Posterior Decompression and Fixation for Thoracic Spine Ossification: A 10-Year Follow-Up Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Clinical Outcomes
2.3. Radiographic Evaluation
2.4. Statistical Analysis
2.5. Ethical Considerations
2.6. Manuscript Preparation and Proofreading
3. Results
3.1. Patient Demographics
3.2. Clinical Outcomes
3.3. Radiographic Evaluation
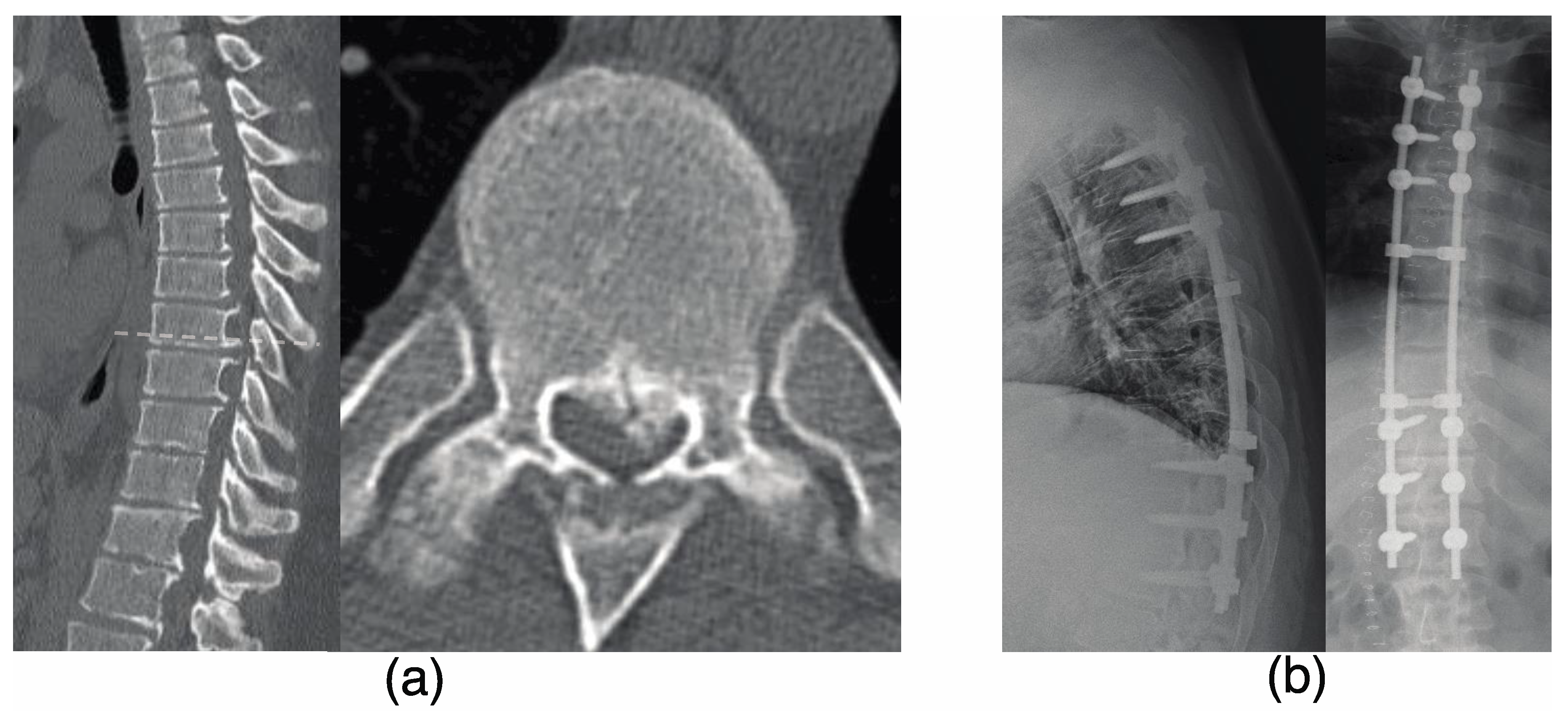
3.4. Representative Case
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T-OPLL | Ossification of the posterior longitudinal ligament of the thoracic spine |
| Posterior Decompression with Instrumented Fusion | |
| JOA | Japanese Orthopedic Association |
References
- Yamazaki, M.; Mochizuki, M.; Ikeda, Y. Clinical Results of Surgery for Thoracic Myelopathy Caused by Ossification of the Posterior Longitudinal Ligament: Operative Indication of Posterior Decompression with Instrumented Fusion. Spine 2006, 31, 1452–1460. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Nakano, M.; Yasuda, T.; Seki, S.; Hori, T.; Kimura, T. Ossification of the Posterior Longitudinal Ligament in Not Only the Cervical Spine, but Also Other Spinal Regions: Analysis Using Multidetector Computed Tomography of the Whole Spine. Spine 2013, 38, E1477–E1482. [Google Scholar] [CrossRef]
- Koda, M.; Furuya, T.; Okawa, A.; Inada, T.; Kamiya, K.; Ota, M.; Maki, S.; Takahashi, K.; Yamazaki, M.; Aramomi, M.; et al. Mid- to Long-Term Outcomes of Posterior Decompression with Instrumented Fusion for Thoracic Ossification of the Posterior Longitudinal Ligament. J. Clin. Neurosci. 2016, 27, 87–90. [Google Scholar] [CrossRef]
- Abiola, R.; Rubery, P.; Mesfin, A. Ossification of the Posterior Longitudinal Ligament: Etiology, Diagnosis, and Outcomes of Nonoperative and Operative Management. Glob. Spine J. 2016, 6, 195–204. [Google Scholar] [CrossRef]
- Nakashima, H.; Tetreault, L.; Kato, S.; Kryshtalskyj, M.T.; Nagoshi, N.; Nouri, A.; Singh, A.; Fehlings, M.G. Prediction of Outcome Following Surgical Treatment of Cervical Myelopathy Based on Features of Ossification of the Posterior Longitudinal Ligament: A Systematic Review. JBJS Rev. 2017, 5, e5. [Google Scholar] [CrossRef]
- Ando, K.; Imagama, S.; Kobayashi, K.; Ito, K.; Tsushima, M.; Morozumi, M.; Tanaka, S.; Machino, M.; Ota, K.; Nakashima, H.; et al. Clinical Features of Thoracic Myelopathy: A Single-Center Study. JAAOS Glob. Res. Rev. 2019, 3, e10.5435. [Google Scholar] [CrossRef]
- Liang, H.; Liu, G.; Lu, S.; Chen, S.; Jiang, D.; Shi, H.; Fei, Q. Epidemiology of Ossification of the Spinal Ligaments and Associated Factors in the Chinese Population: A Cross-Sectional Study of 2000 Consecutive Individuals. BMC Musculoskelet. Disord. 2019, 20, 253. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, H.; Horowitz, E.; Nadarajah, V. Prevalence and Characteristics of Thoracic Ossification of the Posterior Longitudinal Ligament in 3299 Black Patients: A Cross-Sectional Study of a Prospectively Registered Database. BMJ Open 2022, 12, e059238. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Toyama, Y.; Chikuda, H.; Takeshita, K.; Kato, T.; Shindo, S.; Abumi, K.; Takahata, M.; Nohara, Y.; Taneichi, H.; et al. Outcomes of Fusion Surgery for Ossification of the Posterior Longitudinal Ligament of the Thoracic Spine: A Multicenter Retrospective Survey: Clinical Article. J. Neurosurg. Spine 2011, 15, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, Y.; Yoshihara, H.; Tsuji, T.; Sakai, Y.; Yukawa, Y.; Nakamura, H.; Ito, K.; Ishiguro, N. Surgical Outcome of Ossification of the Posterior Longitudinal Ligament (OPLL) of the Thoracic Spine: Implication of the Type of Ossification and Surgical Options. J. Spinal Disord. Tech. 2005, 18, 492–497. [Google Scholar] [CrossRef]
- Matsumoto, M.; Chiba, K.; Toyama, Y.; Takeshita, K.; Seichi, A.; Nakamura, K.; Arimizu, J.; Fujibayashi, S.; Hirabayashi, S.; Hirano, T.; et al. Surgical Results and Related Factors for Ossification of Posterior Longitudinal Ligament of the Thoracic Spine: A Multi-Institutional Retrospective Study. Spine 2008, 33, 1034–1041. [Google Scholar] [CrossRef]
- Matsuyama, Y.; Sakai, Y.; Katayama, Y.; Imagama, S.; Ito, Z.; Wakao, N.; Yukawa, Y.; Ito, K.; Kamiya, M.; Kanemura, T.; et al. Indirect Posterior Decompression with Corrective Fusion for Ossification of the Posterior Longitudinal Ligament of the Thoracic Spine: Is It Possible to Predict the Surgical Results? Eur. Spine J. 2009, 18, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Okawa, A.; Fujiyoshi, T.; Furuya, T.; Koda, M. Posterior Decompression with Instrumented Fusion for Thoracic Myelopathy Caused by Ossification of the Posterior Longitudinal Ligament. Eur. Spine J. 2010, 19, 691–698. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Y.; Yin, P.; Su, Q. A Systematic Review of Surgical Procedures on Thoracic Myelopathy. J. Orthop. Surg. 2020, 15, 595. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Hyun, S.-J.; Kim, K.-J.; Jahng, T.-A. Surgical Outcomes According to Dekyphosis in Patients with Ossification of the Posterior Longitudinal Ligament in the Thoracic Spine. J. Korean Neurosurg. Soc. 2020, 63, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Saiwai, H.; Okada, S.; Hayashida, M.; Harimaya, K.; Matsumoto, Y.; Kawaguchi, K.; Kobayakawa, K.; Maeda, T.; Ohta, H.; Shirasawa, K.; et al. Surgery-Related Predictable Risk Factors Influencing Postoperative Clinical Outcomes for Thoracic Myelopathy Caused by Ossification of the Posterior Longitudinal Ligament: A Multicenter Retrospective Study. J. Neurosurg. Spine 2020, 32, 703–709. [Google Scholar] [CrossRef]
- Ando, K.; Nakashima, H.; Machino, M.; Ito, S.; Segi, N.; Tomita, H.; Koshimizu, H.; Imagama, S. Postoperative Progression of Ligamentum Flavum Ossification after Posterior Instrumented Surgery for Thoracic Posterior Longitudinal Ligament Ossification: Long-Term Outcomes during a Minimum 10-Year Follow-Up. J. Neurosurg. Spine 2022, 36, 986–996. [Google Scholar] [CrossRef]
- Hirabayashi, K.; Miyakawa, J.; Satomi, K. Operative Results and Postoperative Progression of Ossification among Patients with Ossification of Cervical Posterior Longitudinal Ligament. Spine 1981, 6, 354–364. [Google Scholar] [CrossRef]
- Koelé, M.C.; Lems, W.F.; Willems, H.C. The Clinical Relevance of Hyperkyphosis: A Narrative Review. Front. Endocrinol. 2020, 11, 5. [Google Scholar] [CrossRef]
- Hirabayashi, S.; Kitagawa, T.; Yamamoto, I.; Yamada, K.; Kawano, H. Surgical Treatment for Ossification of the Posterior Longitudinal Ligament (OPLL) at the Thoracic Spine: Usefulness of the Posterior Approach. Spine Surg. Relat. Res. 2018, 2, 169–176. [Google Scholar] [CrossRef]
- Ohtsuka, K.; Terayama, K.; Tsuchiya, T. A surgical proce dure of the anterior decompression of the thoracic spinal cord through the posterior approach. Orthop Surg Traumatol. 1983, 26, 1083–1109. [Google Scholar]
- Tomita, K.; Kawahara, N.; Baba, H. Circumspinal Decom Pression for Thoracic Myelopathy Due to Combined Ossification of the Posterior Longitudinal Ligament and Ligamentum Flavum. Spine 1990, 15, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, Y.; Nishi, Y.; Nakamura, M. Long-Term Follow-up Study of Anterior Decompression and Fusion for Thoracic Myelopathy Resulting from Ossification of the Posterior Longitudinal Ligament. Spine 1997, 22, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Murakami, H.; Demura, S.; Yoshioka, K.; Hayashi, H.; Tsuchiya, H. Novel Surgical Technique for Ossification of Posterior Longitudinal Ligament in the Thoracic Spine: Technical Note. J. Neurosurg. Spine 2012, 17, 525–529. [Google Scholar] [CrossRef]
- Kato, S.; Murakami, H.; Demura, S.; Yoshioka, K.; Yokogawa, N.; Takaki, S.; Oku, N.; Tsuchiya, H. Indication for Anterior Spinal Cord Decompression via a Posterolateral Approach for the Treatment of Ossification of the Posterior Longitudinal Ligament in the Thoracic Spine: A Prospective Cohort Study. Eur. Spine J. 2020, 29, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Kanno, H.; Aizawa, T.; Hashimoto, K.; Itoi, E.; Ozawa, H. Anterior Decompression through a Posterior Approach for Thoracic Myelopathy Caused by Ossification of the Posterior Longitudinal Ligament: A Novel Concept in Anterior Decompression and Technical Notes with the Preliminary Outcomes. J. Neurosurg. Spine 2021, 1, 1–11. [Google Scholar] [CrossRef]
- Aizawa, T.; Eto, T.; Hashimoto, K.; Kanno, H.; Itoi, E.; Ozawa, H. Surgical Results of Nonambulatory Patients Caused by Ossification of the Posterior Longitudinal Ligaments in the Thoracic Spine: Retrospective Comparative Study between Posterior Decompression and Instrumented Spinal Fusion versus Anterior Decompression through a Posterior Approach. J. Neurosurg. Spine 2020, 34, 492–497. [Google Scholar] [CrossRef]
- Tsuzuki, N.; Hirabayashi, S.; Abe, R.; Saiki, K. Staged Spinal Cord Decompression through Posterior Approach for Thoracic Myelopathy Caused by Ossification of Posterior Longitudinal Ligament. Spine 2001, 26, 1623–1630. [Google Scholar] [CrossRef]
- Imagama, S.; Ando, K.; Ito, Z.; Kobayashi, K.; Hida, T.; Ito, K.; Ishikawa, Y.; Tsushima, M.; Matsumoto, A.; Tanaka, S.; et al. Resection of Beak-Type Thoracic Ossification of the Posterior Longitudinal Ligament from a Posterior Approach under Intraoperative Neurophysiological Monitoring for Paralysis after Posterior Decompression and Fusion Surgery. Glob. Spine J. 2016, 6, 812–821. [Google Scholar] [CrossRef]
- Hirai, T.; Yoshii, T.; Iwanami, A.; Takeuchi, K.; Mori, K.; Yamada, T.; Wada, K.; Koda, M.; Matsuyama, Y.; Takeshita, K.; et al. Prevalence and Distribution of Ossified Lesions in the Whole Spine of Patients with Cervical Ossification of the Posterior Longitudinal Ligament A Multicenter Study (JOSL CT Study). PLoS ONE 2016, 11, e0160117. [Google Scholar] [CrossRef]
- Fujimori, T.; Watabe, T.; Iwamoto, Y.; Hamada, S.; Iwasaki, M.; Oda, T. Prevalence, Concomitance, and Distribution of Ossification of the Spinal Ligaments: Results of Whole Spine CT Scans in 1500 Japanese Patients. Spine 2016, 41, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Fan, T.; Yang, X.; Sun, C.; Fan, D.; Chen, Z. The Prevalence and Clinical Characteristics of Thoracic Spinal Stenosis: A Systematic Review. Eur. Spine J. 2020, 29, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.A.; Shetty, A.P.; Jakkepally, S.; Kumarasamy, D.; Kanna, R.M.; Rajasekaran, S. Ossification of Posterior Longitudinal Ligament in Cervical Spine and Its Association with Ossified Lesions in the Whole Spine: A Cross-Sectional Study of 2500 CT Scans. Glob. Spine J. 2023, 13, 122–132. [Google Scholar] [CrossRef]
- Özkan, N.; Chihi, M.; Schoemberg, T.; Dinger, T.F.; Helsper, M.; Parlak, A.; Jabbarli, R.; Ahmadipour, Y.; Sure, U.; El Hindy, N.; et al. First Neurological Symptoms in Degenerative Cervical Myelopathy: Does It Predict the Outcome? Eur. Spine J. 2022, 31, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Kalsi-Ryan, S.; Clout, J.; Rostami, P.; Massicotte, E.M.; Fehlings, M.G. Duration of Symptoms in the Quantification of Upper Limb Disability and Impairment for Individuals with Mild Degenerative Cervical Myelopathy (DCM). PLoS ONE 2019, 14, e0222134. [Google Scholar] [CrossRef] [PubMed]



| Age | Sex | BMI | Comorbidity (DM, HT) | Maximum Compression Level | Location of OPLL | Spinal Canal Occupancy Ratio (%) | Type of OPLL | OLF | Duration of Symptoms (Month) | Preoperation JOA Score | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 74 | Female | 20 | middle | T6–8 | 58 | continuous cylindrical | 72 | 6.5 | ||
| 2 | 52 | Female | 26 | DM, HT | middle | T5–7 | 50 | continuous cylindrical | 2 | 4 | |
| 3 | 41 | Female | 35 | DM | upper | T3–7 | 42 | continuous waveform | 4 | 6 | |
| 4 | 55 | Male | 27 | DM | middle | T6–9 | 36 | continuous waveform | positive | 4 | 3 |
| 5 | 65 | Female | 27 | DM, HT | middle | T5–8 | 43 | continuous cylindrical | positive | 8 | 5.5 |
| 6 | 60 | Male | 27 | DM, HT | middle | T4–7 | 79 | beak | positive | 24 | 6 |
| 7 | 64 | Male | 27 | HT | upper | T1–3 | 50 | continuous cylindrical | positive | 5 | 3 |
| 8 | 59 | Male | 32 | DM, HT | middle | T6–9 | 62 | beak | positive | 9 | 2.5 |
| 9 | 42 | Male | 32 | DM | middle | T9–11 | 46 | beak | 7 | 2.5 | |
| 10 | 53 | Female | 35 | middle | T6–8 | 61 | continuous cylindrical | positive | 3 | 1.5 | |
| 11 | 68 | Male | 22 | DM | middle | T4–5 | 72 | beak | positive | 13 | 1.5 |
| 12 | 37 | Male | 34 | DM, HT | middle | T4–7 | 40 | beak | positive | 7 | 1 |
| 13 | 34 | Male | 45 | DM | lower | T8–11 | 45 | continuous waveform | positive | 11 | 4.5 |
| 14 | 49 | Male | 30 | HT | upper | T2–5 | 48 | beak | positive | 20 | 1 |
| 15 | 35 | Male | 37 | upper | C3–T5 | 51 | continuous cylindrical | 4 | 3.5 | ||
| 16 | 72 | Female | 24 | DM, HT | lower | T8–L3 | 60 | continuous cylindrical | 21 | 3.5 | |
| 17 | 22 | Male | 43 | DM | middle | T6–9 | 60 | continuous cylindrical | 7 | 5 | |
| 18 | 36 | Male | 27 | DM | lower | T8–L2 | 53 | beak | 9 | 4 | |
| 19 | 48 | Female | 35 | DM | middle | T5,6 | 57 | beak | positive | 7 | 2.5 |
| 20 | 59 | Female | 22 | middle | T5–10 | 54 | continuous cylindrical | 17 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maruyama, J.; Furuya, T.; Maki, S.; Inoue, T.; Yunde, A.; Miura, M.; Shiratani, Y.; Nagashima, Y.; Shiga, Y.; Inage, K.; et al. Posterior Decompression and Fixation for Thoracic Spine Ossification: A 10-Year Follow-Up Study. J. Clin. Med. 2023, 12, 5701. https://doi.org/10.3390/jcm12175701
Maruyama J, Furuya T, Maki S, Inoue T, Yunde A, Miura M, Shiratani Y, Nagashima Y, Shiga Y, Inage K, et al. Posterior Decompression and Fixation for Thoracic Spine Ossification: A 10-Year Follow-Up Study. Journal of Clinical Medicine. 2023; 12(17):5701. https://doi.org/10.3390/jcm12175701
Chicago/Turabian StyleMaruyama, Juntaro, Takeo Furuya, Satoshi Maki, Takaki Inoue, Atsushi Yunde, Masataka Miura, Yuki Shiratani, Yuki Nagashima, Yasuhiro Shiga, Kazuhide Inage, and et al. 2023. "Posterior Decompression and Fixation for Thoracic Spine Ossification: A 10-Year Follow-Up Study" Journal of Clinical Medicine 12, no. 17: 5701. https://doi.org/10.3390/jcm12175701





