Interrelationships between Peak Strain Dispersion, Myocardial Work Indices, Isovolumetric Relaxation and Systolic–Diastolic Coupling in Middle-Aged Healthy Subjects
Abstract
:1. Introduction
2. Materials and Methods
- No known acute or chronic illness—participants were required to have no documented history of acute or chronic illness;
- Absence of signs and symptoms of acute disease—individuals were assessed for any physical signs or complaints related to underlying disease;
- No chronic medication use, except for oral hormonal contraception in women of reproductive age;
- Smokers were allowed to participate in the study;
- Occasional use of nonsteroid anti-inflammatory drugs for occasional pain relief (e.g., headache) was allowed, except for 48 h before ECG and echocardiography.
- Brachial blood pressure;
- Standard 12-lead ECG recordings were obtained to assess the presence of sinus rhythm and to detect any abnormal findings;
- Transthoracic 2D echocardiography using a GE Vivid E95 platform.
2.1. Echocardiography
2.2. Statistical Analysis
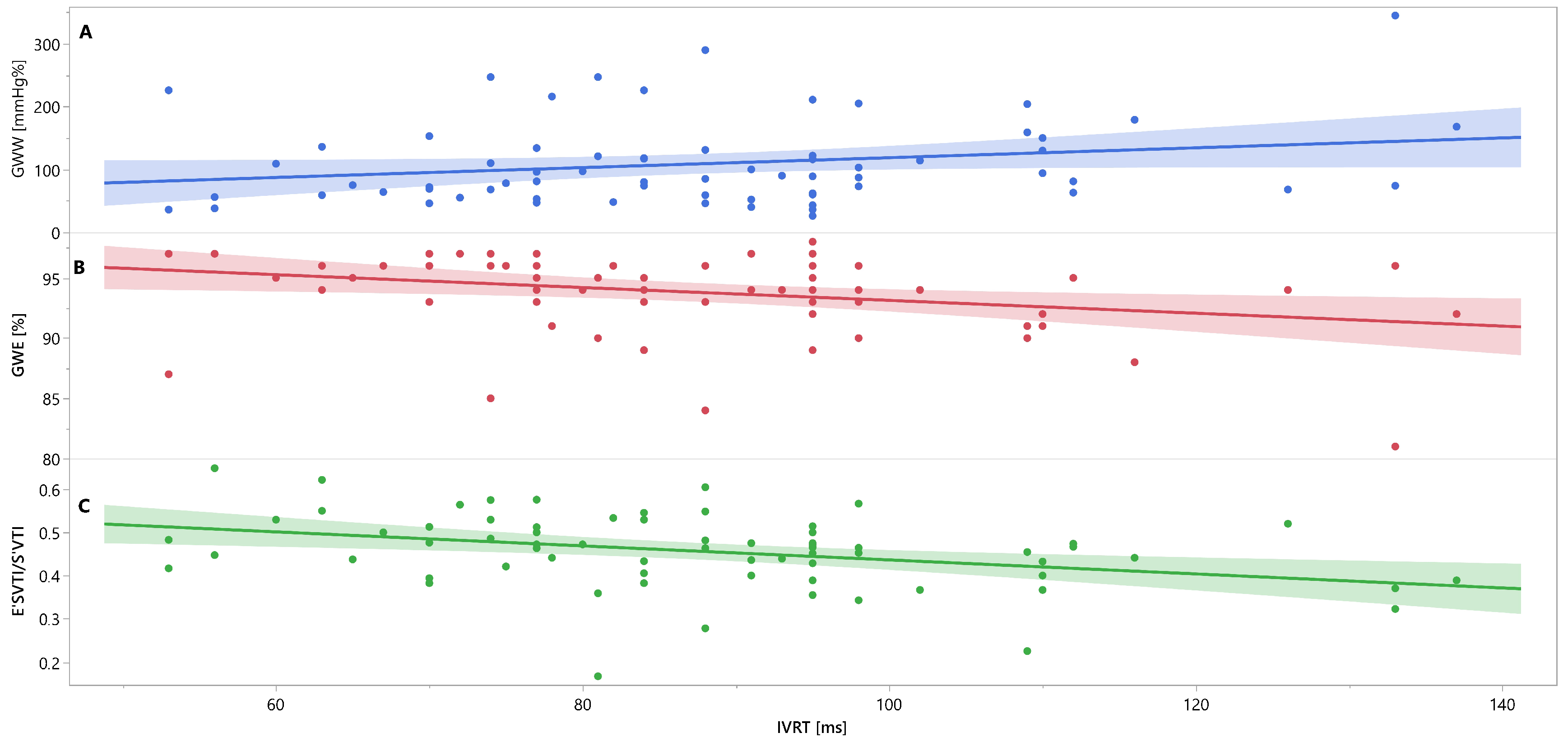
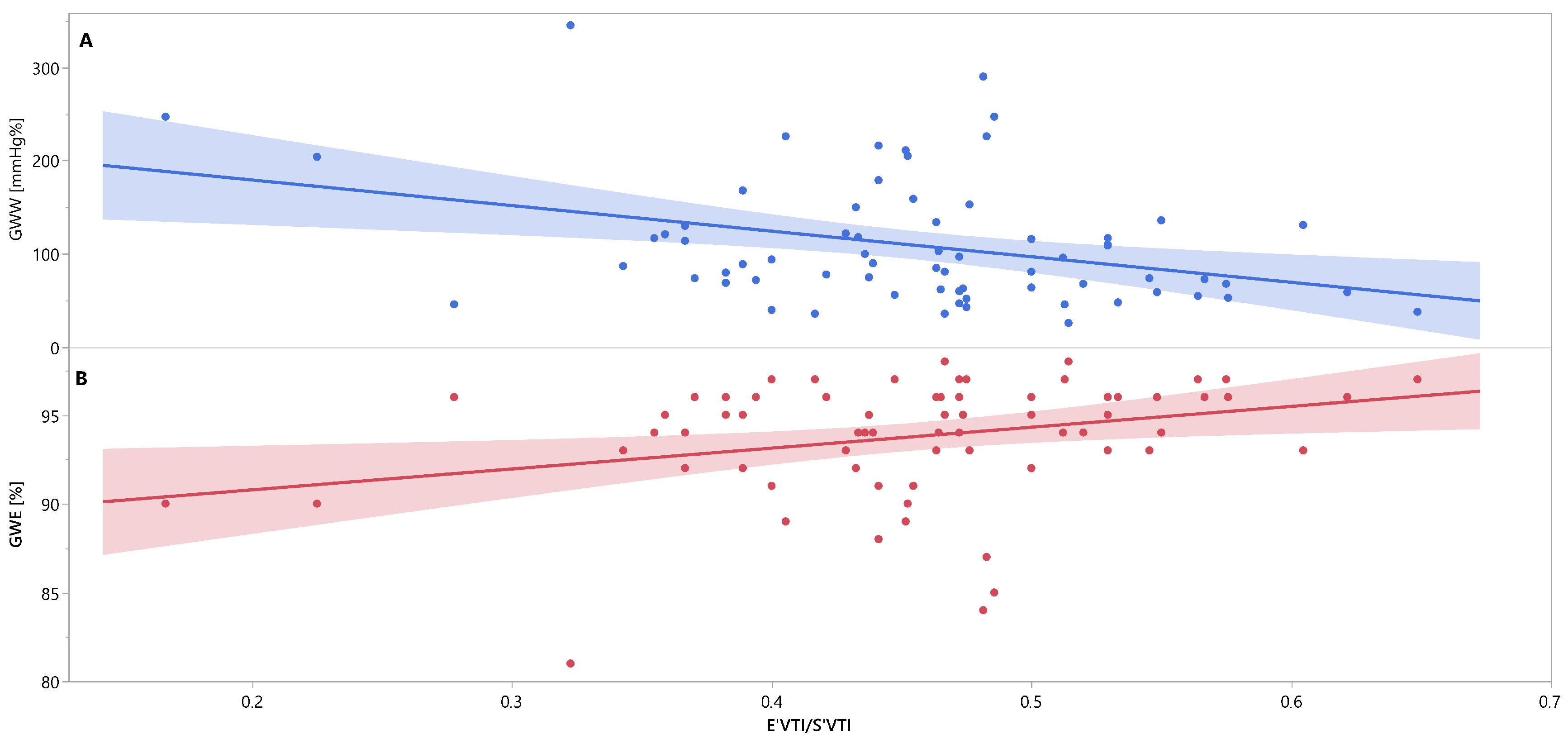
3. Results
4. Discussion
4.1. Study’s Limitations
4.2. Potential Clinical Implications and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Minczykowski, A.; Zwanzig, M.; Dziarmaga, M.; Rutkowska, A.; Baliński, M.; Krauze, T.; Guzik, P.; Wykrętowicz, A. First-Phase Left Ventricular Ejection Fraction as an Early Sign of Left Ventricular Dysfunction in Patients with Stable Coronary Artery Disease. J. Clin. Med. 2023, 12, 868. [Google Scholar] [CrossRef]
- Mornos, C.; Muntean, D.; Mornos, A.; Crisan, S.; Petrescu, L.; Ionac, A.; Sosdean, R.; Cozma, D. Risk stratification in patients with heart failure: The value of considering both global longitudinal left ventricular strain and mechanical dispersion. Can. J. Physiol. Pharmacol. 2017, 95, 1360–1368. [Google Scholar] [CrossRef]
- Pignatelli, R.H.; Ghazi, P.; Reddy, S.C.; Thompson, P.; Cui, Q.; Castro, J.; Okcu, M.F.; Jefferies, J.L. Abnormal Myocardial Strain Indices in Children Receiving Anthracycline Chemotherapy. Pediatr. Cardiol. 2015, 36, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.C.; Mandoli, G.E.; Contorni, F.; Cavigli, L.; Focardi, M.; D’Ascenzi, F.; Patti, G.; Mondillo, S.; Cameli, M. Speckle Tracking Echocardiography: Early Predictor of Diagnosis and Prognosis in Coronary Artery Disease. Biomed. Res. Int. 2021, 2021, 6685378. [Google Scholar] [CrossRef]
- Mandoli, G.E.; Borrelli, C.; Cameli, M.; Mondillo, S.; Ghiadoni, L.; Taddei, C.; Passino, C.; Emdin, M.; Giannoni, A. Speckle tracking echocardiography in heart failure development and progression in patients with apneas. Heart Fail. Rev. 2022, 27, 1869–1881. [Google Scholar] [CrossRef]
- Pastore, M.C.; De Carli, G.; Mandoli, G.E.; D’Ascenzi, F.; Focardi, M.; Contorni, F.; Mondillo, S.; Cameli, M. The prognostic role of speckle tracking echocardiography in clinical practice: Evidence and reference values from the literature. Heart Fail. Rev. 2021, 26, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Bandera, F.; Baghdasaryan, L.; Mandoli, G.E.; Cameli, M. Multimodality imaging predictors of sudden cardiac death. Heart Fail. Rev. 2020, 25, 427–446. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Mandoli, G.E.; Sciaccaluga, C.; Mondillo, S. More than 10 years of speckle tracking echocardiography: Still a novel technique or a definite tool for clinical practice? Echocardiography 2019, 36, 958–970. [Google Scholar] [CrossRef]
- Li, C.; Yuan, M.; Li, K.; Bai, W.; Rao, L. Value of peak strain dispersion in discovering left ventricular dysfunction in diabetes mellitus. Sci. Rep. 2020, 8, 21437. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Hygidio, D.; Le Bihan, D.; Batista Souza, J.; Bellio de Matos Barettto, R.; Mathias, W., Jr.; de Sousa, M. Evaluation of myocardial work in patients with resistant arterial hypertension. Echocardiography 2022, 39, 1412–1419. [Google Scholar] [CrossRef]
- Iwahashi, N.; Kirigaya, J.; Gohbara, M.; Abe, T.; Horii, M.; Hanajima, Y.; Toya, N.; Takahashi, H.; Kirigaya, H.; Minamimoto, Y.; et al. Mechanical dispersion combined with global longitudinal strain estimated by three dimensional speckle tracking in patients with ST elevation myocardial infarction. Int. J. Cardiol. Heart Vasc. 2022, 40, 101028. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Grenne, B.L.; Eek, C.H.; Ersbøll, M.; Valeur, N.; Svendsen, J.H.; Florian, A.; Sjøli, B.; Brunvand, H.; Køber, L.; et al. Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. JACC Cardiovasc. Imaging 2013, 6, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Dziarmaga, M.; Minczykowski, A.; Zwanzig, M.; Krauze, T.; Rutkowska, A.; Morawski, J.; Baliński, M.; Piskorski, J.; Guzik, P.; Wykrętowicz, A. Influence of increased arterial stiffness on myocardial work efficiency in patients with stable coronary artery disease. Kardiol. Pol. 2021, 79, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Cebrowska, K.; Minczykowski, A.; Krauze, T.; Guzik, P.; Szczepanik, A.; Wykrętowicz, A. The pressure-strain work indices in response to isometric handgrip exercise. Kardiol. Pol. 2021, 79, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A novel clinical method for quantification of regional left ventricular pressure-strain loop area: A noninvasive index of myocardial work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Meucci, M.C.; Butcher, S.C.; Galloo, X.; van der Velde, E.T.; Marsan, N.A.; Bax, J.J.; Delgado, V. Noninvasive left ventricular myocardial work in patients with chronic aortic regurgitation and preserved left ventricular ejection fraction. J. Am. Soc. Echocardiogr. 2022, 35, 703–711.e3. [Google Scholar] [CrossRef] [PubMed]
- Einarsen, E.; Hjertaas, J.J.; Gu, H.; Matre, K.; Chowienczyk, P.J.; Gerdts, E.; Chambers, J.B.; Saeed, S. Impact of arterio-ventricular interaction on first-phase ejection fraction in aortic stenosis. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.; Tok, Ö.Ö.; Mitrousi, K.; Ikonomidis, I. Myocardial work: Methodology and clinical applications. Diagnostics 2021, 11, 573. [Google Scholar] [CrossRef] [PubMed]
- Roemer, S.; Jaglan, A.; Santos, D.; Umland, M.; Jain, R.; Tajik, A.J.; Khandheria, B.K. The utility of myocardial work in clinical practice. J. Am. Soc. Echocardiogr. 2021, 34, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Lustosa, R.P.; Butcher, S.C.; van der Bijl, P.; El Mahdiui, M.; Montero-Cabezas, J.M.; Kostyukevich, M.V.; Rocha De Lorenzo, A.; Knuuti, J.; Ajmone Marsan, N.; Bax, J.J.; et al. Global Left Ventricular Myocardial Work Efficiency and Long-Term Prognosis in Patients After ST-Segment-Elevation Myocardial Infarction. Circ. Cardiovasc. Imaging 2021, 14, e012072. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., III; Dakainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Ahrens, T. Stroke volume optimization: The new hemodynamic algorithm. Crit. Care Nurse 2015, 35, 11–27. [Google Scholar] [CrossRef]
- Yamamoto, K. The time constant of left ventricular relaxation: Extrication from load dependence and overestimation of functional abnormality. Circ. Heart Fail. 2010, 3, 178–180. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, M.F. Starling’s law of the heart: An appraisal 70 years on. Aust. N. Z. J. Med. 1984, 14, 879–887. [Google Scholar] [CrossRef]
- Sawicka-Gutaj, N.; Gruszczyński, D.; Guzik, P.; Mostowska, A.; Walkowiak, J. Publication ethics of human studies in the light of the Declaration of Helsinki—A mini-review. JMS 2022, 91, e700. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef]
- MacNamara, J.P.; Koshti, V.; Dias, K.A.; Howden, E.; Hearon, C.M., Jr.; Cheng, I.-J.; Hynan, L.S.; Levine, B.D.; Sarma, S. The impact of cardiac loading on a novel metric of left ventricular diastolic function in healthy middle-aged adults: Systolic-diastolic coupling. Physiol. Rep. 2021, 9, e15129. [Google Scholar] [CrossRef]
- Boe, E.; Skulstad, H.; Smiseth, O.A. Myocardial work by echocardiography: A novel method ready for clinical testing. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 18–20. [Google Scholar] [CrossRef]
- Guzik, P.; Więckowska, B. Data distribution analysis—A preliminary approach to quantitative data in biomedical research. J. Med. Sci. 2023, 92, e869. [Google Scholar] [CrossRef]
- Li, C.; Li, K.; Yuan, M.; Bai, W.; Ra, L. Peak strain dispersion within the left ventricle detected by two-dimensional speckle tracking in patients with uncomplicated systemic lupus erythematosus. Int. J. Cardiovasc. Imaging 2021, 37, 2197–2205. [Google Scholar] [CrossRef]
- Seo, H.-S.; Cho, Y.-H.; Choi, J.H.; Suh, J.; Lee, N.-H.; Lim, O.K. The association of left ventricular hypertrophy with intraventricular dyssynchrony at rest and during exercise in hypertensive patients. J. Cardiovasc. Ultrasound 2012, 20, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Michalik, J.; Dąbrowska-Kugacka, A.; Kosmalska, K.; Moroz, R.; Kot, A.; Lewicka, E.; Szolkiewicz, M. Hemodynamic Effects of Permanent His Bundle Pacing Compared to Right Ventricular Pacing Assessed by Two-Dimensional Speckle-Tracking Echocardiography. Int. J. Environ. Res. Public Health 2021, 18, 11721. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, X.; Feng, H. Evaluation of left ventricular systolic synchrony by peak strain dispersion in patients with rheumatoid arthritis. J. Int. Med. Res. 2021, 49, 3000605211007737. [Google Scholar] [CrossRef] [PubMed]
- Jaglan, A.; Roemer, S.; Perez Moreno, A.C.; Khandheria, B.K. Myocardial work in Stage 1 and 2 hypertensive patients. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Manganaro, R.; Marchetta, S.; Dulgheru, R.; Ilardi, F.; Sugimoto, T.; Robinet, S.; Cimino, S.; Go, Y.Y.; Bernard, A.; Kacharava, G.; et al. Echocardiographic reference ranges for normal non-invasive myocardial work indices: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Hedwig, F.; Nemchyna, O.; Stein, J.; Knosalla, C.; Merke, N.; Knebel, F.; Hagendorff, A.; Schoenrath, F.; Falk, V.; Knierim, J. Myocardial Work Assessment for the Prediction of Prognosis in Advanced Heart Failure. Front. Cardiovasc. Med. 2021, 8, 691611. [Google Scholar] [CrossRef]
- Biering-Sørensen, T.; Mogelvang, R.; Jensen, J.S. Prognostic value of cardiac time intervals measured by tissue Doppler imaging M-mode in the general population. Heart 2015, 101, 954–960. [Google Scholar] [CrossRef]
- Tan, C.; Rubenson, D.; Srivastava, A.; Mohan, R.; Smith, M.R.; Billick, K.; Bardarian, S.; Thomas Heywood, J. Left ventricular outflow tract velocity time integral outperforms ejection fraction and Doppler-derived cardiac output for predicting outcomes in a select advanced heart failure cohort. Cardiovasc. Ultrasound 2017, 15, 18. [Google Scholar] [CrossRef]
- Capone, C.A.; Lamour, J.M.; Lorenzo, J.; Tria, B.; Ye, K.; Hsu, D.T.; Mahgerefteh, J. Ventricular Arterial Coupling: A Novel Echocardiographic Risk Factor for Disease Progression in Pediatric Dilated Cardiomyopathy. Pediatr. Cardiol. 2019, 40, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, M.K.; Margossian, R.; Lu, M.; Mercer-Rosa, L.; Henderson, H.T.; Nutting, A.; Friedman, K.; Molina, K.M.; Altmann, K.; Canter, C.; et al. Pediatric Heart Network Investigators. Systolic-diastolic functional coupling in healthy children and in those with dilated cardiomyopathy. J. Appl. Physiol. 2016, 120, 1301–1318. [Google Scholar] [CrossRef] [PubMed]
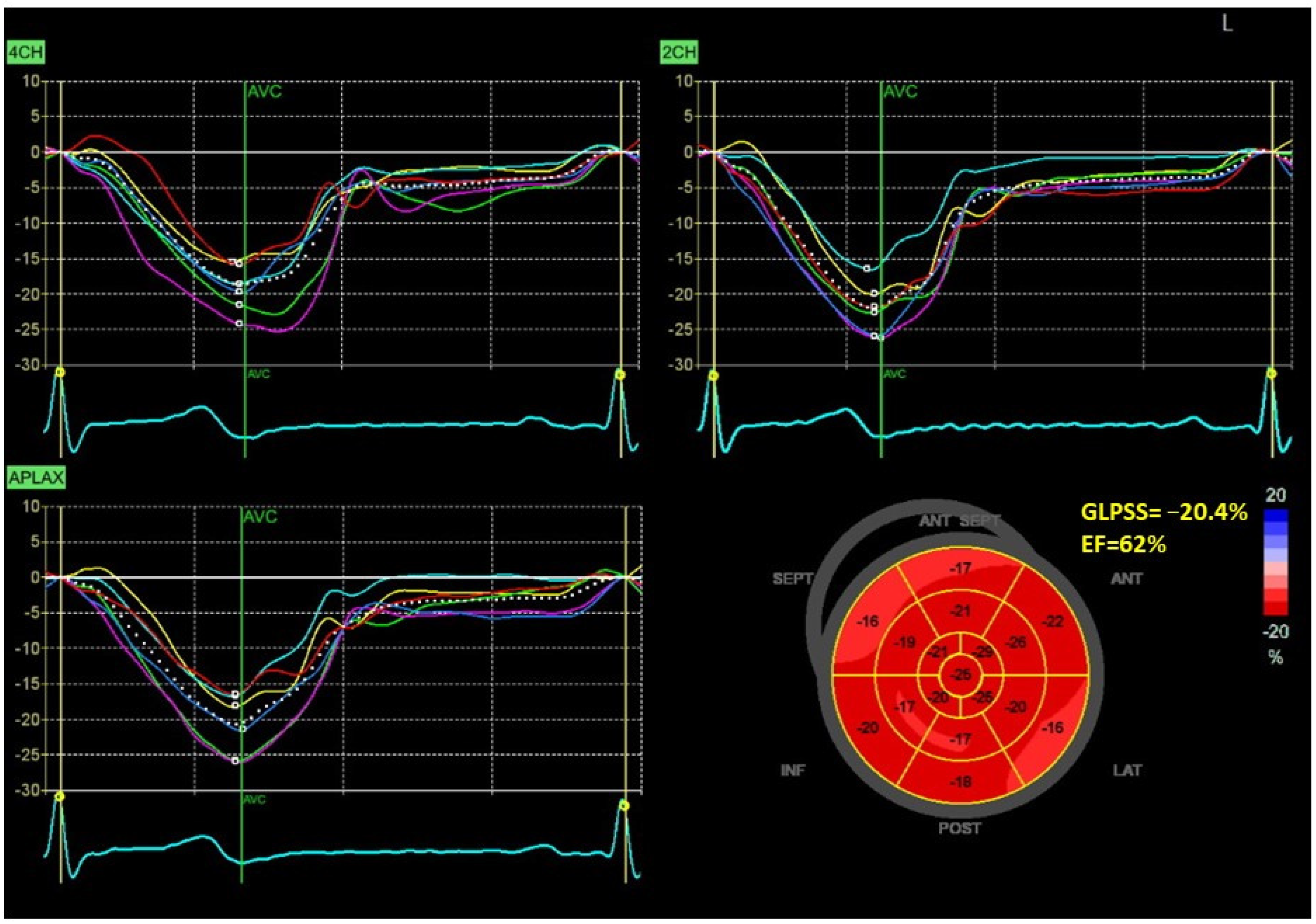
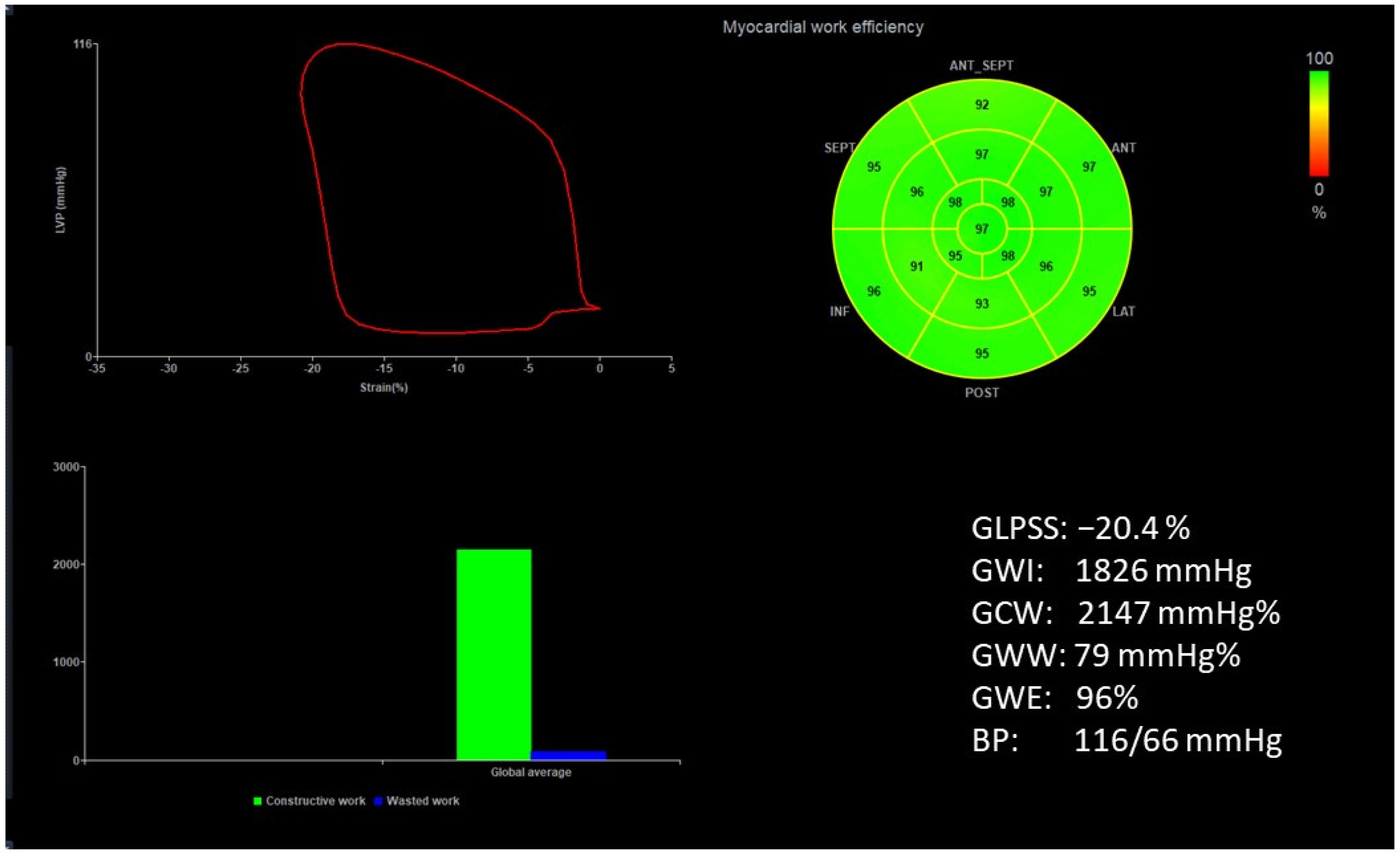
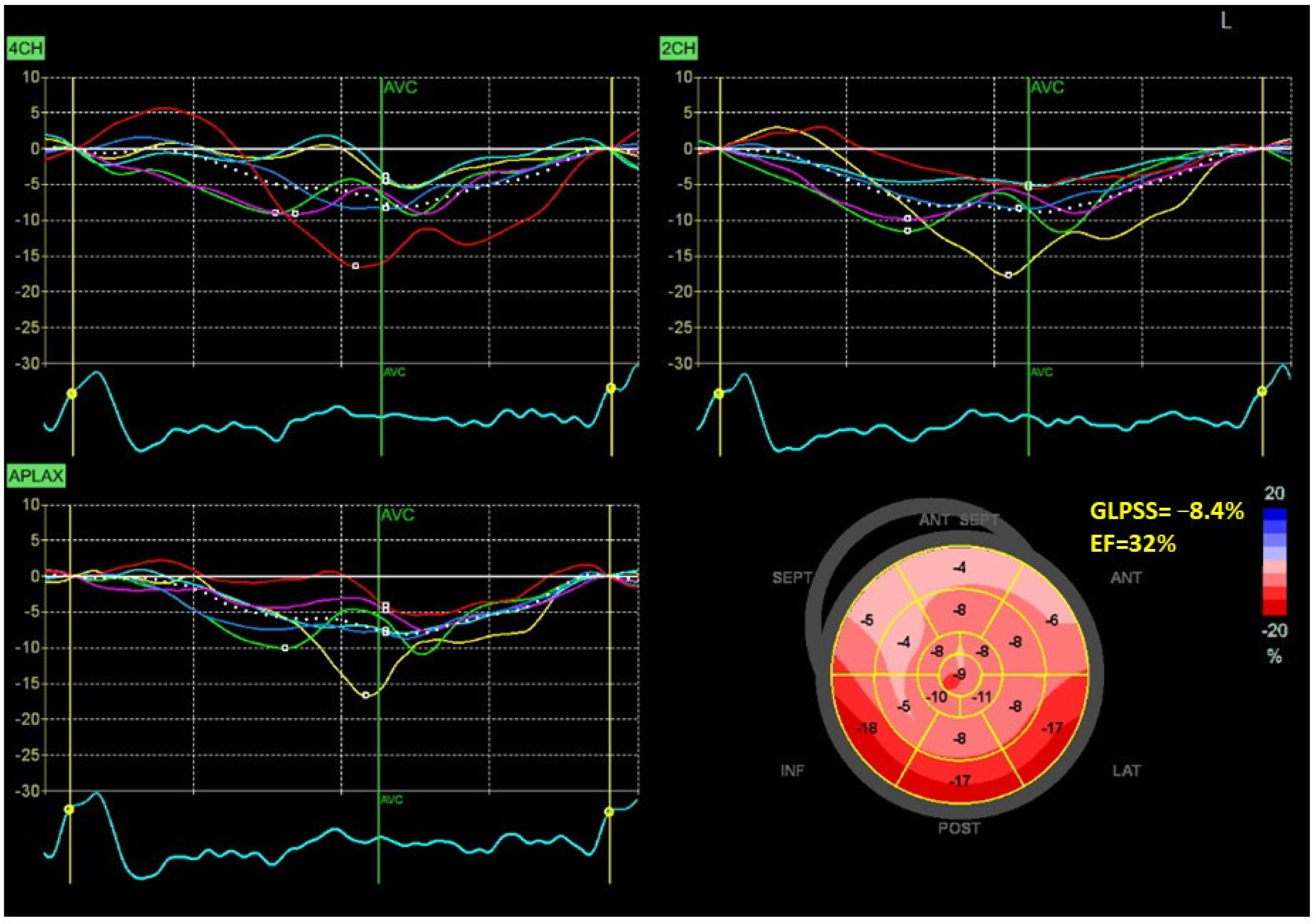
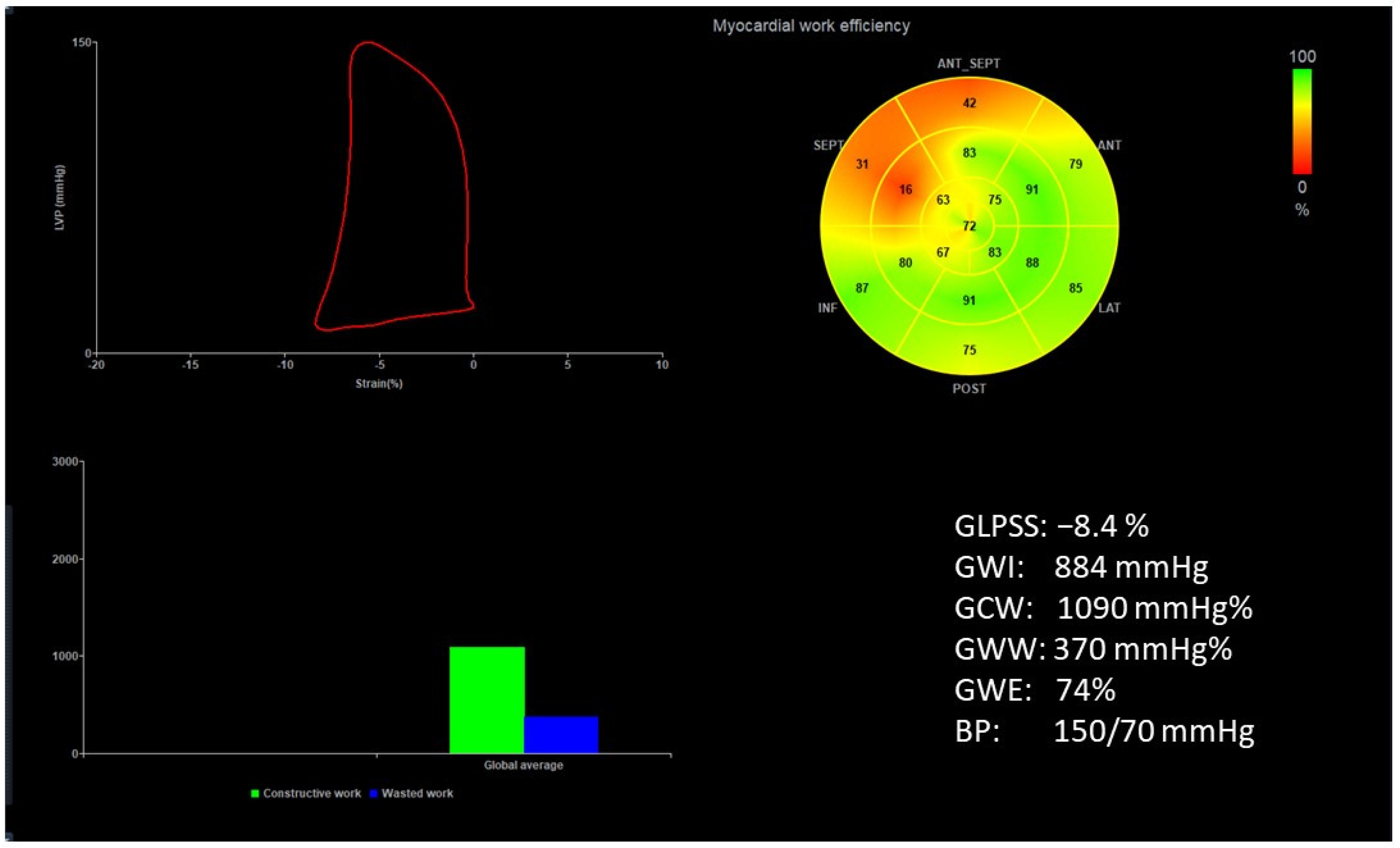

| Characteristics | |
|---|---|
| Age (years) | 58 ± 6 |
| Female/male | 39/30 |
| BP systolic (mmHg) | 122 ± 15 |
| BMI (kg/m2) | 26 ± 4 |
| IVSd (mm) | 11 ± 2 |
| LVPWd (mm) | 10 ± 1 |
| LVEDd (mm) | 43 ± 5 |
| LVEDs (mm) | 28 ± 5 |
| LA (mm) | 33 ± 4 |
| LVMI (g/m2) | 87 ± 19 |
| LVEDV (mL) | 94 ± 27 |
| LVESV (mL) | 36 ± 14 |
| SV (mL) | 55 ± 15 |
| CO (L/min) | 4.0 ± 1.1 |
| EF (%) | 63 ± 5 |
| E/A | 1.1 ± 0.3 |
| E/E′ | 7 ± 2 |
| GLPSS (%) | −18 ± 2 |
| IVRT (ms) | 87.8 ± 18.8 |
| PSD (ms) | 44 ± 12 |
| GWE (%) | 94 ± 3 |
| GWW (mmHg%) | 109 ± 67 |
| E′VTI/S′VTI | 0.46 ± 0.1 |
| Clinical Variable | GWE | GWW | IVRT | E′VTI/S′VTI |
|---|---|---|---|---|
| PSD | −0.49; p < 0.0001 | 0.40; p = 0.0007 | 0.53; p < 0.0001 | −0.44; p = 0.0002 |
| GWE | X | −0.94; p < 0.0001 | −0.30; p = 0.0127 | 0.30; p = 0.0132 |
| GWW | X | 0.22; p = 0.0661 | −0.35; p = 0.0032 | |
| IVRT | X | −0.36; p = 0.0024 |
| Independent variable | ||||||
|---|---|---|---|---|---|---|
| PSD | GWE | GWW | IVRT | E′VTI/S′VTI | ||
| Dependent variable | PSD | X | −1.3421; p = 0.0002 | 0.053; p = 0.004 | 0.24; p = 0.0003 | −45.1867; p = 0.0042 |
| GWE | −0.1471; p = 0.0002 | X | −0.0473; p = <0.001 | −0.0430; p = 0.0611 | 10.9539; p = 0.0391 | |
| GWW | 2.3052; p = 0.004 | −18.7845; p = <0.0001 | X | 0.5842; p = 0.2055 | −267.604; p = 0.0107 | |
| IVRT | 0.7626; p = 0.0003 | −1.2481; p = 0.0611 | 0.0427; p = 0.2055 | X | −64.0973; p = 0.0245 | |
| E′VTI/S′VTI | −0.0027; p = 0.0042 | 0.0059; p = 0.0391 | −0.0004; p = 0.0107 | −0.0012; p = 0.0245 | X | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minczykowski, A.; Guzik, P.; Sajkowska, A.; Pałasz-Borkowska, A.; Wykrętowicz, A. Interrelationships between Peak Strain Dispersion, Myocardial Work Indices, Isovolumetric Relaxation and Systolic–Diastolic Coupling in Middle-Aged Healthy Subjects. J. Clin. Med. 2023, 12, 5623. https://doi.org/10.3390/jcm12175623
Minczykowski A, Guzik P, Sajkowska A, Pałasz-Borkowska A, Wykrętowicz A. Interrelationships between Peak Strain Dispersion, Myocardial Work Indices, Isovolumetric Relaxation and Systolic–Diastolic Coupling in Middle-Aged Healthy Subjects. Journal of Clinical Medicine. 2023; 12(17):5623. https://doi.org/10.3390/jcm12175623
Chicago/Turabian StyleMinczykowski, Andrzej, Przemysław Guzik, Anna Sajkowska, Anna Pałasz-Borkowska, and Andrzej Wykrętowicz. 2023. "Interrelationships between Peak Strain Dispersion, Myocardial Work Indices, Isovolumetric Relaxation and Systolic–Diastolic Coupling in Middle-Aged Healthy Subjects" Journal of Clinical Medicine 12, no. 17: 5623. https://doi.org/10.3390/jcm12175623





