, S.J.; Jeon, D.W.; et al. Efficacy of High-Dose Atorvastatin Loading Before Primary Percutaneous Coronary Intervention in ST-Segment Elevation Myocardial Infarction The STATIN STEMI Trial. JACC Cardiovasc. Interv. 2010, 3, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.Y.; Kim, H.J.; Choi, Y.J.; Jo, S.H.; Kim, H.J.; Lee, S.; Ahn, K.J.; Song, Y.B.; Choi, J.H.; Choi, S.H.; et al. Effects of atorvastatin pretreatment on infarct size in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am. Heart J. 2011, 162, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Berwanger, O.; Santucci, E.V.; de Barros, E.S.P.G.M.; Jesuino, I.A.; Damiani, L.P.; Barbosa, L.M.; Santos, R.H.N.; Laranjeira, L.N.; Egydio, F.M.; Borges de Oliveira, J.A.; et al. Effect of Loading Dose of Atorvastatin Prior to Planned Percutaneous Coronary Intervention on Major Adverse Cardiovascular Events in Acute Coronary Syndrome: The SECURE-PCI Randomized Clinical Trial. JAMA 2018, 319, 1331–1340. [Google Scholar] [CrossRef]
- McCartney, P.J.; Eteiba, H.; Maznyczka, A.M.; McEntegart, M.; Greenwood, J.P.; Muir, D.F.; Chowdhary, S.; Gershlick, A.H.; Appleby, C.; Cotton, J.M.; et al. Effect of Low-Dose Intracoronary Alteplase During Primary Percutaneous Coronary Intervention on Microvascular Obstruction in Patients with Acute Myocardial Infarction a Randomized Clinical Trial. Jama-J. Am. Med. Assoc. 2019, 321, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Alyamani, M.; Campbell, S.; Navarese, E.; Welsh, R.C.; Bainey, K.R. Safety and Efficacy of Intracoronary Thrombolysis as Adjunctive Therapy to Primary PCI in STEMI: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2021, 37, 339–346. [Google Scholar] [CrossRef]
- van Geuns, R.J.; Sideris, G.; Van Royen, N.; El Mahmoud, R.; Diletti, R.; Bal Dit Sollier, C.; Garot, J.; Van Der Hoeven, N.W.; Cortese, B.; Ding, L.; et al. Bivalirudin infusion to reduce ventricular infarction: The open-label, randomised Bivalirudin Infusion for Ventricular InfArction Limitation (BIVAL) study. Eurointervention 2017, 13, e540–e548. [Google Scholar] [CrossRef] [PubMed]
- Navarese, E.P.; Frediani, L.; Kandzari, D.E.; Caiazzo, G.; Cenname, A.M.; Cortese, B.; Piva, T.; Mucaj, A.; Tumscitz, C.; Paparoni, F.; et al. Efficacy and safety of intracoronary epinephrine versus conventional treatments alone in STEMI patients with refractory coronary no-reflow during primary PCI: The RESTORE observational study. Catheter. Cardiovasc. Interv. 2021, 97, 602–611. [Google Scholar] [CrossRef]
- Khan, K.A.; Qamar, N.; Saghir, T.; Sial, J.A.; Kumar, D.; Kumar, R.; Qayyum, D.; Yasin, U.; Jalbani, J.; Karim, M. Comparison of Intracoronary Epinephrine and Adenosine for No-Reflow in Normotensive Patients with Acute Coronary Syndrome (COAR Trial). Circ. Cardiovasc. Interv. 2022, 15, e011408. [Google Scholar] [CrossRef] [PubMed]
- Aksu, T.; Guler, T.E.; Colak, A.; Baysal, E.; Durukan, M.; Sen, T.; Guray, U. Intracoronary epinephrine in the treatment of refractory no-reflow after primary percutaneous coronary intervention: A retrospective study. BMC Cardiovasc. Disord. 2015, 15, 10. [Google Scholar] [CrossRef]
- Darwish, A.; Frere, A.F.; Abdelsamie, M.; Awady, W.E.; Gouda, M. Intracoronary epinephrine versus adenosine in the management of refractory no-reflow phenomenon: A single-center retrospective cohort study. Ann. Saudi Med. 2022, 42, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Massalha, E.; Oren, D.; Goitein, O.; Brodov, Y.; Fardman, A.; Younis, A.; Berkovitch, A.; Raibman-Spector, S.; Konen, E.; Maor, E.; et al. Post-ST-Segment-Elevation Myocardial Infarction Platelet Reactivity Is Associated with the Extent of Microvascular Obstruction and Infarct Size as Determined by Cardiac Magnetic Resonance Imaging. J. Am. Heart Assoc. 2022, 11, e020973. [Google Scholar] [CrossRef] [PubMed]
- Steg, P.G.; James, S.; Harrington, R.A.; Ardissino, D.; Becker, R.C.; Cannon, C.P.; Emanuelsson, H.; Finkelstein, A.; Husted, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: A Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation 2010, 122, 2131–2141. [Google Scholar] [CrossRef]
- Montalescot, G.; van ‘t Hof, A.W.; Lapostolle, F.; Silvain, J.; Lassen, J.F.; Bolognese, L.; Cantor, W.J.; Cequier, A.; Chettibi, M.; Goodman, S.G.; et al. Prehospital ticagrelor in ST-segment elevation myocardial infarction. N. Engl. J. Med. 2014, 371, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, M.A.H.; van der Hoeven, N.W.; Janssens, G.N.; Everaars, H.; Nap, A.; Lemkes, J.S.; de Waard, G.A.; van de Ven, P.M.; van Rossum, A.C.; Ten Cate, T.J.F.; et al. Evaluation of Microvascular Injury in Revascularized Patients with ST-Segment-Elevation Myocardial Infarction Treated with Ticagrelor Versus Prasugrel. Circulation 2019, 139, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Ye, Z.; Li, L.; Su, Q. Effect of preoperative loading dose ticagrelor and clopidogrel on no-reflow phenomenon during intervention in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: A systematic review and meta-analysis. Drug Des. Devel Ther. 2018, 12, 2039–2049. [Google Scholar] [CrossRef]
- Bulluck, H.; Chan, M.H.H.; Bryant, J.A.; Chai, P.; Chawla, A.; Chua, T.S.; Chung, Y.C.; Fei, G.; Ho, H.H.; Ho, A.F.W.; et al. Platelet inhibition to target reperfusion injury trial: Rationale and study design. Clin. Cardiol. 2019, 42, 5–12. [Google Scholar] [CrossRef]
- Piot, C.; Croisille, P.; Staat, P.; Thibault, H.; Rioufol, G.; Mewton, N.; Elbelghiti, R.; Cung, T.T.; Bonnefoy, E.; Angoulvant, D.; et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N. Engl. J. Med. 2008, 359, 473–481. [Google Scholar] [CrossRef]
- Cung, T.T.; Morel, O.; Cayla, G.; Rioufol, G.; Garcia-Dorado, D.; Angoulvant, D.; Bonnefoy-Cudraz, E.; Guerin, P.; Elbaz, M.; Delarche, N.; et al. Cyclosporine before PCI in Patients with Acute Myocardial Infarction. N. Engl. J. Med. 2015, 373, 1021–1031. [Google Scholar] [CrossRef]
- Ottani, F.; Latini, R.; Staszewsky, L.; La Vecchia, L.; Locuratolo, N.; Sicuro, M.; Masson, S.; Barlera, S.; Milani, V.; Lombardi, M.; et al. Cyclosporine A in Reperfused Myocardial Infarction: The Multicenter, Controlled, Open-Label CYCLE Trial. J. Am. Coll. Cardiol. 2016, 67, 365–374. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Martin, J.L.; Dixon, S.R.; Bartorelli, A.L.; Trabattoni, D.; Oemrawsingh, P.V.; Atsma, D.E.; Chang, M.; Marquardt, W.; Oh, J.K.; et al. Acute Myocardial Infarction with Hyperoxemic Therapy (AMIHOT): A prospective, randomized trial of intracoronary hyperoxemic reperfusion after percutaneous coronary intervention. J. Am. Coll. Cardiol. 2007, 50, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Martin, J.L.; de Boer, M.J.; Margheri, M.; Bramucci, E.; Blankenship, J.C.; Metzger, D.C.; Gibbons, R.J.; Lindsay, B.S.; Weiner, B.H.; et al. Effect of supersaturated oxygen delivery on infarct size after percutaneous coronary intervention in acute myocardial infarction. Circ. Cardiovasc. Interv. 2009, 2, 366–375. [Google Scholar] [CrossRef]
- Hofmann, R.; James, S.K.; Jernberg, T.; Lindahl, B.; Erlinge, D.; Witt, N.; Arefalk, G.; Frick, M.; Alfredsson, J.; Nilsson, L.; et al. Oxygen Therapy in Suspected Acute Myocardial Infarction. N. Engl. J. Med. 2017, 377, 1240–1249. [Google Scholar] [CrossRef]
- David, S.W.; Khan, Z.A.; Patel, N.C.; Metzger, D.C.; Wood, F.O.; Wasserman, H.S.; Lotfi, A.S.; Hanson, I.D.; Dixon, S.R.; LaLonde, T.A.; et al. Evaluation of intracoronary hyperoxemic oxygen therapy in acute anterior myocardial infarction: The IC-HOT study. Catheter. Cardiovasc. Interv. 2019, 93, 882–890. [Google Scholar] [CrossRef]
- Erlinge, D.; Gotberg, M.; Lang, I.; Holzer, M.; Noc, M.; Clemmensen, P.; Jensen, U.; Metzler, B.; James, S.; Botker, H.E.; et al. Rapid endovascular catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. The CHILL-MI trial: A randomized controlled study of the use of central venous catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. J. Am. Coll. Cardiol. 2014, 63, 1857–1865. [Google Scholar] [CrossRef]
- Nichol, G.; Strickland, W.; Shavelle, D.; Maehara, A.; Ben-Yehuda, O.; Genereux, P.; Dressler, O.; Parvataneni, R.; Nichols, M.; McPherson, J.; et al. Prospective, multicenter, randomized, controlled pilot trial of peritoneal hypothermia in patients with ST-segment- elevation myocardial infarction. Circ. Cardiovasc. Interv. 2015, 8, e001965. [Google Scholar] [CrossRef]
- Keeble, T.R.; Karamasis, G.V.; Noc, M.; Sredniawa, B.; Aradi, D.; Neskovic, A.N.; Arheden, H.; Erlinge, D.; Holzer, M. Effect of Intravascular Cooling on Microvascular Obstruction (MVO) in Conscious Patients with ST-Elevation Myocardial Infarction Undergoing Primary PCI: Results from the COOL AMI EU Pilot Study. Cardiovasc. Revasc. Med. 2019, 20, 799–804. [Google Scholar] [CrossRef]
- El Farissi, M.; Good, R.; Engstrom, T.; Oldroyd, K.G.; Karamasis, G.V.; Vlaar, P.J.; Lonborg, J.T.; Teeuwen, K.; Keeble, T.R.; Mangion, K.; et al. Safety of Selective Intracoronary Hypothermia During Primary Percutaneous Coronary Intervention in Patients with Anterior STEMI. JACC Cardiovasc. Interv. 2021, 14, 2047–2055. [Google Scholar] [CrossRef]
- Kitakaze, M.; Asakura, M.; Kim, J.; Shintani, Y.; Asanuma, H.; Hamasaki, T.; Seguchi, O.; Myoishi, M.; Minamino, T.; Ohara, T.; et al. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): Two randomised trials. Lancet 2007, 370, 1483–1493. [Google Scholar] [CrossRef]
- Lonborg, J.; Vejlstrup, N.; Kelbaek, H.; Botker, H.E.; Kim, W.Y.; Mathiasen, A.B.; Jorgensen, E.; Helqvist, S.; Saunamaki, K.; Clemmensen, P.; et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur. Heart J. 2012, 33, 1491–1499. [Google Scholar] [CrossRef]
- Woo, J.S.; Kim, W.; Ha, S.J.; Kim, J.B.; Kim, S.J.; Kim, W.S.; Seon, H.J.; Kim, K.S. Cardioprotective Effects of Exenatide in Patients with ST-Segment-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention Results of Exenatide Myocardial Protection in Revascularization Study. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2252–2260. [Google Scholar] [CrossRef]
- Roos, S.T.; Timmers, L.; Biesbroek, P.S.; Nijveldt, R.; Kamp, O.; van Rossum, A.C.; van Hout, G.P.J.; Stella, P.R.; Doevendans, P.A.; Knaapen, P.; et al. No benefit of additional treatment with exenatide in patients with an acute myocardial infarction. Int. J. Cardiol. 2016, 220, 809–814. [Google Scholar] [CrossRef]
- Iwakura, K.; Ito, H.; Okamura, A.; Koyama, Y.; Date, M.; Higuchi, Y.; Inoue, K.; Kimura, R.; Nagai, H.; Imai, M.; et al. Nicorandil Treatment in Patients with Acute Myocardial Infarction—A Meta-Analysis. Circ. J. 2009, 73, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, M.T.; Laarman, G.; van ‘t Hof, A.W.J.; Guagliumi, G.; Tonino, W.A.L.; Tavazzi, L.; Duncker, D.J.G.M.; Simoons, M.L.; Investigators, P.-M. The effect of ITF-1697 on reperfusion in patients undergoing primary angioplasty—Safety and efficacy of a novel tetrapeptide, ITF-1697. Eur. Heart J. 2004, 25, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Lonborg, J.; Holmvang, L.; Kelbaek, H.; Vejlstrup, N.; Jorgensen, E.; Helqvist, S.; Saunamaki, K.; Clemmensen, P.; Treiman, M.; Jensen, J.S.; et al. ST-Segment resolution and clinical outcome with ischemic postconditioning and comparison to magnetic resonance. Am. Heart J. 2010, 160, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Thuny, F.; Lairez, O.; Roubille, F.; Mewton, N.; Rioufol, G.; Sportouch, C.; Sanchez, I.; Bergerot, C.; Thibault, H.; Cung, T.T.; et al. Post-conditioning reduces infarct size and edema in patients with ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2012, 59, 2175–2181. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Patel, V.G.; Mayo, H.G.; de Lemos, J.A.; Brilakis, E.S.; Banerjee, S.; Bavry, A.A.; Bhatt, D.L.; Kumbhani, D.J. Surrogate and Clinical Outcomes Following Ischemic Postconditioning During Primary Percutaneous Coronary Intervention of ST-Segment Elevation Myocardial Infarction: A Meta-Analysis of 15 Randomized Trials. Catheter. Cardiovasc. Interv. 2014, 84, 978–986. [Google Scholar] [CrossRef]
- Engstrom, T.; Kelbaek, H.; Helqvist, S.; Hofsten, D.E.; Klovgaard, L.; Clemmensen, P.; Holmvang, L.; Jorgensen, E.; Pedersen, F.; Saunamaki, K.; et al. Effect of Ischemic Postconditioning During Primary Percutaneous Coronary Intervention for Patients with ST-Segment Elevation Myocardial Infarction A Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 490–497. [Google Scholar] [CrossRef]
- McLeod, S.L.; Iansavichene, A.; Cheskes, S. Remote Ischemic Perconditioning to Reduce Reperfusion Injury During Acute ST-Segment-Elevation Myocardial Infarction: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2017, 6, e005522. [Google Scholar] [CrossRef]
- Elbadawi, A.; Ha, L.D.; Abuzaid, A.S.; Crimi, G.; Azzouz, M.S. Meta-Analysis of Randomized Trials on Remote Ischemic Conditioning During Primary Percutaneous Coronary Intervention in Patients with ST-Segment Elevation Myocardial Infarction. Am. J. Cardiol. 2017, 119, 832–838. [Google Scholar] [CrossRef]
- Eitel, I.; Stiermaier, T.; Rommel, K.P.; Fuernau, G.; Sandri, M.; Mangner, N.; Linke, A.; Erbs, S.; Lurz, P.; Boudriot, E.; et al. Cardioprotection by combined intrahospital remote ischaemic perconditioning and postconditioning in ST-elevation myocardial infarction: The randomized LIPSIA CONDITIONING trial. Eur. Heart J. 2015, 36, 3049–3057. [Google Scholar] [CrossRef]
- Stiermaier, T.; Jensen, J.O.; Rommel, K.P.; de Waha-Thiele, S.; Fuernau, G.; Desch, S.; Thiele, H.; Eitel, I. Combined Intrahospital Remote Ischemic Perconditioning and Postconditioning Improves Clinical Outcome in ST-Elevation Myocardial Infarction. Circ. Res. 2019, 124, 1482–1491. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Kharbanda, R.K.; Moller, U.K.; Ramlall, M.; Aaroe, J.; Butler, R.; Bulluck, H.; Clayton, T.; Dana, A.; Dodd, M.; et al. Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): A single-blind randomised controlled trial. Lancet 2019, 394, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
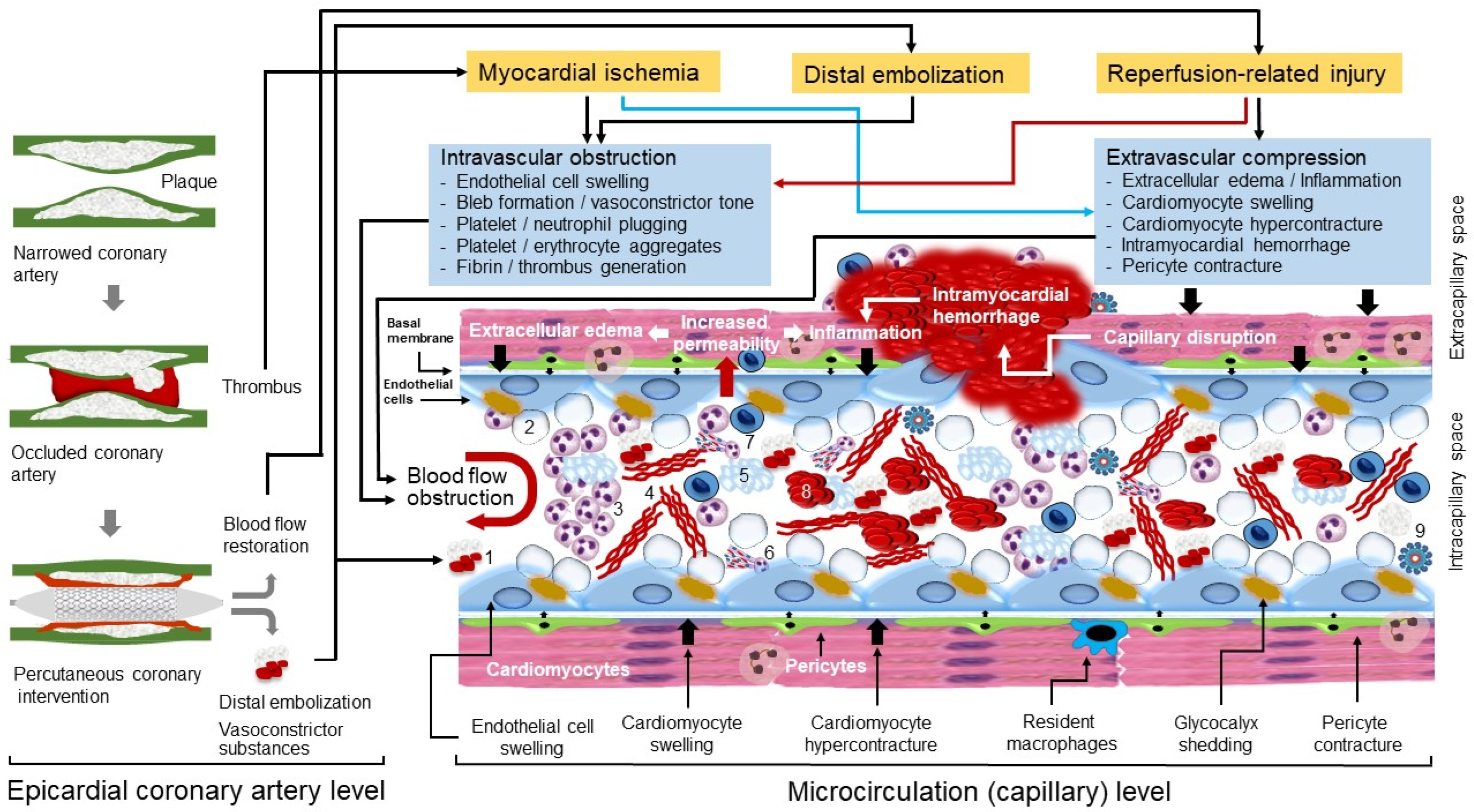
| Author (Year) | Patients | Reperfusion Strategy | Diagnostic Method/CNR Criteria | Frequency of CNR or MVO |
|---|---|---|---|---|
| Rezkalla et al. [170] (2010) | 347 patients with STEMI | Primary PCI | Coronary angiography TIMI < 3; MBG < 3 | 32% (TIMI) 57% (MBG) |
| Hamada et al. [176] (2001) | 104 patients with first AMI | Primary PCI with TIMI flow 3 restoration | Coronary angiography Slow (≤23) CTFC | 43% |
| van ‘t Hof et al. [178] (1998) | 777 patients with STEMI | Primary PCI | Coronary angiography MBG 0–1 | 30% |
| Henriques et al. [171] (2003) | 924 patients with STEMI | Primary PCI with TIMI flow 3 restoration | Coronary angiography MBG 0–1 | 11% |
| Appelbaum et al. [188] (2009) | 31 patients with STEMI | Primary PCI | Coronary angiography TMPG < 3 | 52% |
| Ndrepepa et al. [185] (2010) | 1406 patients with STEMI | Primary PCI | Coronary angiography TIMI < 3; TIMI 3 with TMPG 0–1 | 29% |
| Ito et al. [193] (1996) | 126 patients with first STEMI of anterior wall | Primary PCI or thrombolysis | MCE Residual contrast defects within area at risk | 37% |
| Galiuto et al. [133] (2003) | 24 patients with first STEMI | Primary PCI or thrombolysis | MCE Reduced or absent opacification | 66% |
| Taylor et al. [201] (2004) | 20 patients with STEMI | Primary PCI | CMR Delayed wash-in of gadolinium | 95% |
| De Waha et al. [202] (2017) | 1688 patients with STEMI | Primary PCI | CMR Late gadolinium enhancement | 57% |
| Author (Year) | Type of Study | Patients | Reperfusion Strategy | Coronary No-Reflow/Clinical Outcome |
|---|---|---|---|---|
| Stone et al. [109] (2005) | RT (PCI + distal protection or PCI alone) | 501 patients with STEMI | Primary PCI | Complete STR: 63% vs. 62% 6-month MACE: 10% vs.11% |
| Jolly et al. [288] (2015) | RT (PCI + aspiration thrombectomy or PCI alone) | 10,732 patients with STEMI | Primary PCI | CNR: 2.4% vs. 2.8% (p = 0.28) 6-month adverse events: 6.9% vs. 7.0% |
| Lønborg et al. [290] (2017) | RT (deferred or immediate stenting) | 510 patients with STEMI | Primary PCI | MVO: 43% vs. 42% (p = 0.78) Myocardial SI: 66% vs. 67% (p = 0.80) |
| Stone et al. [292] (2012) | RT (mesh-covered stent or conventional stents) | 433 patients with STEMI | Primary PCI | TIMI 3: 91.7% vs. 82.9% (p = 0.006) STR ≥ 70%: 57.8% vs. 44.7% (p = 0.008) MBG: 83.9% vs. 84.7% (p = 0.81) |
| van‘t Hof et al. [296] (2008) | RT (prehospital high-bolus dose tirofiban or placebo) | 936 patients with STEMI | Primary PCI | Complete STR (>70%): 65.6% vs. 60.0% (p = 0.08) |
| Eitel et al. [172] (2013) | RT (intracoronary or intravenous abciximab) | 2065 patients with STEMI | Primary PCI | Myocardial SI: 52% vs. 50% (p = 0.25) Late MVO: 49% vs. 47% (p = 0.19) IMH: 35% vs. 32% (p = 0.19) |
| Niccoli et al. [305] (2013) | RT (intracoronary adenosine or nitroprusside or placebo) | 240 patients with STEMI and TIMI grade 0–1 | Primary PCI | STR >70: 71% vs. 51% (adenosine vs. placebo; p = 0.009) Angiographic MVO: 18% vs. 30% (adenosine vs. placebo; p = 0.06) |
| Nazir et al. [307] (2016) | RT (intravenous adenosine or sodium nitroprusside or standard PCI) | 247 patients with STEMI | Primary PCI | STR >70%: 68.3% vs. 65.1% (p = 0.66) Late MVO: 68.3% vs. 56.9% (p = 0.205) for adenosine vs. standard PCI |
| Siddiqi et al. [309] (2014) | RT (sodium nitrite infusion or placebo) | 229 patients with STEMI | Primary PCI | Median infarct size at 6–8 days: 22% (nitrite) vs. 20% (placebo); (p = 0.30) |
| Roolvink et al. [319] (2016) | RT (intravenous metoprolol or placebo) | 683 patients with STEMI | Primary PCI | Infarct size by CMR: 15.3% vs. 14.9% (p = 0.616) |
| Kim et al. [320] (2010) | RT (80-mg or. 10-mg atorvastatin) | 171 patients with STEMI | Primary PCI | CTFC (80 mg vs.10 mg): 26.9 vs.34.1 (p= 0.01) MBG: 2.2 vs. 1.9 (p = 0.02) STR >70%: 39.5% vs. 23.8% (p = 0.03) |
| McCartney et al. [323] (2019) | RT (intracoronary alteplase 20 mg, alteplase 10 mg, or placebo) | 440 patients with STEMI | Primary PCI | MVO by CMR (3.5% (20 mg) vs. 2.6% (10 mg) vs. 2.3% (placebo): All p values =NS |
| Navarese et al. [326] (2021) | Observational study (intracoronary epinephrine or no epinephrine) | 30 patients with persistent CNR | Primary PCI | TIMI grade 3, 2 and 0–1: 28.6%, 64.3% 7.1%(epinephrine) and vs. 18.8%, 12.5% and 68.8% (no epinephrine); p = 0.004 |
| Stone et al. [340] (2009) | RT (intracoronary supersaturated oxygen or control) | 301 patients with STEMI of anterior wall | Primary PCI | Scintigraphic infarct size: 26.5% vs. 20% (adjusted p = 0.03) 30-day MACE: 5.4% vs. 3.8% (p = 0.77) |
| Roos et al. [350] (2016) | RT (intravenous exenatide or placebo) | 191 patients with STEMI | Primary PCI | Infarct size by CMR: 18.8% vs. 18.8% (p = 0.96) |
| Hausenloy et al. [361] (2019) | RT (remote ischemic conditioning or standard treatment) | 5401 patients with STEMI | Primary PCI | Death of hospitalization for heart failure: 8.6% vs. 9.4% (p = 0.32) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndrepepa, G.; Kastrati, A. Coronary No-Reflow after Primary Percutaneous Coronary Intervention—Current Knowledge on Pathophysiology, Diagnosis, Clinical Impact and Therapy. J. Clin. Med. 2023, 12, 5592. https://doi.org/10.3390/jcm12175592
Ndrepepa G, Kastrati A. Coronary No-Reflow after Primary Percutaneous Coronary Intervention—Current Knowledge on Pathophysiology, Diagnosis, Clinical Impact and Therapy. Journal of Clinical Medicine. 2023; 12(17):5592. https://doi.org/10.3390/jcm12175592
Chicago/Turabian StyleNdrepepa, Gjin, and Adnan Kastrati. 2023. "Coronary No-Reflow after Primary Percutaneous Coronary Intervention—Current Knowledge on Pathophysiology, Diagnosis, Clinical Impact and Therapy" Journal of Clinical Medicine 12, no. 17: 5592. https://doi.org/10.3390/jcm12175592
APA StyleNdrepepa, G., & Kastrati, A. (2023). Coronary No-Reflow after Primary Percutaneous Coronary Intervention—Current Knowledge on Pathophysiology, Diagnosis, Clinical Impact and Therapy. Journal of Clinical Medicine, 12(17), 5592. https://doi.org/10.3390/jcm12175592







