The Diagnostic and Prognostic Roles Played by Homocysteine and Other Aminothiols in Patients with Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Patients and Controls
2.3. Clinical and Instrumental Methods
2.4. Sample Collection
2.5. Laboratory Procedures
2.5.1. Determination of Cys and Hcy in Plasma and Urine
2.5.2. Determination of SAM and SAH in Urine
2.6. Outcome
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Changes in Aminothiol Levels Depending on the Histories of CKD Patients and the Stage of the Disease
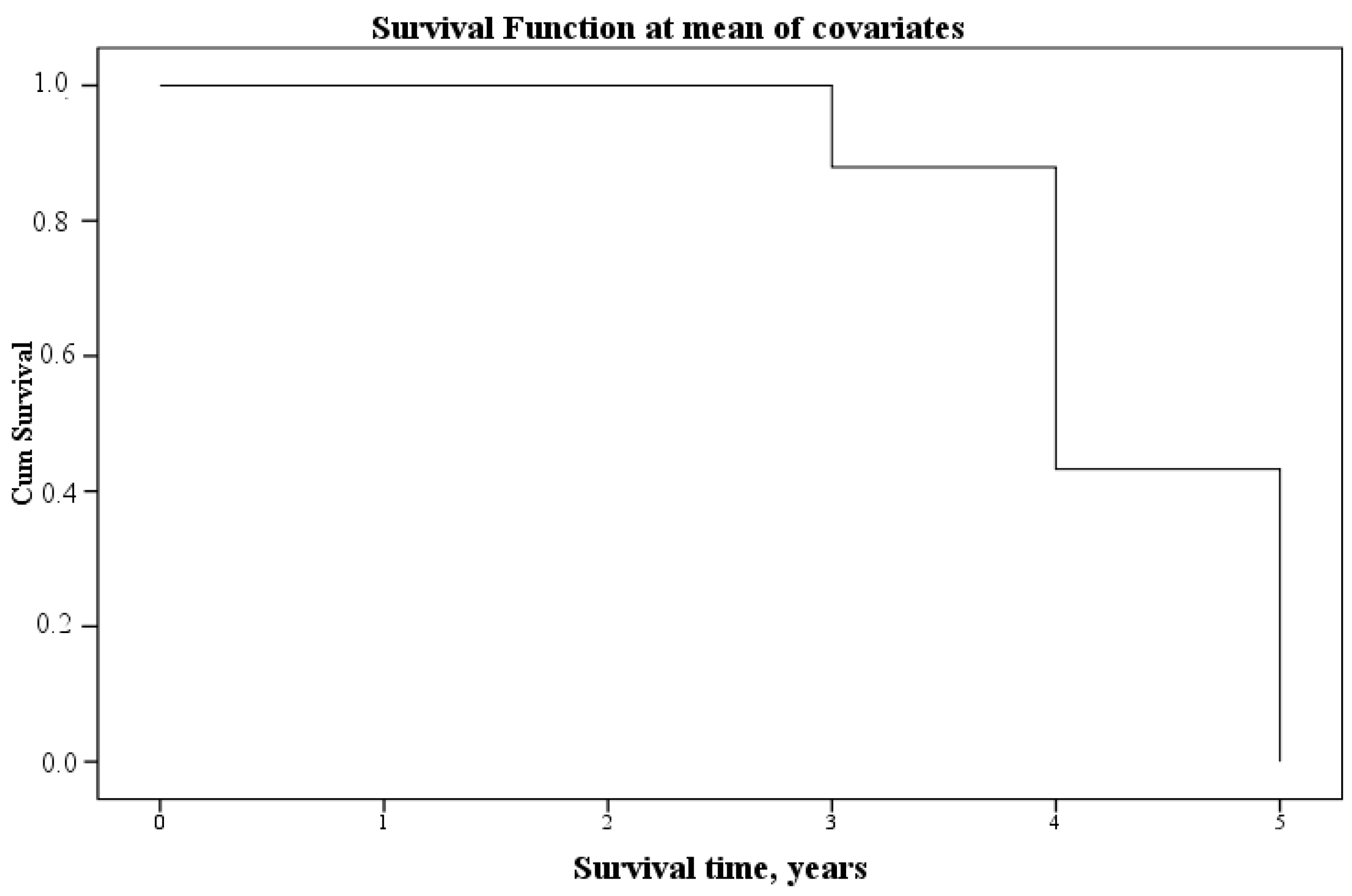
3.3. Analysis of the Relationship between Aminothiols in Blood Plasma and Urine and the Survival of Patients with CKD after Five Years
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Charles, C.; Ferris, A.H. Chronic Kidney Disease. Prim. Care Clin. Off. Pract. 2020, 47, 585–595. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2022, 102, S1–S127. [Google Scholar] [CrossRef]
- Jeyaruban, A.; Hoy, W.; Cameron, A.L.; Healy, H.G.; Wang, Z.; Zhang, J.; Mallett, A. Determining the association between the type of intervention for ischaemic heart disease and mortality and morbidity in patients with chronic kidney disease. Intern. Med. J. 2022, 52, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Nakagawa, T.; Jalal, D.; Sánchez-Lozada, L.G.; Kang, D.-H.; Ritz, E. Uric acid and chronic kidney disease: Which is chasing which? Nephrol. Dial. Transplant. 2013, 28, 2221–2228. [Google Scholar] [CrossRef]
- Olsen, E.; van Galen, G. Chronic Renal Failure-Causes, Clinical Findings, Treatments and Prognosis. Veter. Clin. N. Am. Equine Pract. 2022, 38, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiologi-cal Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Ballew, S.H.; Wang, A.Y.-M.; Kalyesubula, R.; Schaeffner, E.; Agarwal, R. Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat. Rev. Nephrol. 2022, 18, 696–707. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Zhang, W.R.; Parikh, C.R. Biomarkers of Acute and Chronic Kidney Disease. Annu. Rev. Physiol. 2019, 81, 309–333. [Google Scholar] [CrossRef]
- Krstic, D.; Tomic, N.; Radosavljevic, B.; Avramovic, N.; Dragutinovic, V.; Skodric, S.R.; Colovic, M. Biochemical Markers of Renal Function. Curr. Med. Chem. 2016, 23, 2018–2040. [Google Scholar] [CrossRef]
- Wasung, M.E.; Chawla, L.S.; Madero, M. Biomarkers of renal function, which and when? Clin. Chim. Acta 2015, 438, 350–357. [Google Scholar] [CrossRef]
- Benoit, S.W.; Ciccia, E.A.; Devarajan, P. Cystatin C as a biomarker of chronic kidney disease: Latest developments. Expert Rev. Mol. Diagn. 2020, 20, 1019–1026. [Google Scholar] [CrossRef]
- Sinna, M.M.; Altaf, F.M.; Mosa, O.F. Serum and Urinary NGAL and Cystatin C Levels as Diagnostic Tools for Acute Kidney Injury and Chronic Kidney Disease: A Histobiochemical Comparative Study. Curr. Pharm. Des. 2019, 25, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.M.; Rifkin, D.; Lee, A.K.; Tamura, M.K.; Pajewski, N.M.; Weiner, D.E.; Al-Rousan, T.; Shlipak, M.; Ix, J.H. Association of Urine Biomarkers of Kidney Tubule Injury and Dysfunction with Frailty Index and Cognitive Function in Persons with CKD in SPRINT. Am. J. Kidney Dis. 2021, 78, 530–540.e1. [Google Scholar] [CrossRef]
- Sandokji, I.; Greenberg, J.H. Plasma and Urine Biomarkers of CKD: A Review of Findings in the CKiD Study. Semin. Nephrol. 2021, 41, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Stopic, B.; Medic-Brkic, B.; Savic-Vujovic, K.; Davidovic, Z.; Todorovic, J.; Dimkovic, N. Biomarkers and Predictors of Adverse Cardiovascular Events in Different Stages of Chronic Kidney Disease. Dose-Response 2022, 20, 15593258221127568. [Google Scholar] [CrossRef]
- Liu, C.; Debnath, N.; Mosoyan, G.; Chauhan, K.; Vasquez-Rios, G.; Soudant, C.; Menez, S.; Parikh, C.R.; Coca, S.G. Systematic Review and Meta-Analysis of Plasma and Urine Biomarkers for CKD Outcomes. J. Am. Soc. Nephrol. 2022, 33, 1657–1672. [Google Scholar] [CrossRef]
- Finkelstein, J.D.; Martin, J.J. Methionine metabolism in mammals. Distribution of homocysteine between competing pathways. J. Biol. Chem. 1984, 259, 9508–9513. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.A.M.E.S.D. Pathways and Regulation of Homocysteine Metabolism in Mammals. Semin. Thromb. Hemost. 2000, 26, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B12, folate, and the methionine remethylation cycle—Biochemistry, pathways, and regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef]
- Cao, L.; Lou, X.; Zou, Z.; Mou, N.; Wu, W.; Huang, X.; Tan, H. Folic acid attenuates hyperhomocysteinemia-induced glomerular damage in rats. Microvasc. Res. 2013, 89, 146–152. [Google Scholar] [CrossRef]
- Kuz’menko, N.V.; Alexandrova, L.A.; Chefu, S.G.; Blashko, E.L.; Zhloba, A.A.; Cirlin, V.A. Content of total homocysteine and major aminothiols in rats with experimental renal ischemia. Bull. Exp. Biol. Med. 2011, 151, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.-Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Wang, X.; Greene, T.; Beck, G.J.; Kusek, J.W.; Selhub, J.; Levey, A.S.; Sarnak, M.J. Homocysteine in chronic kidney disease: Effect of low protein diet and repletion with B vitamins. Kidney Int. 2005, 67, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, Q.; Liu, Z. Serum homocysteine concentration as a marker for advanced diabetic nephropathy in a cohort of elderly patients. BMC Endocr. Disord. 2023, 23, 114. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhang, Z.; Zhao, W. Relationship between plasma homocysteine and chronic kidney disease in US patients with type 2 diabetes mellitus: A cross-sectional study. BMC Nephrol. 2022, 23, 419. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Chai, K.; Jiang, Y.; Liu, X. Characterization of urinary biomarkers and their relevant mechanisms of zoledronate-induced nephrotoxicity using rats and HK-2 cells. Hum. Exp. Toxicol. 2019, 38, 598–609. [Google Scholar] [CrossRef]
- Kruglova, M.P.; Grachev, S.V.; Bulgakova, P.O.; Ivanov, A.V.; Virus, E.D.; Nikiforova, K.A.; Fedoseev, A.N.; Savina, G.D.; Kubatiev, A.A. Low S-adenosylmethionine/ S-adenosylhomocysteine Ratio in Urine is Associated with Chronic Kidney Disease. Lab. Med. 2020, 51, 80–85. [Google Scholar] [CrossRef]
- Kruglova, M.P.; Ivanov, A.V.; Virus, E.D.; Bulgakova, P.O.; Samokhin, A.S.; Fedoseev, A.N.; Grachev, S.V.; Kubatiev, A.A. Urine S-Adenosylmethionine are Related to Degree of Renal Insufficiency in Patients with Chronic Kidney Disease. Lab. Med. 2021, 52, 47–56. [Google Scholar] [CrossRef]
- Valli, A.; Carrero, J.J.; Qureshi, A.R.; Garibotto, G.; Bárány, P.; Axelsson, J.; Lindholm, B.; Stenvinkel, P.; Anderstam, B.; Suliman, M.E. Elevated serum levels of S-adenosylhomocysteine, but not homocysteine, are associated with cardiovascular disease in stage 5 chronic kidney disease patients. Clin. Chim. Acta 2008, 395, 106–110. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, F.; Shen, A.-W.; Sun, B.; Xia, T.-Y.; Chen, W.-S.; Tao, X.; Yu, S.-Q. Metabolomics Evaluation of Patients with Stage 5 Chronic Kidney Disease Before Dialysis, Maintenance Hemodialysis, and Peritoneal Dialysis. Front. Physiol. 2021, 11, 630646. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, Q.; Yan Li Wang, C.; Zhang, J.; Ma, X.; Peng, H.; Lou, T. High Prevalence of Hyperhomocysteinemia and Its Association with Target Organ Damage in Chinese Patients with Chronic Kidney Disease. Nutrients 2016, 8, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Otaki, Y.; Hasuike, Y.; Nanami, M.; Itahana, R.; Miyagawa, K.; Nishikage, H.; Izumi, M.; Takamitsu, Y. Association of hyperhomocysteinemia with plasma sulphate and urine excretion in patients with progressive renal disease. Am. J. Kidney Dis. 2002, 40, 909–915. [Google Scholar] [CrossRef]
- Garibotto, G.; Sofia, A.; Saffioti, S.; Russo, R.; Deferrari, G.; Rossi, D.; Verzola, D.; Gandolfo, M.T.; Sala, M.R. Interorgan exchange of aminothiols in humans. Am. J. Physiol. Metab. 2003, 284, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Guttormsen, A.B.; Ueland, P.M.; Svarstad, E.; Refsum, H. Kinetic basis of hyperhomocysteinemia in patients with chronic renal failure. Kidney Int. 1997, 52, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Garibotto, G.; Valli, A.; Anderstam, B.; Eriksson, M.; Suliman, M.E.; Balbi, M.; Rollando, D.; Vigo, E.; Lindholm, B. The kidney is the major site of S-adenosylhomocysteine disposal in humans. Kidney Int. 2009, 76, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Bulgakova, P.O.; Virus, E.D.; Kruglova, M.P.; Alexandrin, V.V.; Gadieva, V.A.; Luzyanin, B.P.; Kushlinskii, N.E.; Fedoseev, A.N.; Kubatiev, A.A. Capillary electrophoresis coupled with chloroform-acetonitrile extraction for rapid and highly selective determination of cysteine and homocysteine levels in human blood plasma and urine. Electrophoresis 2017, 38, 2646–2653. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Virus, E.D.; Nikiforova, K.A.; Kushlinskii, N.E.; Luzyanin, B.P.; Maksimova, M.Y.; Piradov, M.A.; Kubatiev, A.A. Capillary electrophoresis and phenylboronic acid solid phase extraction for the determination of S-adenosylmethionine/S-adenosylhomocysteine ratio in human urine. Electrophoresis 2016, 37, 2663–2669. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Kruglova, M.P.; Virus, E.D.; Bulgakova, P.O.; Grachev, S.V.; Kubatiev, A.A. Determination of S -adenosylmethionine, S-adenosylhomocysteine, and methylthioadenosine in urine using solvent-modified micellar electrokinetic chromatography. Electrophoresis 2020, 41, 209–214. [Google Scholar] [CrossRef]
- Perna, A.F.; Ingrosso, D. Atherosclerosis determinants in renal disease: How much is homocysteine involved? Nephrol Dial Transplant 2016, 31, 860–863. [Google Scholar] [CrossRef]
- Perna, A.F.; Ingrosso, D.; Satta, E.; Lombardi, C.; Acanfora, F.; De Santo, N.G. Homocysteine metabolism in renal failure. Curr. Opin. Clin. Nutr. Metab Care 2004, 78, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Ingrosso, D. Homocysteine and chronic kidney disease: An ongoing narrative. J. Nephrol. 2019, 32, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Benito, S.; Sánchez-Ortega, A.; Unceta, N.; Jansen, J.J.; Postma, G.; Andrade, F.; Aldámiz-Echevarria, L.; Buydens, L.M.C.; Goicolea, M.A.; Barrio, R.J. Plasma biomarker discovery for early chronic kidney disease diagnosis based on chemometric approaches using LC-QTOF targeted metabolomics data. J. Pharm. Biomed. Anal. 2018, 149, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Sarnak, M.J.; Wang, S.-R.; Beck, G.J.; Kusek, J.W.; Selhub, J.; Greene, T.; Levey, A.S. Homocysteine, cysteine, and B vitamins as predictors of kidney disease progression. Am. J. Kidney Dis. 2002, 40, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.J.; Pimpão, A.B.; Fernandes, D.G.F.; Morello, J.; Sequeira, C.O.; Calado, J.; Antunes, A.M.M.; Almeida, M.S.; Branco, P.; Monteiro, E.C.; et al. Cysteine as a Multifaceted Player in Kidney, the Cysteine-Related Thiolome and Its Implications for Precision Medicine. Molecules 2022, 27, 1416. [Google Scholar] [CrossRef] [PubMed]
- States, B.; Foreman, J.W.; Segal, S. Cysteine and Glutathione Levels in Developing Rat Kidney and Liver. Pediatr. Res. 1987, 22, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M.; Sanchez, W.Y.; Roy, S.F.; Morais, C.; Brooks, H.L.; Coombes, J.S.; Johnson, D.W.; Gobe, G.C. N-acetyl-cysteine increases cellular dysfunction in progressive chronic kidney damage after acute kidney injury by dampening endogenous antioxidant responses. Am. J. Physiol. Physiol. 2018, 314, F956–F968. [Google Scholar] [CrossRef]
- Wlodek, P.J.; Iciek, M.B.; Milkowski, A.; Smolenski, O.B. Various forms of plasma cysteine and its metabolites in patients undergoing hemodialysis. Clin. Chim. Acta 2001, 304, 9–18. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Guan, Y.F. Hyperhomocysteinemia and kidney diseases. Sheng Li Xue Bao 2018, 70, 607–611. (In Chinese) [Google Scholar]
- Zhao, W.; Zhou, L.; Novák, P.; Shi, X.; Lin, C.B.; Zhu, X.; Yin, K. Metabolic Dysfunction in the Regulation of the NLRP3 Inflammasome Activation: A Potential Target for Diabetic Nephropathy. J. Diabetes Res. 2022, 2022, 2193768. [Google Scholar] [CrossRef]
- De Martinis, M.; Sirufo, M.M.; Nocelli, C.; Fontanella, L.; Ginaldi, L. Hyperhomocysteinemia is Associated with Inflammation, Bone Resorption, Vitamin B12 and Folate Deficiency and MTHFR C677T Polymorphism in Postmenopausal Women with Decreased Bone Mineral Density. Int. J. Environ. Res. Public Health 2020, 17, 4260. [Google Scholar] [CrossRef]
- Liu, S.; Tao, J.; Duan, F.; Li, H.; Tan, H. HHcy Induces Pyroptosis and Atherosclerosis via the Lipid Raft-Mediated NOX-ROS-NLRP3 Inflammasome Pathway in apoE−/− Mice. Cells 2022, 11, 2438. [Google Scholar] [CrossRef]
- Lai, W.K.C.; Kan, M.Y. Homocysteine-Induced Endothelial Dysfunction. Ann. Nutr. Metab. 2015, 67, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Koklesova, L.; Mazurakova, A.; Samec, M.; Biringer, K.; Samuel, S.M.; Büsselberg, D.; Kubatka, P.; Golubnitschaja, O. Homocysteine metabolism as the target for predictive medical approach, disease prevention, prognosis, and treatments tailored to the person. EPMA J. 2021, 12, 477–505. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Zheng, K. Homocysteine and the Mortality of Critically Ill Patients: A Meta-Analysis. Horm. Metab. Res. 2022, 54, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Barron, E.; Lara, J.; White, M.; Mathers, J.C. Blood-Borne Biomarkers of Mortality Risk: Systematic Review of Cohort Studies. PLoS ONE 2015, 10, e0127550. [Google Scholar] [CrossRef]
| Variables | All | Stage II | Stages IIIa–IIIb | Stages IV–V | p-Value |
|---|---|---|---|---|---|
| General characteristics | |||||
| N | n = 110 | n = 33 (30%) | n = 46 (41.8%) | n = 31 (28.2%) | - |
| Gender, men | n = 46 (41.8%) | n = 17 (51.5%) | n = 17 (36.9%) | n = 12 (38.7%) | 0.594 |
| Age, full years | 67 (56; 76) | 52 (38.5; 61.5) | 70.5 (59; 78) * | 74 (67; 79) * | <0.001 |
| BMI, kg/m2 | 28.9 (23.4; 34.0) | 26.2 (23.0; 32.8) | 29.4 (24.8; 34.3) | 29.4 (22.1; 33.1) | 0.335 |
| Obesity | n = 45 (40.9%) | n = 11 (33.3%) | n = 20 (43.5%) | n = 14 (45.2%) | 0.565 |
| Edema | n = 32 (29%) | n = 6 (18%) | n = 12 (26%) | n = 14 (45%) | <0.001 |
| Duration of CKD, years | 2 (1; 5) | 1 (1; 3.5) | 2 (1; 4) | 1 (1; 5) | 0.609 |
| Clinical and laboratory parameters | |||||
| Blood plasma creatinine, µmol/L | 118.0 (96.0; 198.0) | 85.0 (83.0; 97.0) | 118.5 (107.0; 144.0) * | 357.0 (245.5; 481.0) * | <0.001 IIIa–IIIb–IV–V (0.001) |
| GFR, mL/min/1.73 m2 | 44.1 (28.0; 64.8) | 74.4 (68.2; 80.7) | 43.5 (38.0; 49.2) * | 10.6 (8.35; 19.6) * | <0.001 IIIa–IIIb–IV–V (0.001) |
| Uric acid, µmol/L | 399.5 (314.0; 483.0) | 315.0 (247.0; 403.0) | 403.0 (340.0; 465.0) * | 462.0 (374.5; 564.0) * | <0.001 |
| Urea, µmol/L | 8.1 (5.2; 14.6) | 4.6 (3.8; 5.4) | 8.3 (6.5; 10.7) | 18.0 (15.7; 23.4) * | <0.001 IIIa–IIIb–IV–V (<0.001) |
| Sodium, mmol/L | 142.0 (141.0; 144.0) | 142.0 (141.0; 144.0) | 143.5 (142.0; 145.0) | 141.0 (138.5; 143.5) * | <0.001 IIIa–IIIb–IV–V (<0.001) |
| Potassium, mmol/L | 4.6 (4.3; 5.0) | 4.4 (4.2; 4.6) | 4.6 (4.3; 4.9) | 5.0 (4.5;5.4) * | <0.001 |
| Blood plasma albumin, g/L | 42.4 (38.9; 45.8) | 43.6 (40.9; 47.2) | 42.8 (40.8; 45.7) | 38.0 (35.2; 42.3) * | <0.001 IIIa–IIIb–IV–V (0.003) |
| Daily urine protein, g/L | 0.2 (0.1; 0.8) | 0.1 (0.1; 0.2) | 0.1 (0.1; 0.4) | 0.9 (0.3; 2.7) * | <0.001 IIIa–IIIb–IV–V (<0.001) |
| Hemoglobin g/L | 127.5 (107.0; 144.0) | 144.0 (134.0; 147.0) | 134.0 (117.0; 145.0) | 101.0 (90.0; 116.5) * | <0.001 |
| Variables | Controls (n = 50) | CKD (n = 110) | p-Value (Mann–Whitney Test) |
|---|---|---|---|
| urine | |||
| Cr, µmol/L | 3.9 (2.5; 5.4) | 2.3 (1.3; 3.3) | <0.001 * |
| SAM, µmol/L | 9.5 (6.1; 12.1) | 3.3 (1.7; 5.9) | <0.001 * |
| SAH, µmol/L | 0.9 (0.4; 1.6) | 1.1 (0.5; 1.7) | 0.262 |
| SAM/SAH | 8.2 (3.7; 26.0) | 3.3 (1.9; 6.8) | <0.001 * |
| Cys, µmol/L | 136.8 (95.7; 211.1) | 59.0 (31.6; 120.9) | <0.001 * |
| Hcy, µmol/L | 2.9 (1.8; 4.5) | 1.6 (0.8; 2.9) | <0.001 * |
| blood | |||
| Cr, µmol/L | 76.0 (71.0; 88.0) | 118.0 (96.0; 198.0) | <0.001 * |
| Cys, µmol/L | 264.0 (214.4; 303.2) | 420.1 (331.4; 513.1) | <0.001 * |
| Hcy, µmol/L | 10.9 (8.6; 12.5) | 20.9 (15.9; 28.8) | <0.001 * |
| Variables | Control Group (n = 50) | CKD at Stage II (n = 33) | CKD at Stages IIIa–IIIb (n = 46) | CKD at Stages IV–V (n = 31) | p-Value (Kruskal–Wallis Test) |
|---|---|---|---|---|---|
| urine | |||||
| Cr, µmol/L | 3.9 (2.5; 5.4) | 2.8 (1.4; 3.5) | 2.3 * (1.5; 3.4) | 1.6 * (1.3; 2.7) | <0.001 |
| SAM, µmol/L | 9.5 (6.1; 12.1) | 6.7 (4.3; 9.2) | 3.2 * (1.8; 5.5) | 2.1 * (1.5; 2.8) | <0.001 II–IIIa–IIIb (0.019); II–IV–V (0.000) |
| SAH, µmol/L | 0.9 (0.4; 1.6) | 1.0 (0.6; 1.5) | 1.3 (0.5; 1.7) | 0.9 (0.6; 1.9) | 0.694 |
| SAM/SAH | 8.2 (3.7; 26.0) | 6.8 (4.7; 8.6) | 2.8 * (1.9; 4.8) | 1.9 * (1.2; 2.8) | <0.001 II–IIIa–IIIb (0.002); II–IV–V (0.000) |
| Cys, µmol/L | 136.8 (95.7; 211.1) | 117.6 (54.8; 190.8) | 42.8 * (26.0; 70.4) | 53.6 * (29.9; 128.3) | <0.001 II–IIIa–IIIb (0.000) |
| Hcy, µmol/L | 2.9 (1.8; 4.5) | 2.5 (1.3; 3.3) | 1.3 * (0.7; 2.5) | 1.5 * (1.0; 2.5) | <0.001 |
| blood | |||||
| Cr, µmol/L | 76.0 (71.0; 88.0) | 85.0 (83.0; 97.0) | 118.5 * (107.0; 144.0) | 357.0 * (245.5; 481.0) | <0.001 II–IIIa–IIIb (0.000); II–IV–V (0.000); IIIa–IIIb–IV–V (0.001) |
| Cys, µmol/L | 264.0 (214.4; 303.2) | 410.3 * (319.4; 513.1) | 424.2 * (334.7; 471.5) | 413.7 * (336.4; 569.8) | <0.001 |
| Hcy, µmol/L | 10.9 (8.6; 12.5) | 16.7 * (13.5; 22.7) | 19.2 * (15.7; 23.3) | 28.7 * (20.8; 47.9) | <0.001 II–IV–V (0.004); IIIa–IIIb–IV–V (0.031) |
| Control–CKD | AUC | p | Control–Stage II of CKD | AUC | p |
|---|---|---|---|---|---|
| Cr, blood plasma, µmol/L | 0.915 | 0.022 | Cys, blood plasma, µmol/L | 0.857 | 0.000 |
| Hcy, blood plasma, µmol/L | 0.905 | 0.025 | Hcy, blood plasma, µmol/L | 0.848 | 0.000 |
| Cys, blood plasma, µmol/L | 0.880 | 0.026 | Cr, blood plasma, µmol/L | 0.755 | 0.000 |
| SAH, urine, µmol/L | 0.555 | 0.050 | SAH, urine µmol/L | 0.538 | 0.564 |
| Hcy, urine, µmol/L | 0.310 | 0.043 | SAM/SAH, urine, µmol/L | 0.416 | 0.199 |
| Cr, urine, µmol/L | 0.275 | 0.045 | Hcy, urine, µmol/L | 0.409 | 0.161 |
| SAM/SAH, urine | 0.263 | 0.045 | Cys, urine, µmol/L | 0.405 | 0.147 |
| Cys, urine, µmol/L | 0.255 | 0.039 | SAM, urine, µmol/L | 0.388 | 0.086 |
| SAM, urine, µmol/L | 0.240 | 0.044 | Cr, urine, µmol/L | 0.323 | 0.007 |
| CKD at stages II and IIIa–IIIв | AUC | p | CKD at stages IIIa–IIIb and IV–V | AUC | p |
| Cr, blood plasma, µmol/L | 0.938 | 0.000 | Cr, blood plasma, µmol/L | 0.985 | 0.011 |
| Hcy, blood plasma, µmol/L | 0.567 | 0.311 | Hcy, blood plasma µmol/L | 0.756 | 0.057 |
| SAH, urine, µmol/L | 0.531 | 0.644 | Cys, urine, µmol/L | 0.572 | 0.068 |
| Cys, blood plasma, µmol/L | 0.495 | 0.937 | Hcy, urine, µmol/L | 0.559 | 0.067 |
| Cr, urine, µmol/L | 0.440 | 0.366 | Cys, blood plasma, µmol/L | 0.531 | 0.070 |
| Hcy, urine, µmol/L | 0.336 | 0.013 | SAH, urine, µmol/L | 0.509 | 0.067 |
| SAM, urine, µmol/L | 0.282 | 0.001 | Cr, urine, µmol/L | 0.392 | 0.066 |
| Cys, urine, µmol/L | 0.221 | 0.000 | SAM/SAH, urine, µmol/L | 0.327 | 0.064 |
| SAM/SAH, urine, µmol/L | 0.209 | 0.000 | SAM, urine, µmol/L | 0.325 | 0.061 |
| № | Variables | Survival (Spearman Correlation Coefficient) | AUC | p |
|---|---|---|---|---|
| 1 | Blood plasma urea, mmol/L | 0.538 ** | 0.845 | 0.000 |
| 2 | Blood plasma creatinine, µmol/L | 0.746 ** | 0.836 | 0.000 |
| 3 | Age, full years | 0.588 ** | 0.828 | 0.000 |
| 4 | CKD stage | 0.577 ** | 0.827 | 0.000 |
| 5 | Protein in urine, g/L | 0.425 ** | 0.772 | 0.000 |
| 6 | Urine protein/creatinine ratio (PCR), mg/mmol | 0.405 ** | 0.760 | 0.000 |
| 7 | ESR, mm/h | 0.396 ** | 0.754 | 0.000 |
| 8 | Daily urine protein, g/L | 0.380 ** | 0.744 | 0.000 |
| 9 | Blood plasma homocysteine, µmol/L | 0.685 ** | 0.733 | 0.000 |
| 10 | Granulocytes, % | 0.349 ** | 0.724 | 0.000 |
| 11 | Arterial pressure systolic adapted, mm Hg. | 0.351 ** | 0.717 | 0.000 |
| 12 | Leukocytes * 109/L | 0.320 ** | 0.705 | 0.001 |
| 13 | Segmented neutrophils, % | 0.317 ** | 0.703 | 0.001 |
| 14 | SAM urine, µmol/L | −0.314 ** | 0.292 | 0.006 |
| 15 | Bilirubin total, µmol/L | −0.347 ** | 0.278 | 0.000 |
| 16 | SAM/SAH urine | −0.356 ** | 0.276 | 0.001 |
| 17 | Lymphocytes, % | −0.350 ** | 0.275 | 0.025 |
| 18 | Iron, µmol/L | −0.422 ** | 0.229 | 0.000 |
| 19 | Hematocrit, % | −0.427 ** | 0.226 | 0.000 |
| 20 | Daily diuresis, L/day | −0.441 ** | 0.217 | 0.000 |
| 21 | Hemoglobin, g/L | −0.485 ** | 0.189 | 0.000 |
| 22 | Red blood cells * 1012/L | −0.511 ** | 0.172 | 0.000 |
| 23 | Glomerular filtration rate, mL/min/1.73 m2 | −0.576 ** | 0.130 | 0.000 |
| B | SE | Wald | df | Sig. | Exp(B) | 95% CI for Exp(B) | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Blood plasma homocysteine, µmol/L | 0.029 | 0.012 | 5.353 | 1 | 0.021 | 1.029 | 1.004 | 1.054 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruglova, M.P.; Ivanov, A.V.; Fedoseev, A.N.; Virus, E.D.; Stupin, V.A.; Parfenov, V.A.; Titova, S.A.; Lazareva, P.I.; Kubatiev, A.A.; Silina, E.V. The Diagnostic and Prognostic Roles Played by Homocysteine and Other Aminothiols in Patients with Chronic Kidney Disease. J. Clin. Med. 2023, 12, 5653. https://doi.org/10.3390/jcm12175653
Kruglova MP, Ivanov AV, Fedoseev AN, Virus ED, Stupin VA, Parfenov VA, Titova SA, Lazareva PI, Kubatiev AA, Silina EV. The Diagnostic and Prognostic Roles Played by Homocysteine and Other Aminothiols in Patients with Chronic Kidney Disease. Journal of Clinical Medicine. 2023; 12(17):5653. https://doi.org/10.3390/jcm12175653
Chicago/Turabian StyleKruglova, Maria Petrovna, Alexander Vladimirovich Ivanov, Anatolij Nikolaevich Fedoseev, Edward Danielevich Virus, Victor Aleksandrovich Stupin, Vladimir Anatolyevich Parfenov, Svetlana Andreevna Titova, Polina Igorevna Lazareva, Aslan Amirkhanovich Kubatiev, and Ekaterina Vladimirovna Silina. 2023. "The Diagnostic and Prognostic Roles Played by Homocysteine and Other Aminothiols in Patients with Chronic Kidney Disease" Journal of Clinical Medicine 12, no. 17: 5653. https://doi.org/10.3390/jcm12175653
APA StyleKruglova, M. P., Ivanov, A. V., Fedoseev, A. N., Virus, E. D., Stupin, V. A., Parfenov, V. A., Titova, S. A., Lazareva, P. I., Kubatiev, A. A., & Silina, E. V. (2023). The Diagnostic and Prognostic Roles Played by Homocysteine and Other Aminothiols in Patients with Chronic Kidney Disease. Journal of Clinical Medicine, 12(17), 5653. https://doi.org/10.3390/jcm12175653






