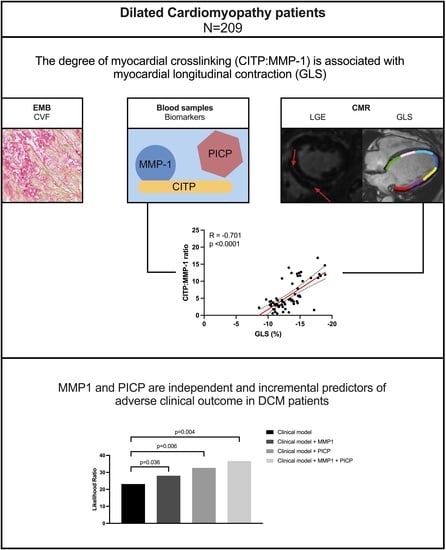
Biomarkers of Collagen Metabolism Are Associated with Left Ventricular Function and Prognosis in Dilated Cardiomyopathy: A Multi-Modal Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CMR Acquisitions and Analysis
2.3. Biochemical Studies
2.4. Endomyocardial Biopsy
2.5. Prognosis
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Associations of Collagen Cross-Linking Biomarkers with Myocardial Fibrosis Based on Histology and Imaging
3.3. Associations of Collagen Biomarkers with Cardiac Function
3.4. Association of Collagen Biomarkers with Event-Free Survival
4. Discussion
4.1. The Association of Collagen Biomarkers with Cardiac Function
4.2. Prognostic Value of Collagen Metabolism-Related Biomarkers
4.3. Clinical Implications and Future Directions
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verdonschot, J.A.J.; Hazebroek, M.R.; Ware, J.S.; Prasad, S.K.; Heymans, S.R.B. Role of Targeted Therapy in Dilated Cardiomyopathy: The Challenging Road Toward a Personalized Approach. J. Am. Heart Assoc. 2019, 8, e012514. [Google Scholar] [CrossRef] [PubMed]
- De Boer, R.A.; De Keulenaer, G.; Bauersachs, J.; Brutsaert, D.; Cleland, J.G.; Diez, J.; Du, X.; Ford, P.; Heinzel, F.R.; Lipson, K.E.; et al. Towards better definition, quantification and treatment of fibrosis in heart failure. A scientific roadmap by the Committee of Translational Research of the Heart Failure Association (HFA) of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Díez, J.; González, A.; Kovacic, J.C. Myocardial Interstitial Fibrosis in Nonischemic Heart Disease, Part 3/4: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 2204–2218. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Schelbert, E.B.; Diez, J.; Butler, J. Myocardial Interstitial Fibrosis in Heart Failure: Biological and Translational Perspectives. J. Am. Coll. Cardiol. 2018, 71, 1696–1706. [Google Scholar] [CrossRef] [PubMed]
- Raafs, A.G.; Verdonschot, J.A.; Henkens, M.T.; Adriaans, B.P.; Wang, P.; Derks, K.; Hamid, M.A.A.; Knackstedt, C.; Empel, V.P.; Díez, J.; et al. The combination of carboxy-terminal propeptide of procollagen type I blood levels and late gadolinium enhancement at cardiac magnetic resonance provides additional prognostic information in idiopathic dilated cardiomyopathy—A multilevel assessment of myocardial fibrosis in dilated cardiomyopathy. Eur. J. Heart Fail. 2021, 23, 933–944. [Google Scholar] [PubMed]
- Querejeta, R.; López, B.; González, A.; Sánchez, E.; Larman, M.; Martínez Ubago, J.L.; Díez, J. Increased collagen type I synthesis in patients with heart failure of hypertensive origin: Relation to myocardial fibrosis. Circulation 2004, 110, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- López, B.; González, A.; Ravassa, S.; Beaumont, J.; Moreno, M.U.; San José, G.; Querejeta, R.; Díez, J. Circulating Biomarkers of Myocardial Fibrosis: The Need for a Reappraisal. J. Am. Coll. Cardiol. 2015, 65, 2449–2456. [Google Scholar] [CrossRef]
- López, B.; Ravassa, S.; González, A.; Zubillaga, E.; Bonavila, C.; Bergés, M.; Echegaray, K.; Beaumont, J.; Moreno, M.U.; José, G.S.; et al. Myocardial Collagen Cross-Linking Is Associated with Heart Failure Hospitalization in Patients With Hypertensive Heart Failure. J. Am. Coll. Cardiol. 2016, 67, 251–260. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Maceira, A.M.; Prasad, S.K.; Khan, M.; Pennell, D.J. Normalized Left Ventricular Systolic and Diastolic Function by Steady State Free Precession Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2006, 8, 417–426. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013, 128, e240–e327. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, e137–e161. [Google Scholar] [PubMed]
- Flett, A.S.; Hasleton, J.; Cook, C.; Hausenloy, D.; Quarta, G.; Ariti, C.; Muthurangu, V.; Moon, J.C. Evaluation of Techniques for the Quantification of Myocardial Scar of Differing Etiology Using Cardiac Magnetic Resonance. JACC Cardiovasc. Imaging 2011, 4, 150–156. [Google Scholar] [CrossRef]
- Ravassa, S.; Kuznetsova, T.; Varo, N.; Thijs, L.; Delles, C.; Dominiczak, A.; Díez, J.; Staessen, J.A. Biomarkers of cardiomyocyte injury and stress identify left atrial and left ventricular remodelling and dysfunction: A population-based study. Int. J. Cardiol. 2015, 185, 177–185. [Google Scholar] [CrossRef]
- Hazebroek, M.R.; Moors, S.; Dennert, R.; van den Wijngaard, A.; Krapels, I.; Hoos, M.; Verdonschot, J.; Merken, J.J.; de Vries, B.; Wolffs, P.F.; et al. Prognostic Relevance of Gene-Environment Interactions in Patients with Dilated Cardiomyopathy: Applying the MOGE(S) Classification. J. Am. Coll. Cardiol. 2015, 66, 1313–1323. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Pichler, G.; Redon, J.; Martínez, F.; Solaz, E.; Calaforra, O.; Andrés, M.S.; Lopez, B.; Díez, J.; Oberbauer, R.; Adlbrecht, C.; et al. Cardiac magnetic resonance-derived fibrosis, strain and molecular biomarkers of fibrosis in hypertensive heart disease. J. Hypertens. 2020, 38, 2036–2042. [Google Scholar] [CrossRef]
- Pedrizzetti, G.; Claus, P.; Kilner, P.J.; Nagel, E. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J. Cardiovasc. Magn. Reson. 2016, 18, 51. [Google Scholar] [CrossRef]
- Kanoupakis, E.M.; Manios, E.G.; Kallergis, E.M.; Mavrakis, H.E.; Goudis, C.A.; Saloustros, I.G.; Milathianaki, M.E.; Chlouverakis, G.I.; Vardas, P.E. Serum Markers of Collagen Turnover Predict Future Shocks in Implantable Cardioverter-Defibrillator Recipients With Dilated Cardiomyopathy on Optimal Treatment. J. Am. Coll. Cardiol. 2010, 55, 2753–2759. [Google Scholar] [CrossRef] [PubMed]
- López, B.; Querejeta, R.; González, A.; Larman, M.; Díez, J. Collagen cross-linking but not collagen amount associates with elevated filling pressures in hypertensive patients with stage C heart failure: Potential role of lysyl oxidase. Hypertension 2012, 60, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Ravassa, S.; López, B.; Querejeta, R.; Echegaray, K.; San José, G.; Moreno, M.U.; Beaumont, F.J.; González, A.; Díez, J. Phenotyping of myocardial fibrosis in hypertensive patients with heart failure. Influence on clinical outcome. J. Hypertens. 2017, 35, 853–861. [Google Scholar] [CrossRef] [PubMed]
- López, B.; Ravassa, S.; Moreno, M.U.; José, G.S.; Beaumont, J.; González, A.; Díez, J. Diffuse myocardial fibrosis: Mechanisms, diagnosis and therapeutic approaches. Nat. Rev. Cardiol. 2021, 18, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Bayes-Genis, A.; Aimo, A.; Jhund, P.; Richards, M.; de Boer, R.A.; Arfsten, H.; Fabiani, I.; Lupón, J.; Anker, S.D.; González, A.; et al. Biomarkers in heart failure clinical trials. A review from the Biomarkers Working Group of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2022, 24, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Ravassa, S.; López, B.; Ferreira, J.P.; Girerd, N.; Bozec, E.; Pellicori, P.; Mariottoni, B.; Cosmi, F.; Hazebroek, M.; Verdonschot, J.A.; et al. Biomarker-based assessment of collagen cross-linking identifies patients at risk of heart failure more likely to benefit from spironolactone effects on left atrial remodelling. Insights from the HOMAGE clinical trial. Eur. J. Heart Fail. 2022, 24, 321–331. [Google Scholar] [CrossRef]




| All (N = 209) | |
|---|---|
| Demographics | |
| Age at diagnosis (years) | 54 ± 13 (18–80) |
| Male (%) | 136/209 (65%) |
| NYHA class III or IV (%) | 61/209 (29%) |
| C-reactive protein | 3 (1–7) |
| NTproBNP | 65.9 (22.5–193.5) |
| Medical history | |
| Hypertension (%) | 84/209 (40%) |
| Diabetes mellitus (%) | 21/209 (10%) |
| Atrial fibrillation (%) | 52/209 (25%) |
| Medication | |
| β-blocker (%) | 174/209 (83%) |
| ACE-inhibitor/ARB (%) | 185/209 (89%) |
| Loop diuretic (%) | 112/209 (54%) |
| Aldosterone antagonist (%) | 74/209 (35%) |
| Fibrosis biomarkers | |
| PICP (ng/mL) | 78 (64–102) |
| CITP (ng/mL) | 5.97 (5.33–6.95) |
| MMP-1 (ng/mL) | 5.79 (3.61–9.43) |
| CITP:MMP-1 | 3.69 (2.54–6.65) |
| Cardiac MRI | |
| LVEDVi (mL/m2) | 136 ± 53 |
| LVESVi (mL/m2) | 92 ± 50 |
| LVEF (%) | 34 ± 12 |
| LV mass index (g/m2) | 75 ± 27 |
| GLS (n = 203, %) | −10 ± 4 |
| GCS (n = 203, %) | −9 ± 4 |
| GRS (n = 203, %) | 17 ± 8 |
| LGE (%) | 65/209 (31%) |
| LGE extent (%) | 2.5 [1.1–5.4] |
| Endomyocardial biopsy | |
| Chronic low-grade inflammation | 71/209 (34%) |
| Collagen volume fraction (%) | 7 (4–11) |
| Time between CMR and EMB (days) | 30 (6–50) |
| Unadjusted Analysis | Adjusted Analysis * | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| CITP | 1.053 (0.891–1.245) | 0.543 | - | - |
| MMP-1 | 1.026 (1.003–1.049) | 0.023 | 1.026 (1.002–1.051) | 0.037 |
| CITP:MMP-1 | 0.985 (0.918–1.058) | 0.678 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raafs, A.G.; Adriaans, B.P.; Henkens, M.T.H.M.; Verdonschot, J.A.J.; Abdul Hamid, M.A.; Díez, J.; Knackstedt, C.; van Empel, V.P.M.; Brunner-La Rocca, H.-P.; González, A.; et al. Biomarkers of Collagen Metabolism Are Associated with Left Ventricular Function and Prognosis in Dilated Cardiomyopathy: A Multi-Modal Study. J. Clin. Med. 2023, 12, 5695. https://doi.org/10.3390/jcm12175695
Raafs AG, Adriaans BP, Henkens MTHM, Verdonschot JAJ, Abdul Hamid MA, Díez J, Knackstedt C, van Empel VPM, Brunner-La Rocca H-P, González A, et al. Biomarkers of Collagen Metabolism Are Associated with Left Ventricular Function and Prognosis in Dilated Cardiomyopathy: A Multi-Modal Study. Journal of Clinical Medicine. 2023; 12(17):5695. https://doi.org/10.3390/jcm12175695
Chicago/Turabian StyleRaafs, Anne G., Bouke P. Adriaans, Michiel T. H. M. Henkens, Job A. J. Verdonschot, Myrurgia A. Abdul Hamid, Javier Díez, Christian Knackstedt, Vanessa P. M. van Empel, Hans-Peter Brunner-La Rocca, Arantxa González, and et al. 2023. "Biomarkers of Collagen Metabolism Are Associated with Left Ventricular Function and Prognosis in Dilated Cardiomyopathy: A Multi-Modal Study" Journal of Clinical Medicine 12, no. 17: 5695. https://doi.org/10.3390/jcm12175695