Hypertrophic Cardiomyopathy in a Latin American Center: A Single Center Observational Study
Abstract
1. Introduction
2. Methods
Statistical Analyses
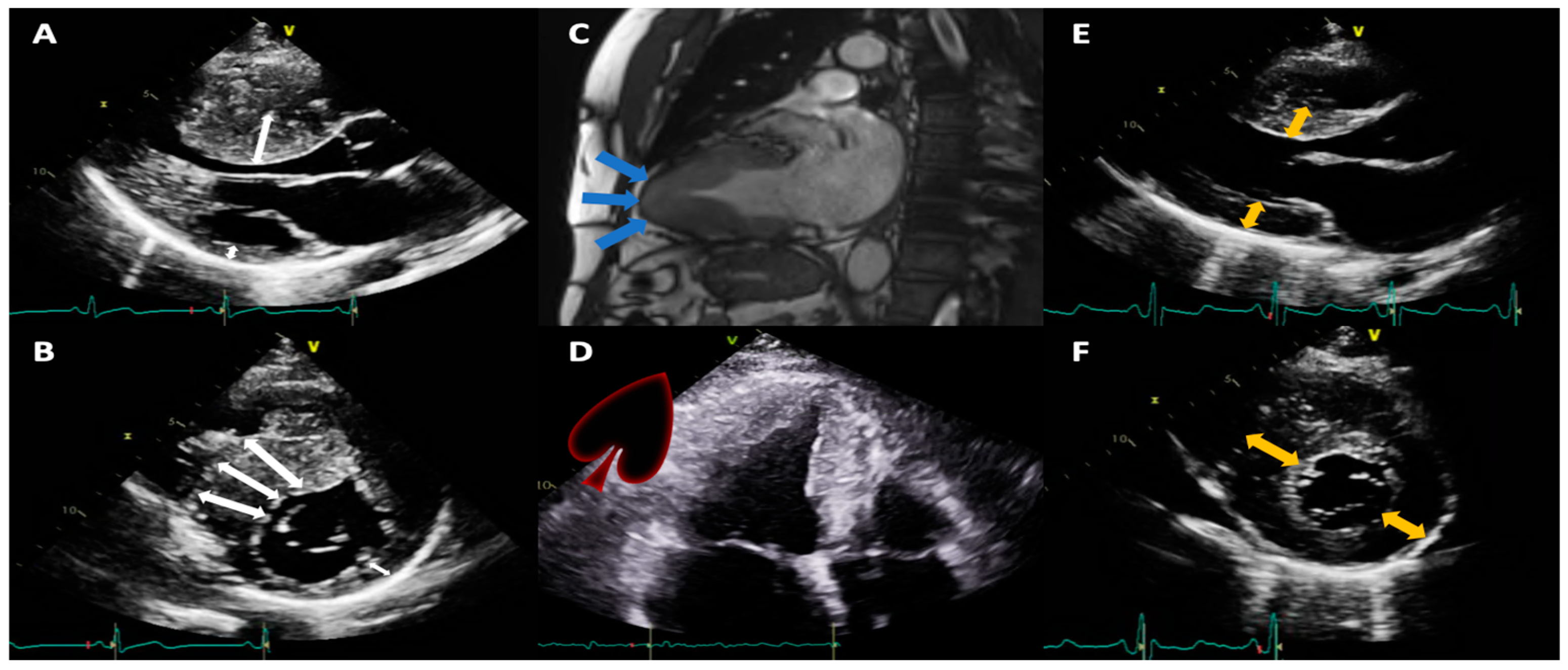
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar] [CrossRef]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the european society of cardiology working group on myocardial and pericardial diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef]
- Zipes, D.P. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. BMH Med. J. 2018, 5, 63. [Google Scholar]
- McKenna, W.J.; Judge, D.P. Epidemiology of the inherited cardiomyopathies. Nat. Rev. Cardiol. 2021, 18, 22–36. [Google Scholar] [CrossRef]
- Marian, A.J.; Braunwald, E. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ. Res. 2017, 121, 749–770. [Google Scholar] [CrossRef]
- Bocchi, E.A.; Arias, A.; Verdejo, H.; Diez, M.; Gómez, E.; Castro, P. The Reality of Heart Failure in Latin America. J. Am. Coll. Cardiol. 2013, 62, 949–958. [Google Scholar] [CrossRef]
- Bocchi, E.A. Heart Failure in South America. Curr. Cardiol. Rev. 2013, 9, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.; Vigliano, C.A.; Casabé, J.H.; Diez, M.; Favaloro, L.E.; Guevara, E.; Favaloro, R.R.; Laguens, R.P. Comparison of Prevalence, Clinical Course, and Pathological Findings of Left Ventricular Systolic Impairment Versus Normal Systolic Function in Patients with Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2011, 108, 548–555. [Google Scholar] [CrossRef]
- Espinola-Zavaleta, N.; Vega, A.; Basto, D.M.; Alcantar-Fernández, A.C.; Lans, V.G.; Soto, M.E. Survival and Clinical Behavior of Hypertrophic Cardiomyopathy in a Latin American Cohort in Contrast to Cohorts from the Developed World. J. Cardiovasc. Ultrasound 2015, 23, 20–26. [Google Scholar] [CrossRef][Green Version]
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006, 113, 1807–1816. [Google Scholar] [CrossRef]
- Ho, C.Y.; Day, S.M.; Ashley, E.A.; Michels, M.; Pereira, A.C.; Jacoby, D.; Cirino, A.L.; Fox, J.C.; Lakdawala, N.K.; Ware, J.; et al. Genotype and Lifetime Burden of Disease in Hypertrophic Cardiomyopathy: Insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation 2018, 138, 1387–1398. [Google Scholar] [CrossRef]
- Cardim, N.; Brito, D.; Lopes, L.R.; Freitas, A.; Araújo, C.; Belo, A.; Gonçalves, L.; Mimoso, J.; Olivotto, I.; Elliott, P.; et al. The Portuguese Registry of Hypertrophic Cardiomyopathy: Overall results. Rev. Port. Cardiol. 2018, 37, 1–10. [Google Scholar] [CrossRef]
- Neubauer, S.; Kolm, P.; Ho, C.Y.; Kwong, R.Y.; Desai, M.Y.; Dolman, S.F.; Appelbaum, E.; Desvigne-Nickens, P.; DiMarco, J.P.; Friedrich, M.G.; et al. Distinct Subgroups in Hypertrophic Cardiomyopathy in the NHLBI HCM Registry. J. Am. Coll. Cardiol. 2019, 74, 2333–2345. [Google Scholar] [CrossRef]
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Diagnosis and Evaluation of Hypertrophic Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 372–389. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Wigle, E.D.; Rakowski, H.; Kimball, B.P.; Williams, W.G. Hypertrophic Cardiomyopathy: Clinical spectrum and treatment. Circulation 1995, 92, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Jan, M.F.; Todaro, M.C.; Oreto, L.; Tajik, A.J. Apical hypertrophic cardiomyopathy: Present status. Int. J. Cardiol. 2016, 222, 745–759. [Google Scholar] [CrossRef]
- Kitaoka, H.; Doi, Y.; A Casey, S.; Hitomi, N.; Furuno, T.; Maron, B.J. Comparison of prevalence of apical hypertrophic cardiomyopathy in Japan and the United States. Am. J. Cardiol. 2003, 92, 1183–1186. [Google Scholar] [CrossRef]
- Huang, G.; Fadl, S.A.; Sukhotski, S.; Matesan, M. Apical variant hypertrophic cardiomyopathy “multimodality imaging evaluation”. Int. J. Cardiovasc. Imaging 2020, 36, 553–561. [Google Scholar] [CrossRef]
- Maron, M.S.; Olivotto, I.; Zenovich, A.G.; Link, M.S.; Pandian, N.G.; Kuvin, J.T.; Nistri, S.; Cecchi, F.; Udelson, J.E.; Maron, B.J.; et al. Hypertrophic Cardiomyopathy Is Predominantly a Disease of Left Ventricular Outflow Tract Obstruction. Circulation 2006, 114, 2232–2239. [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.T.; Dweck, M.R.; Prasher, S.; Shah, A.; Humphries, S.E.; Pennell, D.J.; Montgomery, H.E.; Payne, J.R. Left Ventricular Wall Thickness and the Presence of Asymmetric Hypertrophy in Healthy Young Army Recruits: Data from the LARGE heart study. Circ. Cardiovasc. Imaging 2013, 6, 262–267. [Google Scholar] [CrossRef]
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients with Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2020, 76, e159–e240. [Google Scholar] [CrossRef]
- Maron, B.J.; Yacoub, M.; Dearani, J.A. Benefits of surgery in obstructive hypertrophic cardiomyopathy: Bring septal myectomy back for European patients. Eur. Heart J. 2011, 32, 1055–1058. [Google Scholar] [CrossRef]
- Kim, L.K.; Swaminathan, R.V.; Looser, P.; Minutello, R.M.; Wong, S.C.; Bergman, G.; Naidu, S.S.; Gade, C.L.F.; Charitakis, K.; Singh, H.S.; et al. Hospital Volume Outcomes After Septal Myectomy and Alcohol Septal Ablation for Treatment of Obstructive Hypertrophic Cardiomyopathy. JAMA Cardiol. 2016, 1, 324–332. [Google Scholar] [CrossRef]
- van Velzen, H.G.; Theuns, D.A.; Yap, S.-C.; Michels, M.; Schinkel, A.F. Incidence of Device-Detected Atrial Fibrillation and Long-Term Outcomes in Patients With Hypertrophic Cardiomyopathy: US nationwide inpatient database, 2003–2011. Am. J. Cardiol. 2017, 119, 100–105. [Google Scholar] [CrossRef]
- Wilke, I.; Witzel, K.; Münch, J.; Pecha, S.; Blankenberg, S.; Reichenspurner, H.; Willems, S.; Patten, M.; Aydin, A. High Incidence of De Novo and Subclinical Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy and Cardiac Rhythm Management Device. J. Cardiovasc. Electrophysiol. 2016, 27, 779–784. [Google Scholar] [CrossRef]
- Guttmann, O.P.; Rahman, M.S.; O’Mahony, C.; Anastasakis, A.; Elliott, P.M. Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: Systematic review. Heart 2014, 100, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.; Semsarian, C.; Chan, K.H.; Sy, R.W. Sudden Cardiac Death and Ventricular Arrhythmias in Hypertrophic Cardiomyopathy. Heart Lung Circ. 2019, 28, 146–154. [Google Scholar] [CrossRef]
- Hershberger, R.E.; Givertz, M.M.; Ho, C.Y.; Judge, D.P.; Kantor, P.F.; McBride, K.L.; Morales, A.; Taylor, M.R.; Vatta, M.; Ware, S.M. Genetic Evaluation of Cardiomyopathy—A Heart Failure Society of America Practice Guideline. J. Card. Fail. 2018, 24, 281–302. [Google Scholar] [CrossRef]
- Colombo, M.G.; Botto, N.; Vittorini, S.; Paradossi, U.; Andreassi, M.G. Clinical utility of genetic tests for inherited hypertrophic and dilated cardiomyopathies. Cardiovasc. Ultrasound 2008, 6, 62. [Google Scholar] [CrossRef]
- Lopes, L.R.; Futema, M.; Akhtar, M.M.; Lorenzini, M.; Pittman, A.; Syrris, P.; Elliott, P.M. Prevalence of TTR variants detected by whole-exome sequencing in hypertrophic cardiomyopathy. Amyloid 2019, 26, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Wijngaard, A.v.D.; Volders, P.; Van Tintelen, J.P.; Jongbloed, J.D.H.; Berg, M.P.v.D.; Deprez, R.H.L.; Mannens, M.M.A.M.; Hofmann, N.; Slegtenhorst, M.; Dooijes, D.; et al. Recurrent and founder mutations in the Netherlands: Cardiac Troponin I (TNNI3) gene mutations as a cause of severe forms of hypertrophic and restrictive cardiomyopathy. Neth. Heart J. 2011, 19, 344–351. [Google Scholar] [CrossRef]
- Gigli, M.; Begay, R.L.; Morea, G.; Graw, S.L.; Sinagra, G.; Taylor, M.R.G.; Granzier, H.; Mestroni, L. A Review of the Giant Protein Titin in Clinical Molecular Diagnostics of Cardiomyopathies. Front. Cardiovasc. Med. 2016, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.R.; Zekavati, A.; Syrris, P.; Hubank, M.; Giambartolomei, C.; Dalageorgou, C.; Jenkins, S.; McKenna, W.; Plagnol, V.; Elliott, P.M.; et al. Genetic complexity in hypertrophic cardiomyopathy revealed by high-throughput sequencing. J. Med. Genet. 2013, 50, 228–239. [Google Scholar] [CrossRef]


| Variable | HCM (n = 82) |
|---|---|
| Age at clinical diagnosis, n | |
| Median (IQR) | 49.0 (37.5–61.0) |
| Gender, n | |
| Male, n (%) | 55 (67.1%) |
| Family history of cardiomyopathy, n, (%) | 12 (25.0%) |
| Family history of cardiomyopathy by level of consanguinity, n | |
| First degree, n (%) | 8 (66.7%) |
| Second degree, n (%) | 3 (25.0%) |
| NYHA functional class, n (%) | |
| Class I | 43 (59.7%) |
| Class II | 17 (23.6%) |
| Class III | 10 (13.9%) |
| Class IV | 2 (2.8%) |
| Angina, n (%) | 16 (19.5%) |
| Dyspnea, n (%) | 31 (37.8%) |
| Palpitations, n (%) | 9 (11.0%) |
| Syncope, n (%) | 12 (14.6%) |
| Personal history and comorbidities, n (%) | |
| Atrial fibrillation | 11 (15.1%) |
| Stroke | 7 (9.5%) |
| Diabetes mellitus type 2 | 9 (11.2%) |
| Arterial hypertension | 24 (30.4%) |
| Dyslipidemia | 17 (21.2%) |
| Overweight/Obesity | 6 (7.6%) |
| Chronic kidney disease | 3 (3.8%) |
| Hypothyroidism | 7 (8.8%) |
| Smoking | 9 (17.0%) |
| Pharmacologic treatment, n (%) | |
| Beta-blockers | 58 (85.3%) |
| Diuretics, oral | 13 (19.1%) |
| ACE-inhibitors | 1 (1.5%) |
| Angiotensin II receptor blockers | 17 (25.0%) |
| Mineralocorticoid receptor antagonists | 7 (10.3%) |
| Calcium channel blockers | 19 (27.9%) |
| Acetylsalicylic acid | 8 (11.8%) |
| SGLT2 inhibitors | 1 (1.5%) |
| ARNI | 2 (2.9%) |
| Statins | 13 (19.1%) |
| Oral anticoagulants | 14 (20.5%) |
| Vitamin K antagonists | 4 (5.9%) |
| Apixaban | 5 (7.4%) |
| Rivaroxaban | 4 (5.9%) |
| Non-specified | 1 (1.5%) |
| Non-pharmacological treatment n (%) | |
| Angiography | 7 (19.4%) |
| Alcohol septal ablation | 5 (6.1%) |
| Septal myectomy | 4 (4.9%) |
| Ventricular assist device | 1 (1.3%) |
| Cardiac resynchronization therapy | 2 (2.4%) |
| Implantable cardioverter-defibrillators | 29 (35.4%) |
| Variable, n (%) | HCM (n = 82) |
|---|---|
| Ejection fraction n = 73 | |
| Reduced (<40%) | 4 (5.8%) |
| Slightly reduced (40–49%) | 4 (5.8%) |
| Preserved (>50%) | 65 (89%) |
| Global longitudinal strain, median (IQR) | −13.4 (−18.1, −11.7) |
| Wall diameters | |
| Abnormal interventricular septum (>9 mm female; >10 mm male) | 63 (100%) |
| Abnormal posterior wall (>9 mm female; >10 mm male) | 36 (76.6%) |
| HCM subtype according to LV hypertrophy pattern | |
| Asymmetric septal | 37 (64.9%) |
| Concentric | 16 (28.1%) |
| Predominantly apical | 4 (7.0%) |
| No data | 25/82 (30.5%) |
| Left atrial volume indexed | 53 (65.4%) |
| Normal: 16 to 34 mL/m2 | 8 (15.1%) |
| Slightly abnormal: 35 to 41 mL/m2 | 20 (37.7%) |
| Moderately abnormal: 42 to 48 mL/m2 | 9 (17.0%) |
| Severely abnormal: >48 mL/m2 | 16 (30.2%) |
| E/A ratio | |
| Normal (0.8–2) | 20 (69%) |
| Abnormal (>2) | 9 (31.0%) |
| Right atrial area | |
| Normal ≤19 cm2 | 23(57.5%) |
| Abnormal >19 cm2 | 17(42.5%) |
| TAPSE | |
| Normal >17 | 32 (91.4%) |
| Decreased <17 | 3 (8.6%) |
| S’ wave | |
| Normal | 18 (78.3%) |
| Abnormal | 5 (21.7%) |
| Valvular heart disease | |
| Mitral regurgitation | 43 (52.4%) |
| Aortic insufficiency | 6 (7.3%) |
| Tricuspid insufficiency | 34 (41.5%) |
| Aortic stenosis | 4 (4.9%) |
| Mitral stenosis | 0 (0.0%) |
| LV outflow tract gradient | |
| Normal | 15 (27.3%) |
| <30 mmHg | 11 (20.0%) |
| 30–49 mmHg | 8 (14.5%) |
| >50 mmHg | 21 (38.2%) |
| Unreported | 27/82 (32.9%) |
| SAM | 21 (47.7%) |
| Pericardial effusion | 2 (3.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Ponce de Leon, J.D.; Estacio, M.; Giraldo, N.; Escalante, M.; Rodas, Y.; Largo, J.; Lores, J.; Victoria, M.C.; Argote, D.; Florez, N.; et al. Hypertrophic Cardiomyopathy in a Latin American Center: A Single Center Observational Study. J. Clin. Med. 2023, 12, 5682. https://doi.org/10.3390/jcm12175682
López-Ponce de Leon JD, Estacio M, Giraldo N, Escalante M, Rodas Y, Largo J, Lores J, Victoria MC, Argote D, Florez N, et al. Hypertrophic Cardiomyopathy in a Latin American Center: A Single Center Observational Study. Journal of Clinical Medicine. 2023; 12(17):5682. https://doi.org/10.3390/jcm12175682
Chicago/Turabian StyleLópez-Ponce de Leon, Juan David, Mayra Estacio, Natalia Giraldo, Manuela Escalante, Yorlany Rodas, Jessica Largo, Juliana Lores, María Camila Victoria, Diana Argote, Noel Florez, and et al. 2023. "Hypertrophic Cardiomyopathy in a Latin American Center: A Single Center Observational Study" Journal of Clinical Medicine 12, no. 17: 5682. https://doi.org/10.3390/jcm12175682
APA StyleLópez-Ponce de Leon, J. D., Estacio, M., Giraldo, N., Escalante, M., Rodas, Y., Largo, J., Lores, J., Victoria, M. C., Argote, D., Florez, N., Carrillo, D., Olaya, P., Mejia, M., & Gomez, J. E. (2023). Hypertrophic Cardiomyopathy in a Latin American Center: A Single Center Observational Study. Journal of Clinical Medicine, 12(17), 5682. https://doi.org/10.3390/jcm12175682






