Hepatic Involvement across the Metabolic Syndrome Spectrum: Non-Invasive Assessment and Risk Prediction Using Machine Learning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Statistical Analysis
2.3. Machine-Learning Approach
3. Results
3.1. Description of the Study Population
3.1.1. General Characteristics and Measured Parameters
3.1.2. Distribution of Weight Categories
3.2. Study Population According to the New Fatty Liver Disease Nomenclature
3.3. Analysis of Liver Steatosis and Fibrosis
3.3.1. Steatosis Measured Using Transient Elastography
3.3.2. Fibrosis Measured via Transient Elastography
3.3.3. Machine-Learning Algorithms for Risk Stratification
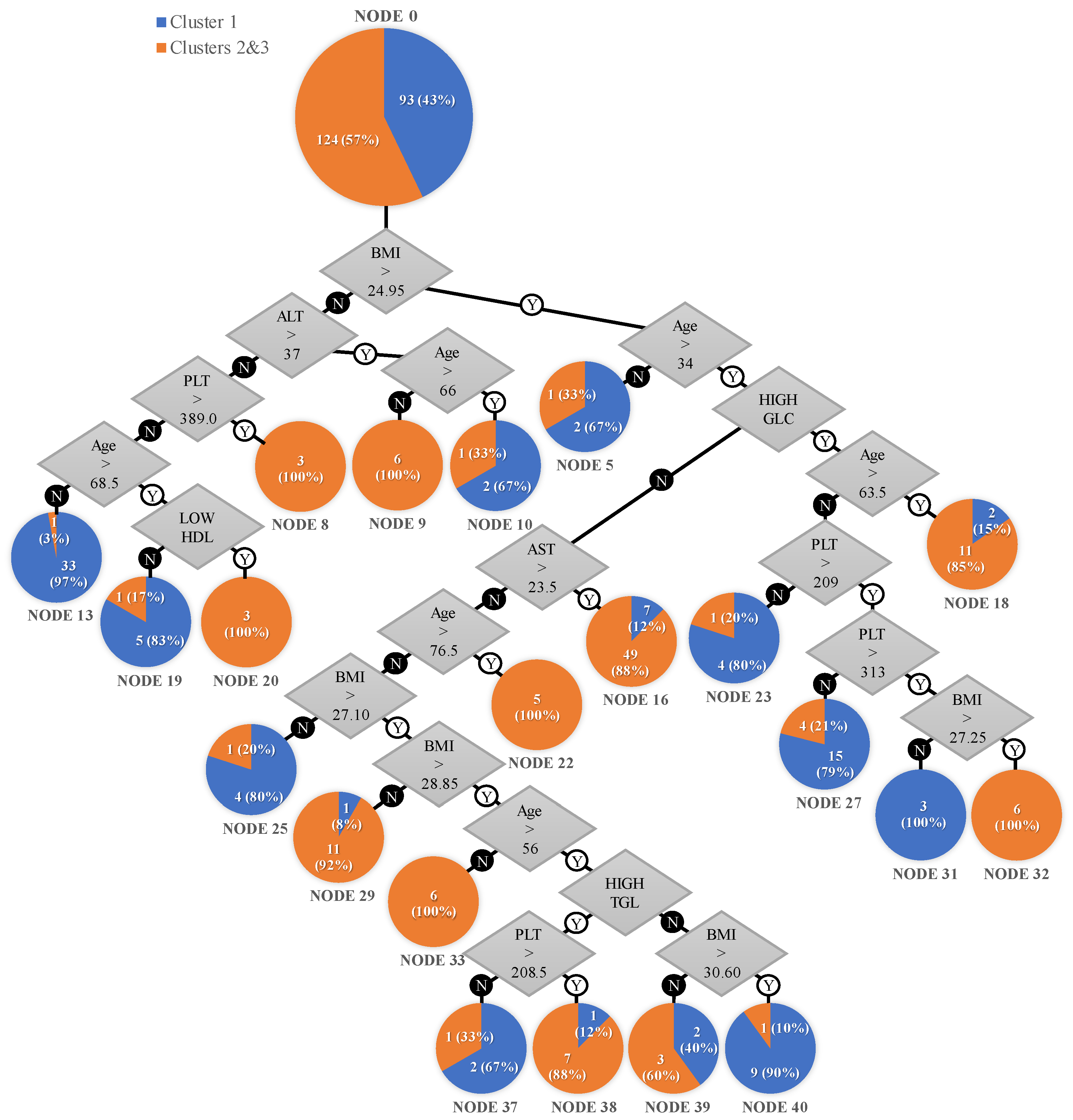
- A total of 12 patients with a normal BMI (<24.95 kg/m²), 6 aged under 66, with an ALT > 37 U/L, 3 with an AST < 37 U/L and platelet count > 389 × 109/L, and 3 aged over 68.5, with an ALT < 37 U/L and low HDL cholesterol, all 12 (100%) belonging to the hepatic involvement cluster.
- A total of 17 patients with a high BMI (>24.95 kg/m²), 5 aged over 76.5, 6 aged 34–63.5 with diabetes mellitus or impaired fasting glucose, BMI > 27.25, and platelet count > 313 × 109/L, and 6 aged 34–56 with a BMI > 28.85 kg/m², all 17 (100%) belonging to the hepatic involvement cluster.
- A total of 56 patients with a BMI > 24.95 kg/m², aged > 34, with an AST > 23.5 U/L, of which 49 (88%) were in the hepatic involvement cluster and 7 (12%) were in cluster 1.
- A total of 12 patients with a BMI ranged 27.1–28.85 kg/m², aged 34–76.5, with an AST < 23.5 U/L, of which 11 (92%) were in the hepatic involvement cluster and 1 (8%) was in cluster 1.
- A total of 13 patients with a BMI > 24.95 kg/m², aged > 63.5, with diabetes mellitus or impaired fasting glucose of whom 11 (85%) were in the hepatic involvement cluster and 2 (15%) were in cluster 1.
- A total of 8 patients with a BMI > 28.85 kg/m², aged 56–76.5, with high triglycerides and platelet count > 208.5 × 109/L, of whom 7 (88%) were in the hepatic involvement cluster and 1 (12%) was in cluster 1.
- A total of 5 patients with a BMI ranged 28.85–30.6 kg/m², aged over 56, with an AST < 23.5 U/L and normal fasting glucose, of whom 2 (40%) belonged to cluster 1 and 3 (60%) were in the hepatic involvement cluster.
- A total of 3 patients with a BMI > 24.95 kg/m², aged under 34, of whom 1 (33%) was in the hepatic involvement cluster and 2 (67%) were in cluster 1.
- A total of 3 patients with a BMI < 24.95 kg/m², aged over 66, with an ALT > 37 U/L, of whom 1 (33%) was in the hepatic involvement cluster and 2 (67%) were in cluster 1.
- A total of 34 patients with a BMI < 24.95 kg/m², aged under 68.5, with an ALT < 37 U/L and platelet count < 389 × 109/L, of which 1 (3%) was in the hepatic involvement cluster and 33 (97%) were in cluster 1.
- A total of 6 patients with a BMI < 24.95 kg/m², aged under 68.5, with an ALT < 37 U/L and platelet count < 389 × 109/L, with normal HDL cholesterol, of whom 1 (17%) was in the hepatic involvement cluster and 5 (83%) were in cluster 1.
- A total of 5 patients with a BMI > 24.95 kg/m², aged 34–63.5, with diabetes mellitus or impaired fasting glucose, platelet count < 209 × 109/L, of whom 1 (20%) was in the hepatic involvement cluster and 4 (80%) were in cluster 1.
- A total of 9 patients, aged 34–63.5 with diabetes mellitus or impaired fasting glucose, platelet count of 209–313 × 109/L, of whom 4 (21%) were in the hepatic involvement cluster and 15 (79%) were in cluster 1.
- A total of 10 patients with a BMI > 30.60 kg/m², aged 56–76.5, with normal triglycerides and AST < 23.5 U/L, of whom 1 (10%) was in the hepatic involvement cluster and 9 (90%) were in cluster 1.
- A total of 5 patients with a BMI ranged 24.95–27.1 kg/m², aged 34–76.5, AST < 23.5 U/L, of whom 1 (20%) was in the hepatic involvement cluster and 4 (80%) were in cluster 1.
- A total of 3 patients with BMI > 28.85 kg/m², aged 56–76.5, with high triglycerides and platelet count < 208.5 × 109/L, of whom 1 (33%) was in the hepatic involvement cluster and 2 (67%) were in cluster 1.
- A total of 3 patients with a BMI ranged 24.95–27.25 kg/m², aged 34–63.5, with diabetes mellitus or impaired fasting glucose, platelet count > 313 × 109/L, of whom 0 (0%) were in the hepatic involvement cluster and 3 (100%) were in cluster 1.
4. Discussion
4.1. Characteristics of Patients with MetS
4.1.1. Demographics
4.1.2. Liver Involvement
4.2. Strengths and Limitations
4.2.1. Liver Biopsy
4.2.2. Sample Size
4.2.3. Confounding Factors
4.3. Financial Aspects
4.4. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yunossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The Global Epidemiology of Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH): A Systematic Review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Tinajero, M.G.; Malik, V.S. An Update on the Epidemiology of Type 2 Diabetes. Endocrinol. Metab. Clin. N. Am. 2021, 50, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.-F. Global, Regional, and National Burden and Trend of Diabetes in 195 Countries and Territories: An Analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef]
- Tang, A.S.P.; Chan, K.E.; Quek, J.; Xiao, J.; Tay, P.; Teng, M.; Lee, K.S.; Lin, S.Y.; Myint, M.Z.; Tan, B.; et al. Non-Alcoholic Fatty Liver Disease Increases Risk of Carotid Atherosclerosis and Ischemic Stroke: An Updated Meta-Analysis with 135,602 Individuals. Clin. Mol. Hepatol. 2022, 28, 483–496. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A Multisystem Disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Moschen, A.R. Multiple Parallel Hits Hypothesis in Nonalcoholic Fatty Liver Disease: Revisited After a Decade. Hepatology 2021, 73, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Lake-Bakaar, G.; Robertson, J.; Aardema, C. The Effect of Obeticholic Acid on Hepatic Blood Flow in Isolated, Perfused Porcine Liver: Correction of Oxygen-nutrient Mismatch Might Be a Putative Mechanism of Action in NASH. Clin. Transl. Discov. 2022, 2, e98. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Nascimbeni, F.; Bedossa, P.; Fedchuk, L.; Pais, R.; Charlotte, F.; Lebray, P.; Poynard, T.; Ratziu, V. Clinical Validation of the FLIP Algorithm and the SAF Score in Patients with Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2020, 72, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased Risk of Mortality by Fibrosis Stage in Nonalcoholic Fatty Liver Disease: Systematic Review and Meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef]
- Golabi, P.; Otgonsuren, M.; de Avila, L.; Sayiner, M.; Rafiq, N.; Younossi, Z.M. Components of Metabolic Syndrome Increase the Risk of Mortality in Nonalcoholic Fatty Liver Disease (NAFLD). Medicine 2018, 97, e0214. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.E.; Wong, V.W.-S.; Rinella, M. Non-Alcoholic Fatty Liver Disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sanyal, A.J.; George, J.; Sanyal, A.; Neuschwander-Tetri, B.; Tiribelli, C.; Kleiner, D.E.; Brunt, E.; Bugianesi, E.; Yki-Järvinen, H.; et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multi-Society Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. Hepatology 2023. online ahead of print. [Google Scholar] [CrossRef]
- Wong, V.W.-S.; Chu, W.C.-W.; Wong, G.L.-H.; Chan, R.S.-M.; Chim, A.M.-L.; Ong, A.; Yeung, D.K.-W.; Yiu, K.K.-L.; Chu, S.H.-T.; Woo, J.; et al. Prevalence of Non-Alcoholic Fatty Liver Disease and Advanced Fibrosis in Hong Kong Chinese: A Population Study Using Proton-Magnetic Resonance Spectroscopy and Transient Elastography. Gut 2012, 61, 409–415. [Google Scholar] [CrossRef]
- Hardy, T.; Wonders, K.; Younes, R.; Aithal, G.P.; Aller, R.; Allison, M.; Bedossa, P.; Betsou, F.; Boursier, J.; Brosnan, M.J.; et al. The European NAFLD Registry: A Real-World Longitudinal Cohort Study of Nonalcoholic Fatty Liver Disease. Contemp. Clin. Trials 2020, 98, 106175. [Google Scholar] [CrossRef]
- Matteoni, C.; Younossi, Z.; Gramlich, T.; Boparai, N.; Liu, Y.; Mccullough, A. Nonalcoholic Fatty Liver Disease: A Spectrum of Clinical and Pathological Severity. Gastroenterology 1999, 116, 1413–1419. [Google Scholar] [CrossRef]
- Springer, F. Liver Fat Content Determined by Magnetic Resonance Imaging and Spectroscopy. World J. Gastroenterol. 2010, 16, 1560. [Google Scholar] [CrossRef]
- Martí-Aguado, D.; Jiménez-Pastor, A.; Alberich-Bayarri, Á.; Rodríguez-Ortega, A.; Alfaro-Cervello, C.; Mestre-Alagarda, C.; Bauza, M.; Gallén-Peris, A.; Valero-Pérez, E.; Ballester, M.P.; et al. Automated Whole-Liver MRI Segmentation to Assess Steatosis and Iron Quantification in Chronic Liver Disease. Radiology 2022, 302, 345–354. [Google Scholar] [CrossRef]
- Almeida, A.-M. Fatty Liver Disease in Severe Obese Patients: Diagnostic Value of Abdominal Ultrasound. World J. Gastroenterol. 2008, 14, 1415. [Google Scholar] [CrossRef]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Thiele, M. EASL Clinical Practice Guidelines on Non-Invasive Tests for Evaluation of Liver Disease Severity and Prognosis—2021 Update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, D.; Kim, H.J.; Lee, C.-H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.-H.; Cho, S.-H.; Sung, M.-W.; et al. Hepatic Steatosis Index: A Simple Screening Tool Reflecting Nonalcoholic Fatty Liver Disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the Clinical Assessment and Management of Nonalcoholic Fatty Liver Disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.S.; Harrison, S.A.; Abdelmalek, M.F.; Anstee, Q.M.; Bedossa, P.; Castera, L.; Dimick-Santos, L.; Friedman, S.L.; Greene, K.; Kleiner, D.E.; et al. Case Definitions for Inclusion and Analysis of Endpoints in Clinical Trials for Nonalcoholic Steatohepatitis through the Lens of Regulatory Science. Hepatology 2018, 67, 2001–2012. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramaniam, A.; Wolfson, J.; Mitchell, N.; Barnes, T.; JaKa, M.; French, S. Decision Trees in Epidemiological Research. Emerg. Themes Epidemiol. 2017, 14, 11. [Google Scholar] [CrossRef]
- French, S.A.; Mitchell, N.R.; Wolfson, J.; Harnack, L.J.; Jeffery, R.W.; Gerlach, A.F.; Blundell, J.E.; Pentel, P.R. Portion Size Effects on Weight Gain in a Free Living Setting. Obesity 2014, 22, 1400–1405. [Google Scholar] [CrossRef]
- Eletreby, R.; Abdellatif, Z.; Gaber, Y.; Ramadan, A.; Ahmad, N.; Khattab, H.; Said, M.; Saad, Y. Validity of Routine Biochemical and Ultrasound Scores for Prediction of Hepatic Fibrosis and Steatosis in NAFLD. Egypt. Liver J. 2021, 11, 44. [Google Scholar] [CrossRef]
- Ratziu, V.; Bellentani, S.; Cortez-Pinto, H.; Day, C.; Marchesini, G. A Position Statement on NAFLD/NASH Based on the EASL 2009 Special Conference. J. Hepatol. 2010, 53, 372–384. [Google Scholar] [CrossRef]
- CDC. Centers for Disease Control and Prevention. Defining Adult Overweight & Obesity. Available online: https://www.cdc.gov/obesity/basics/adult-defining.html (accessed on 7 July 2023).
- Keys, A.; Fidanza, F.; Karvonen, M.J.; Kimura, N.; Taylor, H.L. Indices of Relative Weight and Obesity. Int. J. Epidemiol. 2014, 43, 655–665. [Google Scholar] [CrossRef]
- EASL–EASD–EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2016, 64, 1388–1402. [CrossRef]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.-G.; Mi, Y.-Q.; de Lédinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.-H.; Cardoso, A.C.; et al. Individual Patient Data Meta-Analysis of Controlled Attenuation Parameter (CAP) Technology for Assessing Steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.A.; Gurusamy, K.S.; Ntaoula, S.; Cholongitas, E.; Davidson, B.R.; Burroughs, A.K. Elastography for the Diagnosis of Severity of Fibrosis in Chronic Liver Disease: A Meta-Analysis of Diagnostic Accuracy. J. Hepatol. 2011, 54, 650–659. [Google Scholar] [CrossRef]
- Angulo, P.; Bugianesi, E.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Barrera, F.; Haflidadottir, S.; Day, C.P.; George, J. Simple Noninvasive Systems Predict Long-Term Outcomes of Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2013, 145, 782–789.e4. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Huynh, D.; Zhang, F.; Nguyen, N.Q. Use of Aspartate Aminotransferase to Platelet Ratio to Reduce the Need for FibroScan in the Evaluation of Liver Fibrosis. World J. Hepatol. 2017, 9, 791. [Google Scholar] [CrossRef]
- National Guideline Centre (UK). Non-Alcoholic Fatty Liver Disease: Assessment and Management; (NICE Guideline, No. 49.) 7, Diagnosing the Severity of NAFLD; National Institute for Health and Care Excellence (NICE): London, UK, 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK384727/ (accessed on 27 August 2023).
- Wong, V.W.-S.; Vergniol, J.; Wong, G.L.-H.; Foucher, J.; Chan, H.L.-Y.; Le Bail, B.; Choi, P.C.-L.; Kowo, M.; Chan, A.W.-H.; Merrouche, W.; et al. Diagnosis of Fibrosis and Cirrhosis Using Liver Stiffness Measurement in Nonalcoholic Fatty Liver Disease. Hepatology 2010, 51, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Bril, F.; McPhaul, M.J.; Caulfield, M.P.; Clark, V.C.; Soldevilla-Pico, C.; Firpi-Morell, R.J.; Lai, J.; Shiffman, D.; Rowland, C.M.; Cusi, K. Performance of Plasma Biomarkers and Diagnostic Panels for Nonalcoholic Steatohepatitis and Advanced Fibrosis in Patients with Type 2 Diabetes. Diabetes Care 2020, 43, 290–297. [Google Scholar] [CrossRef]
- Mózes, F.E.; Lee, J.A.; Selvaraj, E.A.; Jayaswal, A.N.A.; Trauner, M.; Boursier, J.; Fournier, C.; Staufer, K.; Stauber, R.E.; Bugianesi, E.; et al. Diagnostic Accuracy of Non-Invasive Tests for Advanced Fibrosis in Patients with NAFLD: An Individual Patient Data Meta-Analysis. Gut 2022, 71, 1006–1019. [Google Scholar] [CrossRef]
- McPherson, S.; Stewart, S.F.; Henderson, E.; Burt, A.D.; Day, C.P. Simple Non-Invasive Fibrosis Scoring Systems Can Reliably Exclude Advanced Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Gut 2010, 59, 1265–1269. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Foucquier, J.; Younossi, Z.M.; Harrison, S.A.; Newsome, P.N.; Chan, W.-K.; Yilmaz, Y.; De Ledinghen, V.; Costentin, C.; Zheng, M.-H.; et al. Enhanced Diagnosis of Advanced Fibrosis and Cirrhosis in Individuals with NAFLD Using FibroScan-Based Agile Scores. J. Hepatol. 2023, 78, 247–259. [Google Scholar] [CrossRef]
- Pennisi, G.; Enea, M.; Pandolfo, A.; Celsa, C.; Antonucci, M.; Ciccioli, C.; Infantino, G.; La Mantia, C.; Parisi, S.; Tulone, A.; et al. AGILE 3+ Score for the Diagnosis of Advanced Fibrosis and for Predicting Liver-Related Events in NAFLD. Clin. Gastroenterol. Hepatol. 2023, 21, 1293–1302.e5. [Google Scholar] [CrossRef]
- Neamțu, B.M.; Visa, G.; Maniu, I.; Ognean, M.L.; Pérez-Elvira, R.; Dragomir, A.; Agudo, M.; Șofariu, C.R.; Gheonea, M.; Pitic, A.; et al. A Decision-Tree Approach to Assist in Forecasting the Outcomes of the Neonatal Brain Injury. Int. J. Environ. Res. Public. Health 2021, 18, 4807. [Google Scholar] [CrossRef] [PubMed]
- Glen, J.; Floros, L.; Day, C.; Pryke, R. Non-Alcoholic Fatty Liver Disease (NAFLD): Summary of NICE Guidance. BMJ 2016, 354, i4428. [Google Scholar] [CrossRef] [PubMed]
- Lembo, E.; Russo, M.F.; Verrastro, O.; Anello, D.; Angelini, G.; Iaconelli, A.; Guidone, C.; Stefanizzi, G.; Ciccoritti, L.; Greco, F.; et al. Prevalence and Predictors of Non-Alcoholic Steatohepatitis in Subjects with Morbid Obesity and with or without Type 2 Diabetes. Diabetes Metab. 2022, 48, 101363. [Google Scholar] [CrossRef] [PubMed]
- Vernon, G.; Baranova, A.; Younossi, Z.M. Systematic Review: The Epidemiology and Natural History of Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis in Adults. Aliment. Pharmacol. Ther. 2011, 34, 274–285. [Google Scholar] [CrossRef]
- Kim, D.; Kim, W.R. Nonobese Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2017, 15, 474–485. [Google Scholar] [CrossRef]
- Tabatabaei-Malazy, O.; Hasani-Ranjbar, S.; Amoli, M.M.; Heshmat, R.; Sajadi, M.; Derakhshan, R.; Amiri, P.; Namakchian, M.; Rezazadeh, E.; Tavakkoly-Bazzaz, J.; et al. Gender-Specific Differences in the Association of Adiponectin Gene Polymorphisms with Body Mass Index. Rev. Diabet. Stud. 2010, 7, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K. Role of Obesity and Lipotoxicity in the Development of Nonalcoholic Steatohepatitis: Pathophysiology and Clinical Implications. Gastroenterology 2012, 142, 711–725.e6. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and Management of the Metabolic Syndrome. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Giannini, E.; Risso, D.; Botta, F.; Chiarbonello, B.; Fasoli, A.; Malfatti, F.; Romagnoli, P.; Testa, E.; Ceppa, P.; Testa, R. Validity and Clinical Utility of the Aspartate Aminotransferase–Alanine Aminotransferase Ratio in Assessing Disease Severity and Prognosis in Patients with Hepatitis C Virus–Related Chronic Liver Disease. Arch. Intern. Med. 2003, 163, 218. [Google Scholar] [CrossRef]
- Graupera, I.; Thiele, M.; Serra-Burriel, M.; Caballeria, L.; Roulot, D.; Wong, G.L.-H.; Fabrellas, N.; Guha, I.N.; Arslanow, A.; Expósito, C.; et al. Low Accuracy of FIB-4 and NAFLD Fibrosis Scores for Screening for Liver Fibrosis in the Population. Clin. Gastroenterol. Hepatol. 2022, 20, 2567–2576.e6. [Google Scholar] [CrossRef]
- Viganò, M.; Pugliese, N.; Cerini, F.; Turati, F.; Cimino, V.; Ridolfo, S.; Rocchetto, S.; Foglio, F.; Terrin, M.; La Vecchia, C.; et al. Accuracy of FIB-4 to Detect Elevated Liver Stiffness Measurements in Patients with Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study in Referral Centers. Int. J. Mol. Sci. 2022, 23, 12489. [Google Scholar] [CrossRef] [PubMed]
- Le, M.H.; Henry, L.; Cheung, R.; Nguyen, M.H. Transient Elastography and Serum-Based Tests for Diagnosis of Fatty Liver and Advanced Fibrosis in a Community Cohort—A Cross Sectional Analysis. Digestive Diseases 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.S.; Vuppalanchi, R.; Van Natta, M.L.; Hallinan, E.; Kowdley, K.V.; Abdelmalek, M.; Neuschwander-Tetri, B.A.; Loomba, R.; Dasarathy, S.; Brandman, D.; et al. Vibration-Controlled Transient Elastography to Assess Fibrosis and Steatosis in Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2019, 17, 156–163.e2. [Google Scholar] [CrossRef]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef] [PubMed]
- Petroff, D.; Blank, V.; Newsome, P.N.; Shalimar; Voican, C.S.; Thiele, M.; de Lédinghen, V.; Baumeler, S.; Chan, W.K.; Perlemuter, G.; et al. Assessment of Hepatic Steatosis by Controlled Attenuation Parameter Using the M and XL Probes: An Individual Patient Data Meta-Analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 185–198. [Google Scholar] [CrossRef]
- Lin, Y.; Feng, X.; Cao, X.; Miao, R.; Sun, Y.; Li, R.; Ye, J.; Zhong, B. Age Patterns of Nonalcoholic Fatty Liver Disease Incidence: Heterogeneous Associations with Metabolic Changes. Diabetol. Metab. Syndr. 2022, 14, 181. [Google Scholar] [CrossRef]
- Schmitz, S.M.; Storms, S.; Koch, A.; Stier, C.; Kroh, A.; Rheinwalt, K.P.; Schipper, S.; Hamesch, K.; Ulmer, T.F.; Neumann, U.P.; et al. Insulin Resistance Is the Main Characteristic of Metabolically Unhealthy Obesity (MUO) Associated with NASH in Patients Undergoing Bariatric Surgery. Biomedicines 2023, 11, 1595. [Google Scholar] [CrossRef]
- Garjani, A.; Safaeiyan, A.; Khoshbaten, M. Association Between Platelet Count as a Noninvasive Marker and Ultrasonographic Grading in Patients with Nonalcoholic Fatty Liver Disease. Hepat. Mon. 2015, 15, e24449. [Google Scholar] [CrossRef]
- Dowman, J.K.; Tomlinson, J.W.; Newsome, P.N. Systematic Review: The Diagnosis and Staging of Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis. Aliment. Pharmacol. Ther. 2011, 33, 525–540. [Google Scholar] [CrossRef]
- de Alwis, N.M.W.; Day, C.P. Non-Alcoholic Fatty Liver Disease: The Mist Gradually Clears. J. Hepatol. 2008, 48, S104–S112. [Google Scholar] [CrossRef]
- Chitturi, S.; Farrell, G.C.; Hashimoto, E.; Saibara, T.; Lau, G.K.; Sollano, J.D. Non-Alcoholic Fatty Liver Disease in the Asia? Pacific Region: Definitions and Overview of Proposed Guidelines. J. Gastroenterol. Hepatol. 2007, 22, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Esmon, C.T. The Impact of the Inflammatory Response on Coagulation. Thromb. Res. 2004, 114, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zhao, C.; Shen, C.; Wang, Y. Cytokeratin 18, Alanine Aminotransferase, Platelets and Triglycerides Predict the Presence of Nonalcoholic Steatohepatitis. PLoS ONE 2013, 8, e82092. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Jong, S.; Gola, A.; Gailer, R.; Morgan, S.; Sennett, K.; Tanwar, S.; Pizzo, E.; O’Beirne, J.; Tsochatzis, E.; et al. Cost-Comparison Analysis of FIB-4, ELF and Fibroscan in Community Pathways for Non-Alcoholic Fatty Liver Disease. BMC Gastroenterol. 2019, 19, 122. [Google Scholar] [CrossRef]
- Lee, D.H. Noninvasive Evaluation of Nonalcoholic Fatty Liver Disease. Endocrinol. Metab. 2020, 35, 243–259. [Google Scholar] [CrossRef]
- van der Ploeg, T.; Austin, P.C.; Steyerberg, E.W. Modern Modelling Techniques Are Data Hungry: A Simulation Study for Predicting Dichotomous Endpoints. BMC Med. Res. Methodol. 2014, 14, 137. [Google Scholar] [CrossRef]
- Lu, M.-Y.; Liu, T.-W.; Liang, P.-C.; Huang, C.-I.; Tsai, Y.-S.; Tsai, P.-C.; Ko, Y.-M.; Wang, W.-H.; Lin, C.-C.; Chen, K.-Y.; et al. Decision Tree Algorithm Predicts Hepatocellular Carcinoma among Chronic Hepatitis C Patients Following Viral Eradication. Am. J. Cancer Res. 2023, 13, 190–203. [Google Scholar]
- Campos-Varela, I.; Villagrasa, A.; Simon-Talero, M.; Riveiro-Barciela, M.; Ventura-Cots, M.; Aguilera-Castro, L.; Alvarez-Lopez, P.; Nordahl, E.A.; Anton, A.; Bañares, J.; et al. The Role of Liver Steatosis as Measured with Transient Elastography and Transaminases on Hard Clinical Outcomes in Patients with COVID-19. Therap Adv. Gastroenterol. 2021, 14, 175628482110165. [Google Scholar] [CrossRef]
- Wai, J.W.; Fu, C.; Wong, V.W.-S. Confounding Factors of Non-Invasive Tests for Nonalcoholic Fatty Liver Disease. J. Gastroenterol. 2020, 55, 731–741. [Google Scholar] [CrossRef]


| Weight Category | Total | Metabolic Syndrome | p-Value | |
|---|---|---|---|---|
| No | Yes | |||
| Normal | 55 (25.35%) | 50 (46.30%) | 5 (4.59%) | <0.01 |
| Overweight | 76 (35.02%) | 38 (35.19%) | 38 (34.86%) | |
| Obesity class I | 52 (23.96%) | 15 (13.89%) | 37 (33.94%) | |
| Obesity class II | 23 (10.60%) | 4 (3.70%) | 19 (17.43%) | |
| Obesity class III | 11 (5.07%) | 1 (0.93%) | 10 (9.17%) | |
| Cardiometabolic Criteria | Total | Metabolic Syndrome | p-Value | |
|---|---|---|---|---|
| No | Yes | |||
| BMI ≥ 25 kg/m2 | 162 (74.65%) | 58 (53.7%) | 104 (95.41%) | p < 0.01 |
| WC | 103 (47.47%) | 22 (20.37%) | 81 (74.31%) | p < 0.01 |
| BP | 126 (58.06%) | 32 (29.63%) | 94 (86.24%) | p < 0.01 |
| FG/T2DM | 142 (65.44%) | 45 (41.67%) | 97 (88.99%) | p < 0.01 |
| TGL | 80 (36.87%) | 19 (17.59%) | 61 (55.96%) | p < 0.01 |
| HDL | 75 (34.56%) | 9 (8.33%) | 66 (60.55%) | p < 0.01 |
| None | 18 (8.29%) | 18 (16.67%) | 0 (0%) | p < 0.01 |
| Steatosis | Total | Metabolic Syndrome | p-Value | |
|---|---|---|---|---|
| No | Yes | |||
| No steatosis | 45 (20.74%) | 36 (33.33%) | 9 (8.25%) | <0.01 |
| Mild steatosis | 39 (17.97%) | 26 (24.07%) | 13 (11.92%) | |
| Moderate steatosis | 50 (23.04%) | 20 (18.52%) | 30 (27.52%) | |
| Severe steatosis | 83 (38.25%) | 26 (24.07%) | 57 (52.29%) | |
| Fibrosis | Total | Metabolic Syndrome | p-Value | |
|---|---|---|---|---|
| No | Yes | |||
| No fibrosis | 142 (65.40%) | 96 (88.89%) | 46 (42.20%) | <0.01 |
| F1 | 17 (7.80%) | 5 (4.62%) | 12 (11.01%) | |
| F2 | 23 (10.60%) | 3 (2.78%) | 20 (18.35%) | |
| F3 | 21 (9.70%) | 1 (0.93%) | 20 (18.35%) | |
| F4 | 14 (6.5%) | 3 (2.78%) | 11 (10.09%) | |
| Variable | Characteristic | Cluster 1 | Cluster 2 | Cluster 3 |
|---|---|---|---|---|
| Count | - | 93 | 103 | 21 |
| E (kPa) | Mean | 4.99 | 6.19 | 19.01 |
| StdDev | 1.93 | 2.18 | 7.38 | |
| IQR | 2.1 | 3.2 | 10.1 | |
| MIN | 2.1 | 2.1 | 11.4 | |
| MAX | 12.9 | 11.3 | 38 | |
| 95%CI | 4.61–5.37 | 5.77–6.62 | 15.66–22.37 | |
| Predictor importance | 1 | |||
| CAP (dB/m) | Mean | 231.63 | 318.53 | 312.81 |
| StdDev | 34.42 | 29.44 | 50.5 | |
| IQR | 52 | 40 | 70 | |
| MIN | 101 | 276 | 219 | |
| MAX | 276 | 400 | 390 | |
| 95%CI | 224.55–238.72 | 312.78–324.29 | 289.82–335.79 | |
| Predictor importance | 0.91 | |||
| Liver fibrosis | No fibrosis | 71 (76.3%) | 67 (65%) | 4 (19.0%) |
| F1 | 10 (10.8%) | 6 (5.8%) | 1 (4.8%) | |
| F2 | 7 (7.5%) | 14 (13.6%) | 2 (9.5%) | |
| F3 | 4 (4.3%) | 11 (10.7%) | 6 (28.6%) | |
| F4 | 1 (1.1%) | 5 (4.9%) | 8 (38.1%) | |
| Liver steatosis | No steatosis | 38 (40.9%) | 6 (5.8%) | 1 (4.8%) |
| Mild steatosis | 28 (30.1%) | 7 (6.8%) | 4 (19%) | |
| Moderate steatosis | 12 (12.9%) | 35 (34%) | 3 (14.3%) | |
| Severe steatosis | 15 (16,.1%) | 55 (53.4%) | 13 (61.9%) | |
| Variable | Characteristic | Cluster 1 | Clusters 2&3 | p-Value |
|---|---|---|---|---|
| Count | - | 93 | 124 | - |
| Liver fibrosis | No fibrosis | 71 (76.3%) | 71 (57.3%) | <0.01 |
| F1 | 10 (10.8%) | 7 (5.6%) | ||
| F2 | 7 (7.5%) | 16 (12.9%) | ||
| F3 | 4 (4.3%) | 17 (13.7%) | ||
| F4 | 1 (1.1%) | 13 (10.5%) | ||
| Liver steatosis | No steatosis | 38 (40.9%) | 7 (5.6%) | <0.01 |
| Mild steatosis | 28 (30.1%) | 11 (8.9%) | ||
| Moderate steatosis | 12 (12.9%) | 38 (30.6%) | ||
| Severe steatosis | 15 (16.1%) | 68 (54.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solomon, A.; Cipăian, C.R.; Negrea, M.O.; Boicean, A.; Mihaila, R.; Beca, C.; Popa, M.L.; Grama, S.M.; Teodoru, M.; Neamtu, B. Hepatic Involvement across the Metabolic Syndrome Spectrum: Non-Invasive Assessment and Risk Prediction Using Machine Learning. J. Clin. Med. 2023, 12, 5657. https://doi.org/10.3390/jcm12175657
Solomon A, Cipăian CR, Negrea MO, Boicean A, Mihaila R, Beca C, Popa ML, Grama SM, Teodoru M, Neamtu B. Hepatic Involvement across the Metabolic Syndrome Spectrum: Non-Invasive Assessment and Risk Prediction Using Machine Learning. Journal of Clinical Medicine. 2023; 12(17):5657. https://doi.org/10.3390/jcm12175657
Chicago/Turabian StyleSolomon, Adelaida, Călin Remus Cipăian, Mihai Octavian Negrea, Adrian Boicean, Romeo Mihaila, Corina Beca, Mirela Livia Popa, Sebastian Mihai Grama, Minodora Teodoru, and Bogdan Neamtu. 2023. "Hepatic Involvement across the Metabolic Syndrome Spectrum: Non-Invasive Assessment and Risk Prediction Using Machine Learning" Journal of Clinical Medicine 12, no. 17: 5657. https://doi.org/10.3390/jcm12175657






