The Role of Early Serum Biomarkers and Clinical Rating Scales in the Prediction of Delayed Cerebral Ischaemia and Short-Term Outcome after Aneurysmal Subarachnoid Haemorrhage: Single Centre Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Consent to Participate
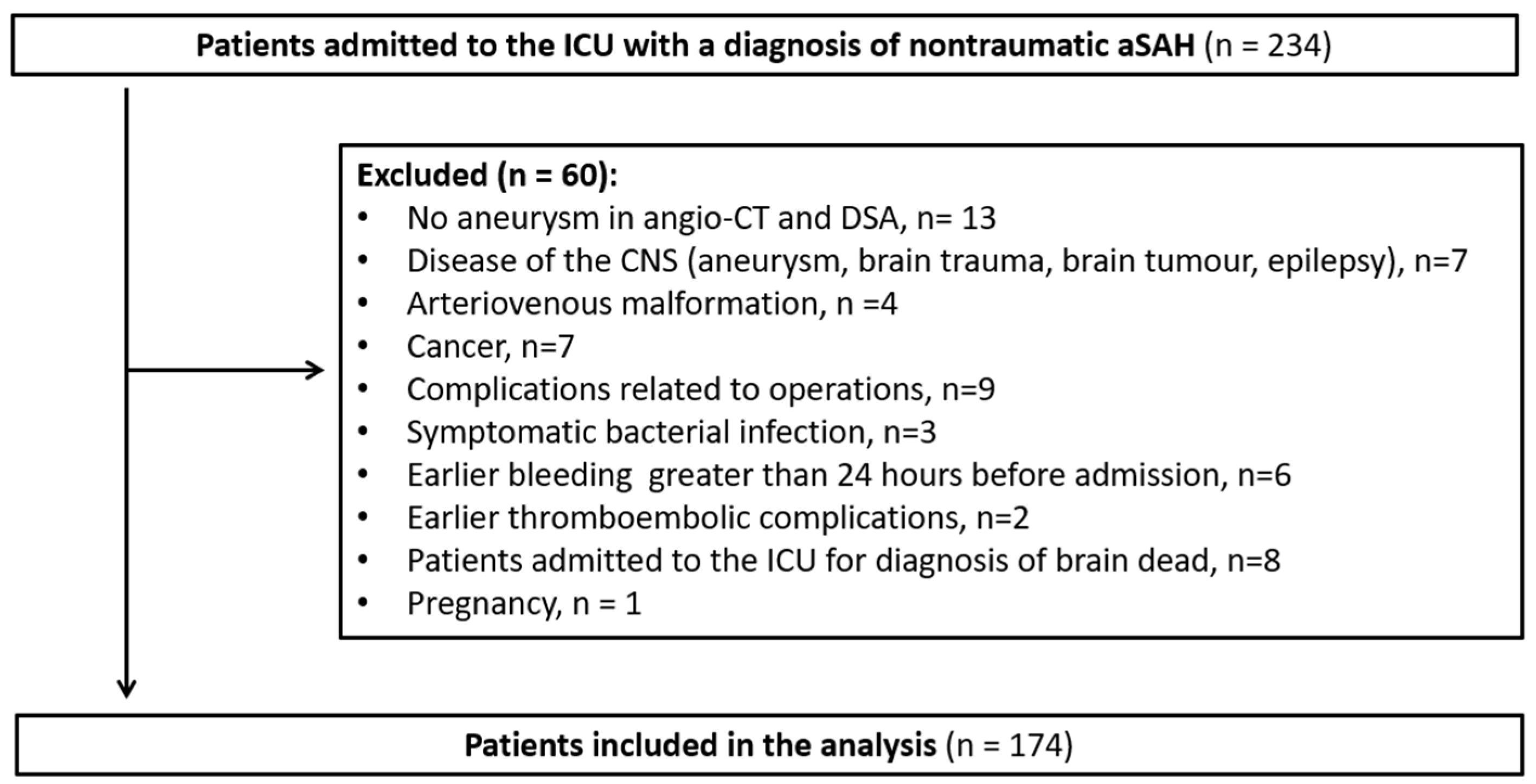
2.2. Study Population
2.3. Data Collection
2.4. Selection of Serum Biomarkers
2.5. Definitions and Endpoints
2.6. Statistical Methods
3. Results
3.1. Baseline Characteristics and Clinical Data
3.2. Comparison between Patients with Poor Outcome vs. Good Outcome
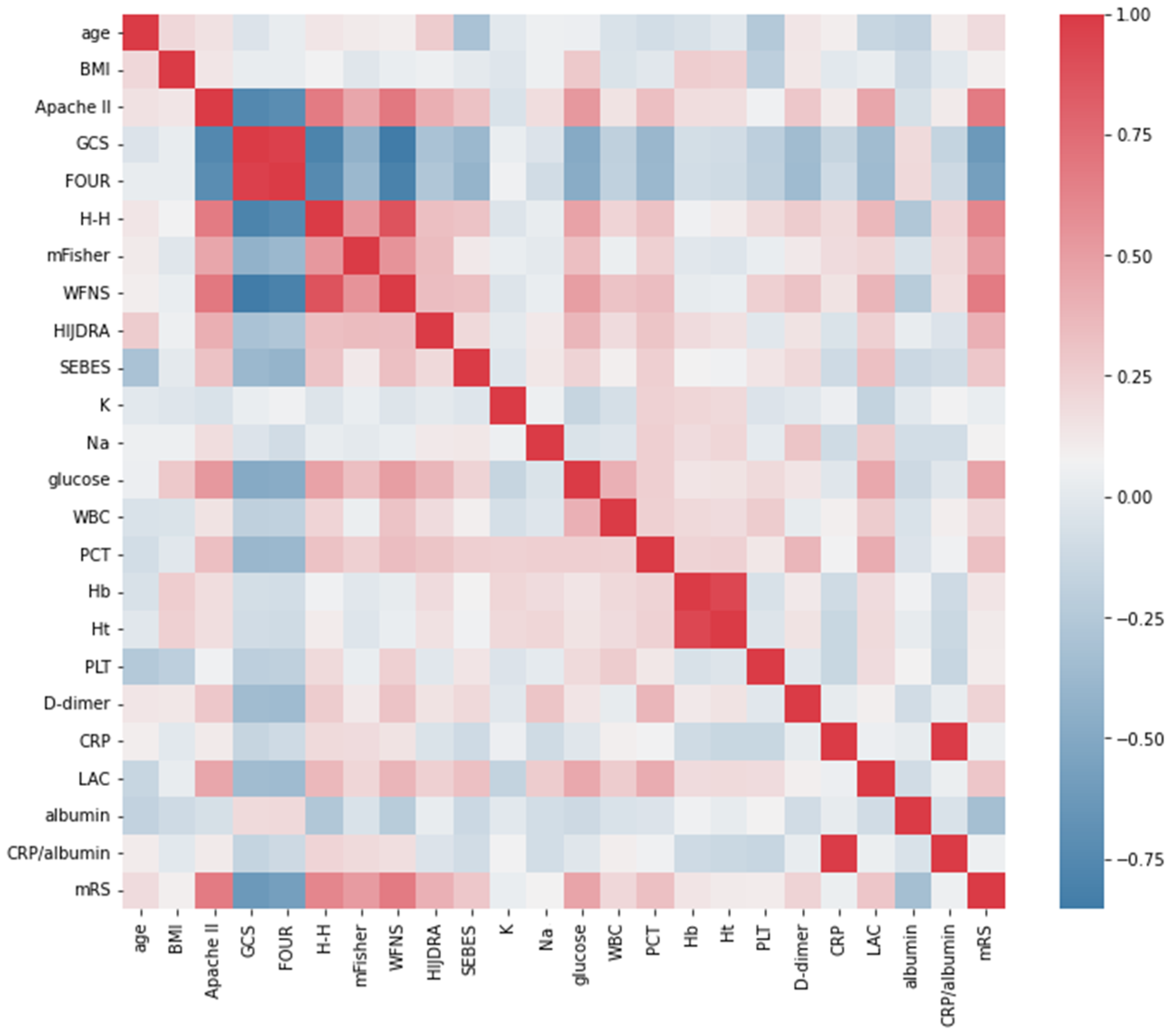
3.3. Correlation between Serum Biomarkers and Poor Outcome
3.4. SEBES vs. DCI Development and Outcome
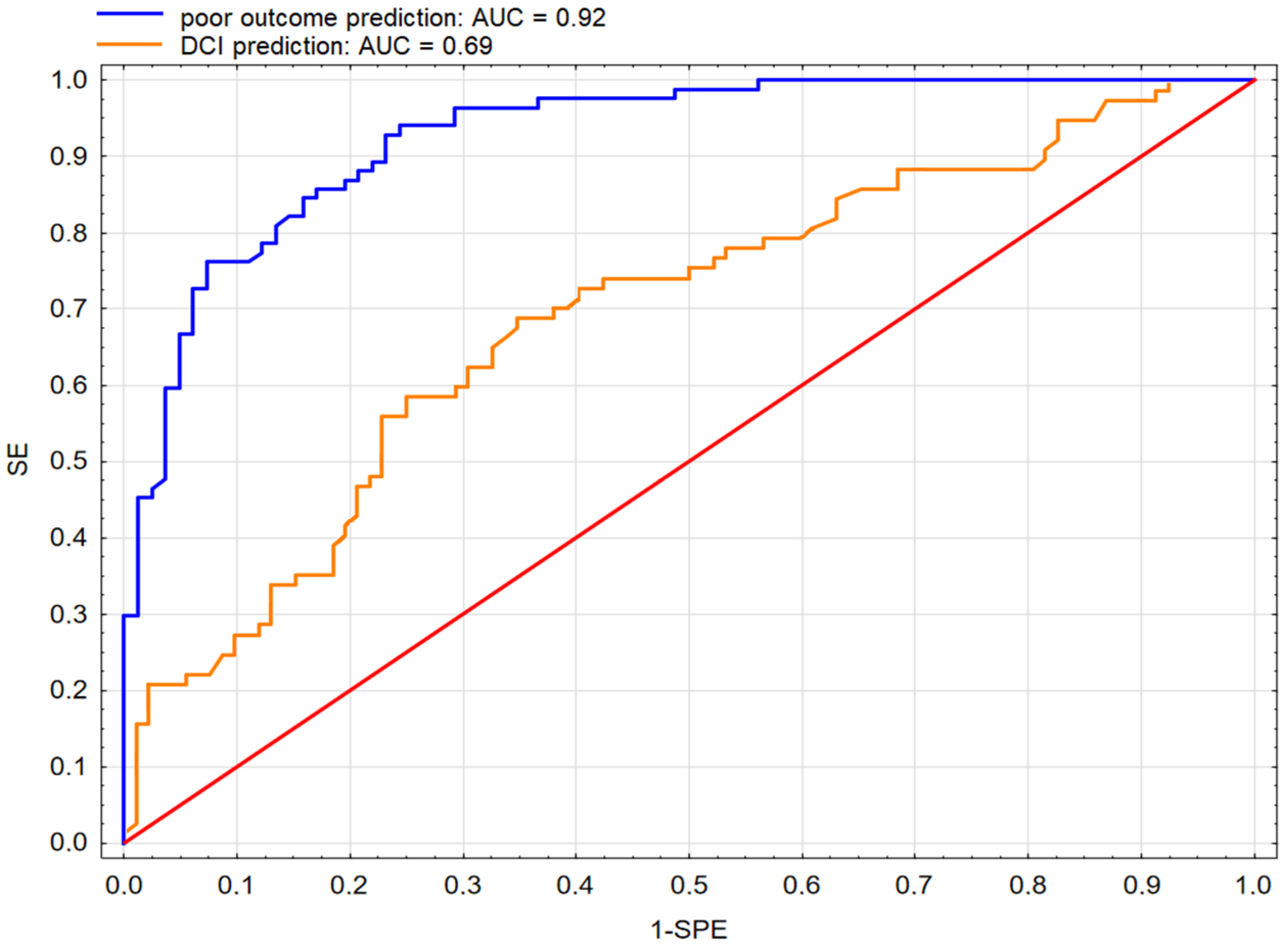
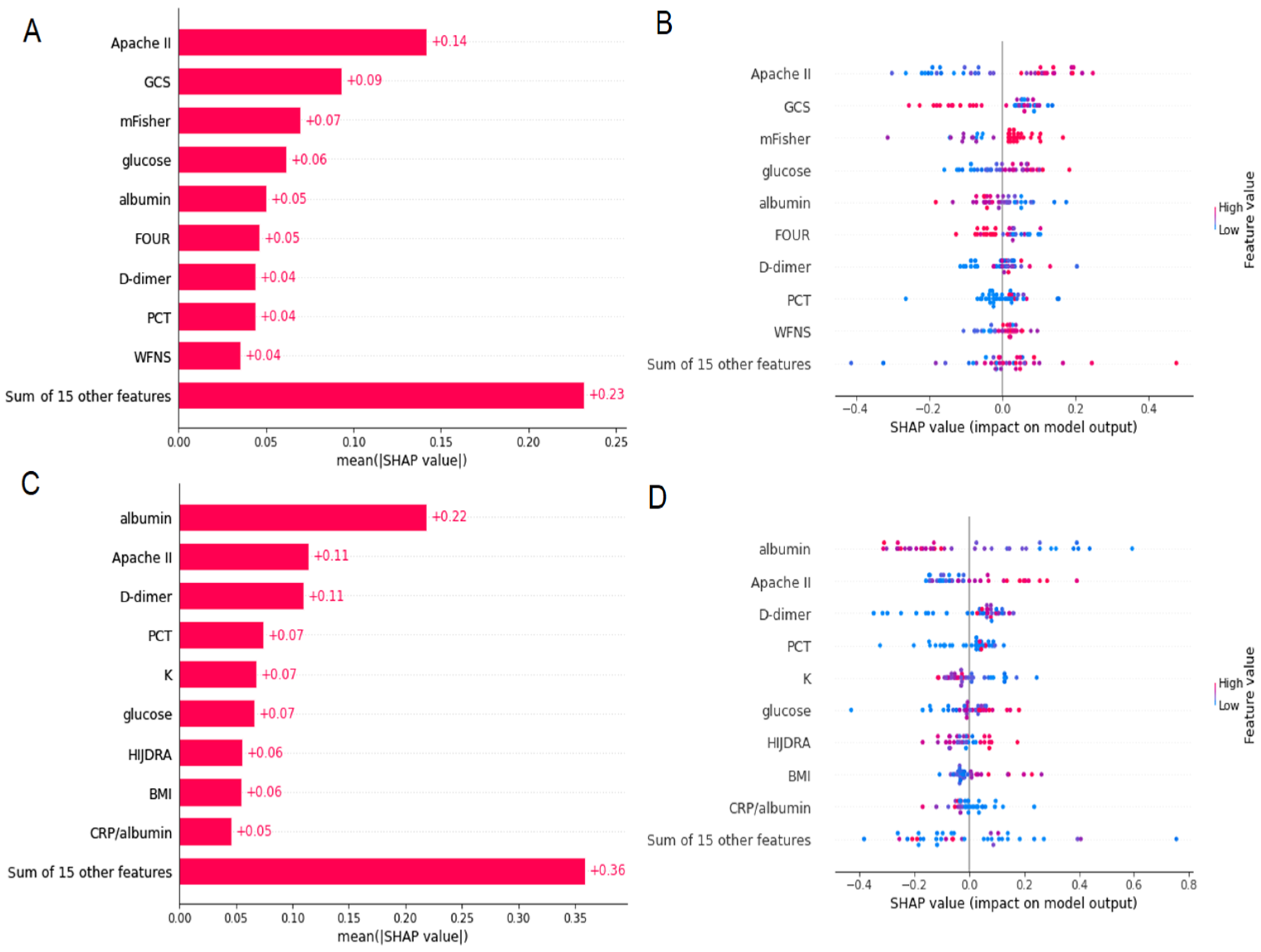
3.5. Prediction of Poor Outcome Using SM and ML Approach
3.6. Prediction of DCI Using SM and ML Approach
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suarez, J.I.; Tarr, R.W.; Selman, W.R. Aneurysmal Subarachnoid Hemorrhage. N. Engl. J. Med. 2006, 354, 387–396. [Google Scholar] [CrossRef]
- Rincon, F.; Rossenwasser, R.H.; Dumont, A. The Epidemiology of Admissions of Nontraumatic Subarachnoid Hemorrhage in the United States. Neurosurgery 2013, 73, 217–223. [Google Scholar] [CrossRef]
- La Pira, B.; Singh, T.D.; Rabinstein, A.A.; Lanzino, G. Time Trends in Outcomes After Aneurysmal Subarachnoid Hemorrhage Over the Past 30 Years. Mayo Clin. Proc. 2018, 93, P1786–P1793. [Google Scholar] [CrossRef] [PubMed]
- Etminan, N.; Chang, H.S.; Hackenberg, K.; De Rooij, N.K.; Vergouwen, M.D.I.; Rinkel, G.J.E.; Algra, A. Worldwide Incidence of Aneurysmal Subarachnoid Hemorrhage According to Region, Time Period, Blood Pressure, and Smoking Prevalence in the Population: A Systematic Review and Meta-Analysis. JAMA Neurol. 2019, 76, 588. [Google Scholar] [CrossRef]
- Macdonald, R.L.; Schweizer, T.A. Spontaneous Subarachnoid Haemorrhage. Lancet 2017, 389, 655–666. [Google Scholar] [CrossRef]
- Shah, V.A.; Kazmi, S.O.; Damani, R.; Harris, A.H.; Hohmann, S.F.; Calvillo, E.; Suarez, J.I. Regional Variability in the Care and Outcomes of Subarachnoid Hemorrhage Patients in the United States. Front. Neurol. 2022, 13, 1268. [Google Scholar] [CrossRef] [PubMed]
- Cossu, G.; Messerer, M.; Oddo, M.; Daniel, R.T. To Look beyond Vasospasm in Aneurysmal Subarachnoid Haemorrhage. Biomed. Res. Int. 2014, 2014, 628597. [Google Scholar] [CrossRef]
- Sehba, F.A.; Hou, J.; Pluta, R.M.; Zhang, J.H. The Importance of Early Brain Injury after Subarachnoid Hemorrhage. Prog. Neurobiol. 2012, 97, 14–37. [Google Scholar] [CrossRef] [PubMed]
- Dodd, W.S.; Laurent, D.; Dumont, A.S.; Hasan, D.M.; Jabbour, P.M.; Starke, R.M.; Hosaka, K.; Polifka, A.J.; Hoh, B.L.; Chalouhi, N. Pathophysiology of Delayed Cerebral Ischemia After Subarachnoid Hemorrhage: A Review. J. Am. Heart Assoc. 2021, 10, 21845. [Google Scholar] [CrossRef]
- McMahon, C.J.; Hopkins, S.; Vail, A.; King, A.T.; Smith, D.; Illingworth, K.J.; Clark, S.; Rothwell, N.J.; Tyrrell, P.J. Inflammation as a Predictor for Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Haemorrhage. J. Neurointerv. Surg. 2013, 5, 512–517. [Google Scholar] [CrossRef]
- Hong, C.M.; Tosun, C.; Kurland, D.B.; Gerzanich, V.; Schreibman, D.; Simard, J.M. Biomarkers as Outcome Predictors in Subarachnoid Hemorrhage-a Systematic Review. Biomarkers 2014, 19, 95–108. [Google Scholar] [CrossRef]
- Lucke-Wold, B.P.; Logsdon, A.F.; Manoranjan, B.; Turner, R.C.; McConnell, E.; Vates, G.E.; Huber, J.D.; Rosen, C.L.; Simard, J.M. Aneurysmal Subarachnoid Hemorrhage and Neuroinflammation: A Comprehensive Review. Int. J. Mol. Sci. 2016, 17, 497. [Google Scholar] [CrossRef] [PubMed]
- Mathieson, T.; Edner, G.; Ulfarsson, E.; Andersson, B. Cerebrospinal Fluid Interleukin-1 Receptor Antagonist and Tumor Necrosis Factor-Alpha Following Subarachnoid Hemorrhage. J. Neurosurg. 1997, 87, 215–220. [Google Scholar] [CrossRef]
- Gruber, A.; Rössler, K.; Graninger, W.; Donner, A.; Illievich, U.M.; Czech, T. Ventricular Cerebrospinal Fluid and Serum Concentrations of STNFR-I, IL-1ra, and IL-6 after Aneurysmal Subarachnoid Hemorrhage. J. Neurosurg. Anesthesiol. 2000, 12, 297–306. [Google Scholar] [CrossRef]
- Veldeman, M.; Lepore, D.; Höllig, A.; Clusmann, H.; Stoppe, C.; Schubert, G.A.; Albanna, W. Procalcitonin in the Context of Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage. J. Neurosurg. 2020, 135, 29–37. [Google Scholar] [CrossRef]
- Chen, Y.; Lian, B.Q.; Peng, L.; Ding, C.Y.; Lin, Y.X.; Yu, L.H.; Wang, D.L.; Kang, D.Z. Neutrophil to Lymphocyte Ratio Is a Prognosis Factor for Post-Operative Pneumonia in Aneurysmal Subarachnoid Hemorrhage Patients. Chin. Med. J. 2020, 134, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Burzyńska, M.; Uryga, A.; Kasprowicz, M.; Czosnyka, M.; Dragan, B.; Kübler, A. The Relationship between the Time of Cerebral Desaturation Episodes and Outcome in Aneurysmal Subarachnoid Haemorrhage: A Preliminary Study. J. Clin. Monit. Comput. 2020, 34, 705. [Google Scholar] [CrossRef] [PubMed]
- Bederson, J.B.; Connolly, E.S.; Batjer, H.H.; Dacey, R.G.; Dion, J.E.; Diringer, M.N.; Duldner, J.E.; Harbaugh, R.E.; Patel, A.B.; Rosenwasser, R.H. Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage: A Statement for Healthcare Professionals from a Special Writing Group of the Stroke Council, American Heart Association. Stroke 2009, 40, 994–1025. [Google Scholar] [CrossRef]
- Diringer, M.N.; Bleck, T.P.; Hemphill, J.C.; Menon, D.; Shutter, L.; Vespa, P.; Bruder, N.; Connolly, E.S.; Citerio, G.; Gress, D.; et al. Critical Care Management of Patients Following Aneurysmal Subarachnoid Hemorrhage: Recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit. Care 2011, 15, 211–240. [Google Scholar] [CrossRef]
- Connolly, E.S.; Rabinstein, A.A.; Carhuapoma, J.R.; Derdeyn, C.P.; Dion, J.; Higashida, R.T.; Hoh, B.L.; Kirkness, C.J.; Naidech, A.M.; Ogilvy, C.S.; et al. Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2012, 43, 1711–1737. [Google Scholar] [CrossRef]
- Hemphill, J.C.; Greenberg, S.M.; Anderson, C.S.; Becker, K.; Bendok, B.R.; Cushman, M.; Fung, G.L.; Goldstein, J.N.; MacDonald, R.L.; Mitchell, P.H.; et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage. Stroke 2015, 46, 2032–2060. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A Practical Scale. Lancet 1974, 304, 81–84. [Google Scholar] [CrossRef]
- Wijdicks, E.F.M.; Bamlet, W.R.; Maramattom, B.V.; Manno, E.M.; McClelland, R.L. Validation of a New Coma Scale: The FOUR Score. Ann. Neurol. 2005, 58, 585–593. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P. Zimmerman JE APACHE II: A Severity of Disease Classification System. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Hunt, W.E.; Hess, R.M. Surgical Risk as Related to Time of Intervention in the Repair of Intracranial Aneurysms. J. Neurosurg. 1968, 28, 14–20. [Google Scholar] [CrossRef]
- Report of World Federation of Neurological Surgeons Committee on a Universal Subarachnoid Hemorrhage Grading Scale. J. Neurosurg. 1988, 68, 985–986. [CrossRef]
- Fisher, C.M.; Kistler, J.P.; Davis, J.M. Relation of Cerebral Vasospasm to Subarachnoid Hemorrhage Visualized by Computerized Tomographic Scanning. Neurosurgery 1980, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hijdra, A.; Brouwers, P.; Vermeulen, M.; Gijn, J. Van Grading the Amount of Blood on Computed Tomograms after Subarachnoid Hemorrhage. Stroke 1990, 21, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Savarraj, J.P.; Pervez, M.; Jones, W.; Park, J.; Jeon, S.B.; Kwon, S.U.; Chang, T.R.; Lee, K.; Kim, D.H.; et al. The Subarachnoid Hemorrhage Early Brain Edema Score Predicts Delayed Cerebral Ischemia and Clinical Outcomes. Neurosurgery 2018, 83, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Rass, V.; Helbok, R. Early Brain Injury After Poor-Grade Subarachnoid Hemorrhage. Curr. Neurol. Neurosci. Rep. 2019, 19, 78. [Google Scholar] [CrossRef]
- Amodio, S.; Bouzat, P.; Robba, C.; Taccone, F.S. Rethinking Brain Injury after Subarachnoid Hemorrhage. Crit. Care 2020, 24, 612. [Google Scholar] [CrossRef]
- Vergouwen, M.D.I.; Vermeulen, M.; van Gijn, J.; Rinkel, G.J.E.; Wijdicks, E.F.; Muizelaar, J.P.; Mendelow, A.D.; Juvela, S.; Yonas, H.; Terbrugge, K.G.; et al. Definition of Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage as an Outcome Event in Clinical Trials and Observational Studies: Proposal of a Multidisciplinary Research Group. Stroke 2010, 41, 2391–2395. [Google Scholar] [CrossRef]
- Vergouwen, M.D.I. Vasospasm versus Delayed Cerebral Ischemia as an Outcome Event in Clinical Trials and Observational Studies. Neurocrit. Care 2011, 15, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.F.; Westover, M.B.; Gaspard, N.; Gilmore, E.J.; Foreman, B.P.; O’Connor, K.L.; Rosenthal, E.S. Inter-Rater Agreement for Consensus Definitions of Delayed Ischemic Events Following Aneurysmal Subarachnoid Hemorrhage. J. Clin. Neurophysiol. 2016, 33, 235. [Google Scholar] [CrossRef] [PubMed]
- Malinova, V.; Dolatowski, K.; Schramm, P.; Moerer, O.; Rohde, V.; Mielke, D. Early Whole-Brain CT Perfusion for Detection of Patients at Risk for Delayed Cerebral Ischemia after Subarachnoid Hemorrhage. J. Neurosurg. 2016, 125, 128–136. [Google Scholar] [CrossRef]
- Rankin, J. Cerebral Vascular Accidents in Patients over the Age of 60. II. Prognosis. Scott. Med. J. 1957, 2, 200–215. [Google Scholar] [CrossRef]
- Van der Steen, W.E.; Leemans, E.L.; van den Berg, R.; Roos, Y.B.W.E.M.; Marquering, H.A.; Verbaan, D.; Majoie, C.B.L.M. Radiological Scales Predicting Delayed Cerebral Ischemia in Subarachnoid Hemorrhage: Systematic Review and Meta-Analysis. Neuroradiology 2019, 61, 247–256. [Google Scholar] [CrossRef]
- Raatikainen, E.; Vahtera, A.; Kuitunen, A.; Junttila, E.; Huhtala, H.; Ronkainen, A.; Pyysalo, L.; Kiiski, H. Prognostic Value of the 2010 Consensus Definition of Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage. J. Neurol. Sci. 2021, 420, 117261. [Google Scholar] [CrossRef]
- Lee, V.H.; Ouyang, B.; John, S.; Conners, J.J.; Garg, R.; Bleck, T.P.; Temes, R.E.; Cutting, S.; Prabhakaran, S. Risk Stratification for the In-Hospital Mortality in Subarachnoid Hemorrhage: The HAIR Score. Neurocrit. Care 2014, 21, 14–19. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Souza, N.V.; Rouanet, C.; Solla, D.J.F.; de Lima, C.V.B.; de Souza, C.A.; Rezende, F.; Alves, M.M.; de Oliveira Manuel, A.L.; Chaddad Neto, F.; Frudit, M.; et al. The Role of VASOGRADE as a Simple Grading Scale to Predict Delayed Cerebral Ischemia and Functional Outcome After Aneurysmal Subarachnoid Hemorrhage. Neurocrit. Care 2023, 38, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Hostettler, I.C.; Sebök, M.; Ambler, G.; Muroi, C.; Prömmel, P.; Neidert, M.C.; Richter, J.K.; Pangalu, A.; Regli, L.; Germans, M.R. Validation and Optimization of Barrow Neurological Institute Score in Prediction of Adverse Events and Functional Outcome After Subarachnoid Hemorrhage-Creation of the HATCH (Hemorrhage, Age, Treatment, Clinical State, Hydrocephalus) Score. Neurosurgery 2020, 88, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.J.; Mei, S.H.; Lu, J.N.; Chen, Y.K.; Chai, Z.H.; Dong, X.; Araujo, C.; Reis, C.; Zhang, J.M.; Chen, S. New Risk Score of the Early Period after Spontaneous Subarachnoid Hemorrhage: For the Prediction of Delayed Cerebral Ischemia. CNS Neurosci. Ther. 2019, 25, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Simon-Pimmel, J.; Foucher, Y.; Léger, M.; Feuillet, F.; Bodet-Contentin, L.; Cinotti, R.; Frasca, D.; Dantan, E. Original Research: Methodological Quality of Multivariate Prognostic Models for Intracranial Haemorrhages in Intensive Care Units: A Systematic Review. BMJ Open 2021, 11, e047279. [Google Scholar] [CrossRef] [PubMed]
- Hostettler, I.C.; Pavlou, M.; Ambler, G.; Alg, V.S.; Bonner, S.; Walsh, D.C.; Bulters, D.; Kitchen, N.; Brown, M.M.; Grieve, J.; et al. Assessment of the Subarachnoid Hemorrhage International Trialists (SAHIT) Models for Dichotomized Long-Term Functional Outcome Prediction After Aneurysmal Subarachnoid Hemorrhage in a United Kingdom Multicenter Cohort Study. Neurosurgery 2020, 87, 1269–1276. [Google Scholar] [CrossRef]
- De Rooij, N.K.; Greving, J.P.; Rinkel, G.J.E.; Frijns, C.J.M. Early Prediction of Delayed Cerebral Ischemia after Subarachnoid Hemorrhage: Development and Validation of a Practical Risk Chart. Stroke 2013, 44, 1288–1294. [Google Scholar] [CrossRef]
- Yin, L.; Ma, C.Y.; Li, Z.K.; Wang, D.D.; Bai, C.M. Predictors Analysis of Symptomatic Cerebral Vasospasm after Subarachnoid Hemorrhage. Acta Neurochir. Suppl. 2011, 110, 175–178. [Google Scholar] [CrossRef]
- Park, S.K.; Chun, H.J.; Kim, D.W.; Im, T.H.; Hong, H.J.; Yi, H.J. Acute Physiology and Chronic Health Evaluation II and Simplified Acute Physiology Score II in Predicting Hospital Mortality of Neurosurgical Intensive Care Unit Patients. J. Korean Med. Sci. 2009, 24, 420–426. [Google Scholar] [CrossRef]
- Akavipat, P.; Thinkhamrop, J.; Thinkhamrop, B.; Sriraj, W. Acute Physiology and Chronic Health Evaluation (APACHE) II Score—The Clinical Predictor in Neurosurgical Intensive Care Unit. Acta Clin. Croat. 2019, 58, 50–56. [Google Scholar] [CrossRef]
- Coulibaly, A.P.; Provencio, J.J. Aneurysmal Subarachnoid Hemorrhage: An Overview of Inflammation-Induced Cellular Changes. Neurotherapeutics 2020, 17, 436. [Google Scholar] [CrossRef]
- Schneider, U.C.; Xu, R.; Vajkoczy, P. Inflammatory Events Following Subarachnoid Hemorrhage (SAH). Curr. Neuropharmacol. 2018, 16, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.A.; Turan, N.; Chau, M.; Pradilla, G. Inflammation, Vasospasm, and Brain Injury after Subarachnoid Hemorrhage. Biomed. Res. Int. 2014, 2014, 384342. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Burkett, A.; Paz, A.; Savarraj, J.P.; Hinds, S.; Hergenroeder, G.; Gusdon, A.M.; Ren, X.; Hong, J.H.; Choi, H.A. Systemic Inflammatory Markers of Persistent Cerebral Edema after Aneurysmal Subarachnoid Hemorrhage. J. Neuroinflamm. 2022, 19, 199. [Google Scholar] [CrossRef] [PubMed]
- Hurth, H.; Birkenhauer, U.; Steiner, J.; Schlak, D.; Hennersdorf, F.; Ebner, F.H. Delayed Cerebral Ischemia in Patients with Aneurysmal Subarachnoid Hemorrhage—Serum D-Dimer and C-Reactive Protein as Early Markers. J. Stroke Cerebrovasc. Dis. 2020, 29, 104558. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhao, Y.; Chen, X.; Hao, Q. Predictive Values of White Blood Cell Count in Peripheral Blood at Admission on In-Hospital Complications and 90-Day Outcomes of Patients with Aneurysmal Subarachnoid Hemorrhage: Insights from the LongTEAM Registry. J. Inflamm. Res. 2022, 15, 6481–6494. [Google Scholar] [CrossRef]
- O’Connor, E.; Venkatesh, B.; Mashongonyika, C.; Lipman, J.; Hall, J.; Thomas, P. Serum Procalcitonin and C-Reactive Protein as Markers of Sepsis and Outcome in Patients with Neurotrauma and Subarachnoid Haemorrhage. Anaesth. Intensive Care 2004, 32, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Taccone, F.S.; Badenes, R.; Arib, S.; Rubulotta, F.; Mirek, S.; Franchi, F.; Gordon, S.; Nadji, A.; Crippa, I.A.; Stazi, E.; et al. Cerebrospinal Fluid Glucose and Lactate Levels After Subarachnoid Hemorrhage: A Multicenter Retrospective Study. J. Neurosurg. Anesthesiol. 2020, 32, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Güresir, E.; Coch, C.; Fimmers, R.; Ilic, I.; Hadjiathanasiou, A.; Kern, T.; Brandecker, S.; Güresir, Á.; Velten, M.; Vatter, H.; et al. Initial Inflammatory Response Is an Independent Predictor of Unfavorable Outcome in Patients with Good-Grade Aneurysmal Subarachnoid Hemorrhage. J. Crit. Care 2020, 60, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Kasius, K.M.; Frijns, C.J.M.; Algra, A.; Rinkel, G.J.E. Association of Platelet and Leukocyte Counts with Delayed Cerebral Ischemia in Aneurysmal Subarachnoid Hemorrhage. Cerebrovasc. Dis. 2010, 29, 576–583. [Google Scholar] [CrossRef]
- Van Donkelaar, C.E.; Dijkland, S.A.; Van Den Bergh, W.M.; Bakker, J.; Dippel, D.W.; Nijsten, M.W.; Van Der Jagt, M. Early Circulating Lactate and Glucose Levels After Aneurysmal Subarachnoid Hemorrhage Correlate with Poor Outcome and Delayed Cerebral Ischemia: A Two-Center Cohort Study. Crit. Care Med. 2016, 44, 966–972. [Google Scholar] [CrossRef]
- Oh, C.H.; Kim, J.W.; Kim, G.H.; Lee, K.R.; Hong, D.Y.; Park, S.O.; Baek, K.J.; Kim, S.Y. Serum Lactate Could Predict Mortality in Patients with Spontaneous Subarachnoid Hemorrhage in the Emergency Department. Front. Neurol. 2020, 11, 975. [Google Scholar] [CrossRef]
- Lehmann, F.; Schenk, L.M.; Schneider, M.; Bernstock, J.D.; Bode, C.; Borger, V.; Gessler, F.; Güresir, E.; Hadjiathanasiou, A.; Hamed, M.; et al. Predictive Relevance of Baseline Lactate and Glucose Levels in Patients with Spontaneous Deep-Seated Intracerebral Hemorrhage. Brain Sci. 2021, 11, 633. [Google Scholar] [CrossRef]
- Aisiku, I.P.; Chen, P.R.; Truong, H.; Monsivais, D.R.; Edlow, J. Admission Serum Lactate Predicts Mortality in Aneurysmal Subarachnoid Hemorrhage. Am. J. Emerg. Med. 2016, 34, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Djangang, N.N.; Ramunno, P.; Izzi, A.; Garufi, A.; Menozzi, M.; Diaferia, D.; Peluso, L.; Prezioso, C.; Talamonti, M.; Njimi, H.; et al. The Prognostic Role of Lactate Concentrations after Aneurysmal Subarachnoid Hemorrhage. Brain Sci. 2020, 10, 1004. [Google Scholar] [CrossRef]
- Shang, F.; Zhao, H.; Cheng, W.; Qi, M.; Wang, N.; Qu, X. Predictive Value of the Serum Albumin Level on Admission in Patients with Spontaneous Subarachnoid Hemorrhage. Front. Surg. 2021, 8, 502. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Dhandapani, S.; Gaudihalli, S.; Dhandapani, M.; Singh, H.; Mukherjee, K.K. Serum Albumin Level in Spontaneous Subarachnoid Haemorrhage: More than a Mere Nutritional Marker! Br. J. Neurosurg. 2018, 32, 47–52. [Google Scholar] [CrossRef]
- Ibrahim, G.M.; Macdonald, R.L. The Network Topology of Aneurysmal Subarachnoid Haemorrhage. J. Neurol. Neurosurg. Psychiatry 2015, 86, 895–901. [Google Scholar] [CrossRef]
- Nicholson, J.P.; Wolmarans, M.R.; Park, G.R. The Role of Albumin in Critical Illness. Br. J. Anaesth. 2000, 85, 599–610. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, Y.; Wang, P.; Wang, X.; Nie, X.Q.; Yao, W.; Li, T.; Chen, L.; Chong, W.; Hai, Y.; et al. Association between Hyperglycemia at Admission and Mortality in Aneurysmal Subarachnoid Hemorrhage. J. Clin. Neurosci. 2022, 103, 172–179. [Google Scholar] [CrossRef]
- Alberti, O.; Becker, R.; Benes, L.; Wallenfang, T.; Bertalanffy, H. Initial Hyperglycemia as an Indicator of Severity of the Ictus in Poor-Grade Patients with Spontaneous Subarachnoid Hemorrhage. Clin. Neurol. Neurosurg. 2000, 102, 78–83. [Google Scholar] [CrossRef]
- Okazaki, T.; Hifumi, T.; Kawakita, K.; Shishido, H.; Ogawa, D.; Okauchi, M.; Shindo, A.; Kawanishi, M.; Tamiya, T.; Kuroda, Y. Blood Glucose Variability: A Strong Independent Predictor of Neurological Outcomes in Aneurysmal Subarachnoid Hemorrhage. J. Intensive Care Med. 2018, 33, 189–195. [Google Scholar] [CrossRef]
- Schlenk, F.; Vajkoczy, P.; Sarrafzadeh, A. Inpatient Hyperglycemia Following Aneurysmal Subarachnoid Hemorrhage: Relation to Cerebral Metabolism and Outcome. Neurocrit. Care 2009, 11, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhang, T.B.; Li, X.F.; Zhang, Z.Y.; Li, Z.J.; Wang, X.L.; Zhao, W.Y. The Prognostic Value of Hyperglycemia in Aneurysmal Subarachnoid Hemorrhage: A Systematic Review and Meta-Analysis. Neurosurg. Rev. 2022, 45, 3717–3728. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.A.; Fernandez, A.; Claassen, J.; Schmidt, M.; Schumacher, H.C.; Wartenberg, K.; Temes, R.; Parra, A.; Ostapkovich, N.D.; Mayer, S.A. Hyperglycemia after SAH: Predictors, Associated Complications, and Impact on Outcome. Stroke 2006, 37, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Dhar, R.; Diringer, M.N. The Burden of the Systemic Inflammatory Response Predicts Vasospasm and Outcome after Subarachnoid Hemorrhage. Neurocrit. Care 2008, 8, 404–412. [Google Scholar] [CrossRef]
- Zheng, K.; Zhong, M.; Zhao, B.; Chen, S.Y.; Tan, X.X.; Li, Z.Q.; Xiong, Y.; Duan, C. Poor-Grade Aneurysmal Subarachnoid Hemorrhage: Risk Factors Affecting Clinical Outcomes in Intracranial Aneurysm Patients in a Multi-Center Study. Front. Neurol. 2019, 10, 123. [Google Scholar] [CrossRef]
- Zheng, Y.K.; Dong, X.Q.; Du, Q.; Wang, H.; Yang, D.B.; Zhu, Q.; Che, Z.H.; Shen, Y.F.; Jiang, L.; Hu, W.; et al. Comparison of Plasma Copeptin and Multiple Biomarkers for Assessing Prognosis of Patients with Aneurysmal Subarachnoid Hemorrhage. Clin. Chim. Acta 2017, 475, 64–69. [Google Scholar] [CrossRef]
| Characteristic | Total N = 174 | mRS 0–2 n = 85 | mRS 3–6 n = 89 | p |
|---|---|---|---|---|
| Age | 56 ± 22 | 52 ± 19 | 61 ± 24 | 0.005 |
| Female | 105 (60%) | 53 (62%) | 52 (58%) | 0.597 |
| BMI | 25.8 ± 6.4 | 25.7 ±5.8 | 26.3 ± 6.3 | 0.404 |
| APACHE II | 15 ± 12 | 11 ±6 | 22 ± 10 | <0.001 |
| GCS | 11 ± 9 | 14 ±3 | 6 ± 7 | <0.001 |
| FOUR Score | 14 ± 9 | 16 ±2 | 8 ± 9 | <0.001 |
| Comorbidities | ||||
| Hypertension | 85 (49%) | 43 (51%) | 42 (47%) | 0.654 |
| Current smoker | 75 (43%) | 36 (42%) | 39 (44%) | 0.845 |
| Diabetes mellitus, n (%) | 10 (6%) | 4 (5%) | 6 (7%) | 0.402 |
| Other | 15 (9%) | 10 (12%) | 5 (5%) | 0.120 |
| Aneurysm | ||||
| ICA | 46 (26%) | 22 (26%) | 24 (27%) | 0.871 |
| MCA | 48 (28%) | 22 (26%) | 26 (29%) | 0.623 |
| ACA | 12 (7%) | 9 (11%) | 3 (3%) | 0.060 |
| ACoA | 49 (28%) | 23 (27%) | 26 (29%) | 0.752 |
| Posterior | 15 (9%) | 6 (7%) | 9 (10%) | 0.329 |
| Size: small | 145 (83) | 75 (88%) | 70 (79%) | 0.112 |
| Size: medium | 26 (15%) | 9 (11%) | 17 (19%) | 0.092 |
| Size: large | 3 (2%) | 1 (1%) | 2 (2%) | 0.570 |
| Multiple aneurysms | 54 (31%) | 26 (31%) | 28 (31%) | 0.901 |
| Clinical assessment | ||||
| H-H | 3 ± 3 | 3 ± 1 | 4 ± 2 | <0.001 |
| Grade I–III | 92 (53%) | 67 (79%) | 25 (27%) | <0.001 |
| Grade IV–V | 82 (47%) | 18 (21%) | 64 (73%) | |
| mFisher | 4 ± 1 | 3 ± 2 | 4 | <0.001 |
| HIJDRA | 18 ± 20 | 12 ± 21 | 23 ± 17 | <0.001 |
| SEBES | 2 ± 4 | 1 ± 3 | 3 ± 3 | <0.001 |
| Grade 0–2 | 100 (57%) | 62 (73%) | 38 (43%) | <0.001 |
| Grade 3–4 | 74 (43%) | 23 (27%) | 51 (57%) | |
| WFNS | 4 ± 3 | 2 ± 2 | 5 ± 1 | <0.001 |
| Grade I–III | 87 (50%) | 65 (76%) | 22 (25%) | <0.001 |
| Grade IV–V | 87 (50%) | 20 (24%) | 67 (75%) | |
| CV in CTA | 21 (13%) | 10 (12%) | 11 (14%) | 0.792 |
| Aneurysm treatment | ||||
| Clipping | 102 (59%) | 49 (58%) | 53 (60%) | 0.603 |
| Coiling | 72 (41%) | 36 (42%) | 36 (40%) | 0.616 |
| EVD | 75 (43%) | 26 (31%) | 49 (55%) | 0.001 |
| Decompressive craniectomy | 36 (21%) | 5 (6%) | 31 (36%) | <0.001 |
| Outcome | ||||
| ICU stay [days] | 9 ± 12 | 6 ± 5 | 13 ± 15 | <0.001 |
| Hospital stay [days] | 19 ± 20 | 18 ±10 | 22 ± 43 | 0.039 |
| Dead | 43 (24%) | 0 | 43 (46%) | <0.001 |
| Neurologic complications | ||||
| Cerebral infarction on follow-up CT | 66 (38%) | 16 (19%) | 50 (56%) | <0.001 |
| DCI | 79 (45%) | 24 (28%) | 55 (62%) | <0.001 |
| CV in TCD | 93 (55%) | 44 (52%) | 49 (58%) | 0.491 |
| Criteria for DCI diagnosis | ||||
| A decline in GCS score and focal deficit | 13 (16%) | 8 (33%) | 5 (9%) | |
| A decline in GCS score, focal deficit, and new infarction | 54 (69%) | 12 (50%) | 42 (76%) | |
| New infarction alone | 12 (15%) | 4 (17%) | 8 (15%) | |
| Systemic complications | ||||
| Pneumonia | 60 (35%) | 12 (14%) | 48 (54%) | <0.001 |
| Inflammation of the urinary tract | 38 (22%) | 8 (9%) | 30 (34%) | <0.001 |
| Cardiac complications | 18 (10%) | 4 (5%) | 14 (16%) | 0.015 |
| SIRS | 62 (36%) | 11 (13%) | 51 (57%) | <0.001 |
| Meningitis/ventriculitis | 7 (4%) | 1 (1%) | 6 (7%) | 0.067 |
| MOF | 24 (14%) | 1 (1%) | 23 (26%) | <0.001 |
| Neurogenic pulmonary oedema | 46 (26%) | 13 (15%) | 33 (37%) | 0.001 |
| Days on MV | 7 ± 7 | 2 ± 4 | 11 ± 7 | <0.001 |
| Characteristic | Total N = 174 | mRS 0–2 n = 85 | mRS 3–6 n = 89 | p |
|---|---|---|---|---|
| CRP [mg/L] (0–5) | 6 ± 102 | 4 ± 95 | 8 ± 104 | 0.239 |
| CRP/albumin [a.u] | 0.1 ± 0.4 | 0.1 ± 0.2 | 0.2 ± 0.5 | 0.040 |
| D-dimer [ng/mL] (0–0.5) | 2.2 ± 3.4 | 1.5 ± 2.8 | 3.0 ± 4.5 | <0.001 |
| WBC [G/L] (4–10 × 103) | 13.5 ± 6.7 | 12.9 ± 5.1 | 13.8 | 0.104 |
| PCT [ng/mL] (0–0.05) | 0.1 ± 0.3 | 0.1 ± 0.1 | 0.2 ± 0.4 | <0.001 |
| PLT [103/μL] (140–440) | 243 ± 90 | 234 ± 83 | 252 ± 81 | 0.144 |
| LAC [mmol/L] (0.5–1.6) | 1.2 ± 1.1 | 1.0 ± 0.8 | 1.6 ± 1.6 | 0.001 |
| Glucose [mmol/L] (70–99) | 156 ± 60 | 137 ± 48 | 169 ± 69 | <0.001 |
| Hb [G/dL] (14–18) | 13.8 ± 2.1 | 13.5 ± 2.1 | 14.1 ± 2.1 | 0.057 |
| Ht [%] (40–54) | 40.8 ± 5.8 | 40.6 ± 6.1 | 41.1 ± 5.8 | 0.119 |
| Na [mmol/L] (136–146) | 138 ± 5 | 138 ± 4 | 138 ± 6 | 0.172 |
| K [mmol/L] (3.5–5.1) | 3.7 ± 0.8 | 3.8 ± 0.7 | 3.6 ± 0.7 | 0.753 |
| Albumin [G/L] (35–52) | 34 ± 6 | 35 ± 5 | 31 ± 7 | <0.001 |
| Parameter | Threshold Value | Z | p | AUC | SE | SPE |
|---|---|---|---|---|---|---|
| Biomarkers: | ||||||
| CRP/albumin | 0.167 | 2.09 | 0.037 | 0.59 | 0.51 | 0.73 |
| D-dimer | 1.62 | 5.57 | <0.001 | 0.72 | 0.81 | 0.55 |
| PCT | 0.08 | 3.79 | <0.001 | 0.67 | 0.69 | 0.62 |
| LAC | 1.3 | 3.38 | <0.001 | 0.67 | 0.64 | 0.67 |
| Glucose | 153 | 6,63 | <0.001 | 0.75 | 0.67 | 0.72 |
| Albumin | 33 | 5.79 | <0.001 | 0.73 | 0.68 | 0.72 |
| Covariates: | ||||||
| Age | 66 | 2.90 | 0.004 | 0.62 | 0.38 | 0.86 |
| Apache II scale | 17 | 12.27 | <0.001 | 0.86 | 0.76 | 0.86 |
| GCS scale | 12 | 11.60 | <0.001 | 0.84 | 0.82 | 0.73 |
| FOUR scale | 15 | 10.33 | <0.001 | 0.83 | 0.88 | 0.66 |
| H-H scale | 4 | 10.69 | <0.001 | 0.83 | 0.72 | 0.79 |
| mFisher scale | 4 | 7.19 | <0.001 | 0.77 | 0.83 | 0.66 |
| WFNS scale | 4 | 10.36 | <0.001 | 0.83 | 0.75 | 0.77 |
| HIJDRA score | 19 | 5.29 | <0.001 | 0.71 | 0.67 | 0.68 |
| SEBES | 3 | 4.26 | <0.001 | 0.67 | 0.56 | 0.73 |
| Poor Outcome | ||||||
|---|---|---|---|---|---|---|
| Factor | B | SE | W | p | OR | 95% CI |
| SEBES | 0.32 | 0.15 | 4.42 | 0.035 | 1.38 | 1.02–1.85 |
| Apache II scale | 0.18 | 0.04 | 19.31 | <0.001 | 1.20 | 1.10–1.30 |
| mFisher scale | 1.23 | 0.36 | 11.72 | 0.001 | 3.43 | 1.70–7.00 |
| albumin | −0.19 | 0.05 | 11.97 | 0.001 | 0.83 | 0.74–0.92 |
| DCI development | ||||||
| Factor | B | SE | W | p | OR | 95% CI |
| Apache II scale | 0.05 | 0.02 | 5.78 | 0.016 | 1.05 | 1.00–1.09 |
| albumin | −0.11 | 0.04 | 10.53 | 0.001 | 0.88 | 0.82–0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burzyńska, M.; Uryga, A.; Woźniak, J.; Załuski, R.; Robba, C.; Goździk, W. The Role of Early Serum Biomarkers and Clinical Rating Scales in the Prediction of Delayed Cerebral Ischaemia and Short-Term Outcome after Aneurysmal Subarachnoid Haemorrhage: Single Centre Experience. J. Clin. Med. 2023, 12, 5614. https://doi.org/10.3390/jcm12175614
Burzyńska M, Uryga A, Woźniak J, Załuski R, Robba C, Goździk W. The Role of Early Serum Biomarkers and Clinical Rating Scales in the Prediction of Delayed Cerebral Ischaemia and Short-Term Outcome after Aneurysmal Subarachnoid Haemorrhage: Single Centre Experience. Journal of Clinical Medicine. 2023; 12(17):5614. https://doi.org/10.3390/jcm12175614
Chicago/Turabian StyleBurzyńska, Małgorzata, Agnieszka Uryga, Jowita Woźniak, Rafał Załuski, Chiara Robba, and Waldemar Goździk. 2023. "The Role of Early Serum Biomarkers and Clinical Rating Scales in the Prediction of Delayed Cerebral Ischaemia and Short-Term Outcome after Aneurysmal Subarachnoid Haemorrhage: Single Centre Experience" Journal of Clinical Medicine 12, no. 17: 5614. https://doi.org/10.3390/jcm12175614





