Risk Stratification in Primary Biliary Cholangitis
Abstract
:1. Introduction
2. Risk Stratification
2.1. Individual Factors
2.1.1. Age
2.1.2. Sex
2.2. Clinical Factors
2.2.1. Symptomatic Disease
2.2.2. Extrahepatic Autoimmune Diseases
2.3. Variant Syndromes
2.3.1. AIH-PBC Overlap Syndrome
- two of the following: (A) alkaline phosphatase (ALP) > 2× upper limit of normal (ULN) or gamma-glutamyltransferase (GGT) > 5× ULN; (B) AMA > 1:40 or PBC-specific antinuclear antibodies (ANA) (immunofluorescent or/and specific anti-sp100/gp-210 test); (C) florid bile duct lesion on histology;
- and two of the following three features: (A) alanine aminotransferase (ALT) > 5× ULN; (B) Immunoglobulin G (IgG) serum levels > 2× ULN or smooth muscle antibody positive (SMA); (C) moderate or severe interface hepatitis on histology.
2.3.2. Premature Ductopenic Variant
2.4. Antibody Profile
2.4.1. AMA
2.4.2. ANA
2.4.3. ACA
2.4.4. Novel Autoantibodies
2.5. Disease Staging
2.5.1. Histological Features
2.5.2. Noninvasive Markers of Fibrosis—Biomarkers
2.5.3. Noninvasive Markers of Fibrosis—Liver Stiffness
2.6. Liver Biochemistry
2.6.1. ALP
2.6.2. Bilirubin
2.7. Assessment of Response to Treatment
2.7.1. Dichotomous Scoring System
2.7.2. Continuous Scoring System
2.7.3. UDCA Predictive Score—UDCA Response Score (URS)
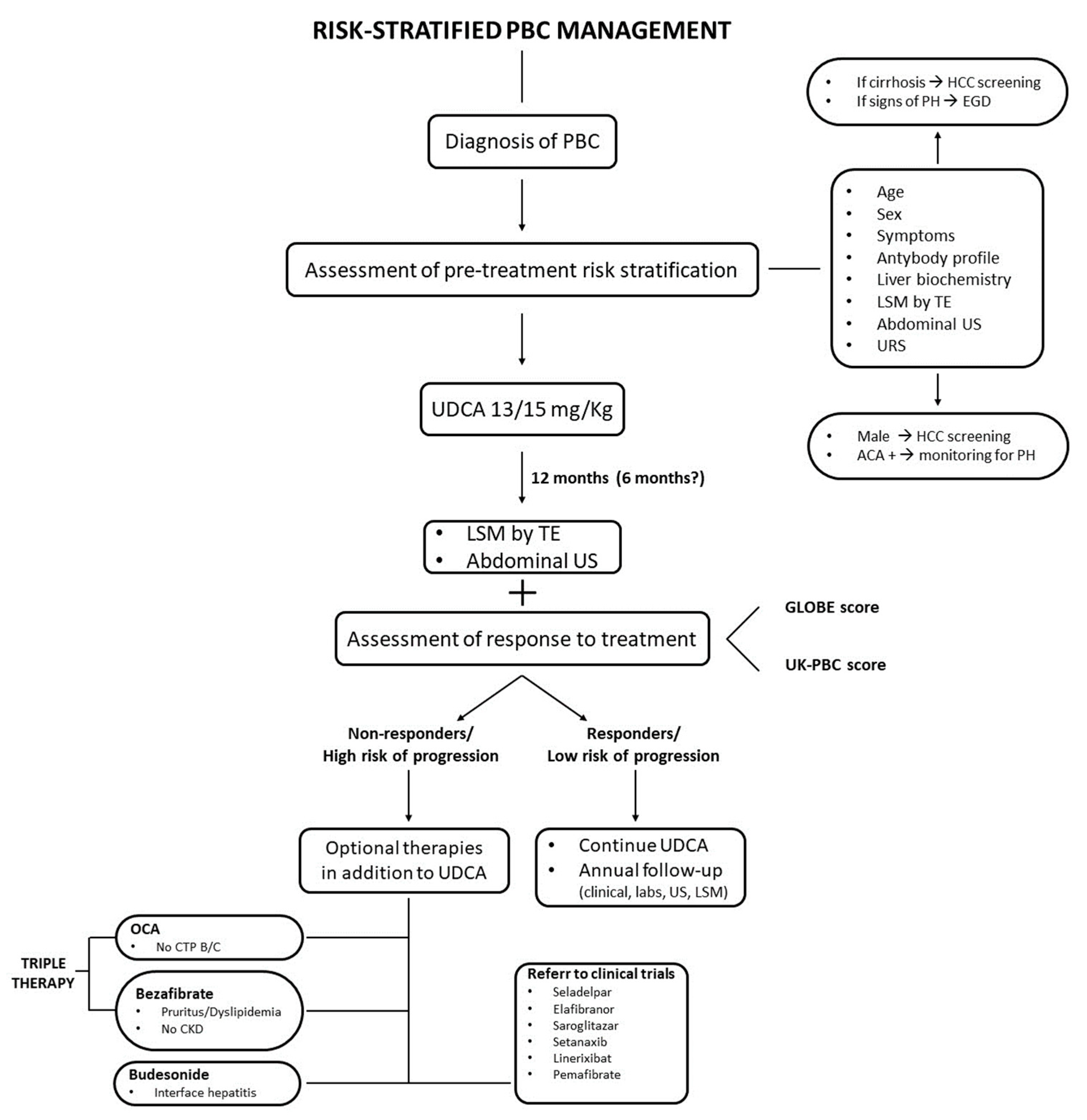
3. Risk-Stratified Management
4. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gulamhusein, A.F.; Hirschfield, G.M. Primary Biliary Cholangitis: Pathogenesis and Therapeutic Opportunities. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Gershwin, M.E.; Ansari, A.A.; Mackay, I.R.; Nakanuma, Y.; Nishio, A.; Rowley, M.J.; Coppel, R.L. Primary Biliary Cirrhosis: An Orchestrated Immune Response against Epithelial Cells. Immunol. Rev. 2000, 174, 210–225. [Google Scholar] [CrossRef]
- Dahlqvist, G.; Gaouar, F.; Carrat, F.; Meurisse, S.; Chazouillères, O.; Poupon, R.; Johanet, C.; Corpechot, C. Large-scale Characterization Study of Patients with Antimitochondrial Antibodies but Nonestablished Primary Biliary Cholangitis. Hepatology 2017, 65, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Lindor, K.D.; Bowlus, C.L.; Boyer, J.; Levy, C.; Mayo, M. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2019, 69, 394–419. [Google Scholar] [CrossRef] [PubMed]
- Hirschfield, G.M.; Beuers, U.; Corpechot, C.; Invernizzi, P.; Jones, D.; Marzioni, M.; Schramm, C. EASL Clinical Practice Guidelines: The Diagnosis and Management of Patients with Primary Biliary Cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef]
- Harms, M.H.; Janssen, Q.P.; Adam, R.; Duvoux, C.; Mirza, D.; Hidalgo, E.; Watson, C.; Wigmore, S.J.; Pinzani, M.; Isoniemi, H.; et al. Trends in Liver Transplantation for Primary Biliary Cholangitis in Europe over the Past Three Decades. Aliment. Pharmacol. Ther. 2019, 49, 285–295. [Google Scholar] [CrossRef]
- Nevens, F.; Andreone, P.; Mazzella, G.; Strasser, S.I.; Bowlus, C.; Invernizzi, P.; Drenth, J.P.H.; Pockros, P.J.; Regula, J.; Beuers, U.; et al. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N. Engl. J. Med. 2016, 375, 631–643. [Google Scholar] [CrossRef]
- Kubota, J.; Ikeda, F.; Terada, R.; Kobashi, H.; Fujioka, S.; Okamoto, R.; Baba, S.; Morimoto, Y.; Ando, M.; Makino, Y.; et al. Mortality Rate of Patients with Asymptomatic Primary Biliary Cirrhosis Diagnosed at Age 55 Years or Older Is Similar to That of the General Population. J. Gastroenterol. 2009, 44, 1000–1006. [Google Scholar] [CrossRef]
- Carbone, M.; Mells, G.F.; Pells, G.; Dawwas, M.F.; Newton, J.L.; Heneghan, M.A.; Neuberger, J.M.; Day, D.B.; Ducker, S.J.; Sandford, R.N.; et al. Sex and Age Are Determinants of the Clinical Phenotype of Primary Biliary Cirrhosis and Response to Ursodeoxycholic Acid. Gastroenterology 2013, 144, 560–569.e7. [Google Scholar] [CrossRef]
- Cheung, A.C.; Lammers, W.J.; Murillo Perez, C.F.; van Buuren, H.R.; Gulamhusein, A.; Trivedi, P.J.; Lazaridis, K.N.; Ponsioen, C.Y.; Floreani, A.; Hirschfield, G.M.; et al. Effects of Age and Sex of Response to Ursodeoxycholic Acid and Transplant-Free Survival in Patients With Primary Biliary Cholangitis. Clin. Gastroenterol. Hepatol. 2019, 17, 2076–2084.e2. [Google Scholar] [CrossRef]
- Drazilova, S.; Babinska, I.; Gazda, J.; Halanova, M.; Janicko, M.; Kucinsky, B.; Safcak, D.; Martinkova, D.; Tarbajova, L.; Cekanova, A.; et al. Epidemiology and Clinical Course of Primary Biliary Cholangitis in Eastern Slovakia. Int. J. Public Health 2020, 65, 683–691. [Google Scholar] [CrossRef]
- Chen, S.; Duan, W.; Li, M.; Li, S.; Lv, T.; Tian, Q.; Wang, Q.; Wu, X.; Zhao, X.; Wang, X.; et al. Prognosis of 732 Ursodeoxycholic Acid-treated Patients with Primary Biliary Cholangitis: A Single Center Follow-up Study from China. J. Gastroenterol. Hepatol. 2019, 34, 1236–1241. [Google Scholar] [CrossRef]
- Abdulkarim, M.; Zenouzi, R.; Sebode, M.; Schulz, L.; Quaas, A.; Lohse, A.W.; Schramm, C.; Weiler-Normann, C. Sex Differences in Clinical Presentation and Prognosis in Patients with Primary Biliary Cholangitis. Scand. J. Gastroenterol. 2019, 54, 1391–1396. [Google Scholar] [CrossRef]
- Cheung, K.-S.; Seto, W.-K.; Fung, J.; Lai, C.-L.; Yuen, M.-F. Epidemiology and Natural History of Primary Biliary Cholangitis in the Chinese: A Territory-Based Study in Hong Kong between 2000 and 2015. Clin. Transl. Gastroenterol. 2017, 8, e116. [Google Scholar] [CrossRef]
- Kim, K.-A.; Ki, M.; Choi, H.Y.; Kim, B.H.; Jang, E.S.; Jeong, S.-H. Population-Based Epidemiology of Primary Biliary Cirrhosis in South Korea. Aliment. Pharmacol. Ther. 2016, 43, 154–162. [Google Scholar] [CrossRef]
- Boonstra, K.; Bokelaar, R.; Stadhouders, P.H.; Tuynman, H.A.; Poen, A.C.; van Nieuwkerk, K.M.; Witteman, E.M.; Hamann, D.; Witteman, B.J.; Beuers, U.; et al. Increased Cancer Risk in a Large Population-Based Cohort of Patients with Primary Biliary Cirrhosis: Follow-up for up to 36 Years. Hepatol. Int. 2014, 8, 268–274. [Google Scholar] [CrossRef]
- Ali, A.H.; Sinakos, E.; Silveira, M.G.; Jorgensen, R.A.; Angulo, P.; Lindor, K.D. Varices in Early Histological Stage Primary Biliary Cirrhosis. J. Clin. Gastroenterol. 2011, 45, e66–e71. [Google Scholar] [CrossRef]
- Marschall, H.U.; Henriksson, I.; Lindberg, S.; Söderdahl, F.; Thuresson, M.; Wahlin, S.; Ludvigsson, J.F. Incidence, Prevalence, and Outcome of Primary Biliary Cholangitis in a Nationwide Swedish Population-Based Cohort. Sci. Rep. 2019, 9, 47890. [Google Scholar] [CrossRef]
- Gatselis, N.K.; Zachou, K.; Lygoura, V.; Azariadis, K.; Arvaniti, P.; Spyrou, E.; Papadamou, G.; Koukoulis, G.K.; Dalekos, G.N.; Rigopoulou, E.I. Geoepidemiology, Clinical Manifestations and Outcome of Primary Biliary Cholangitis in Greece. Eur. J. Intern. Med. 2017, 42, 81–88. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Golabi, P.; Epstein, R.S.; Strauss, M.E.; Nader, F.; Racila, A. Factors Associated With Potential Progressive Course of Primary Biliary Cholangitis. J. Clin. Gastroenterol. 2019, 53, 693–698. [Google Scholar] [CrossRef]
- John, B.V.; Aitcheson, G.; Schwartz, K.B.; Khakoo, N.S.; Dahman, B.; Deng, Y.; Goldberg, D.; Martin, P.; Taddei, T.H.; Levy, C.; et al. Male Sex Is Associated With Higher Rates of Liver-Related Mortality in Primary Biliary Cholangitis and Cirrhosis. Hepatology 2021, 74, 879–891. [Google Scholar] [CrossRef]
- Adejumo, A.C.; Akhtar, D.H.; Dennis, B.B.; Cholankeril, G.; Alayo, Q.; Ogundipe, O.A.; Kim, D.; Ahmed, A. Gender and Racial Differences in Hospitalizations for Primary Biliary Cholangitis in the USA. Dig. Dis. Sci 2021, 66, 1461–1476. [Google Scholar] [CrossRef]
- Lleo, A.; Jepsen, P.; Morenghi, E.; Carbone, M.; Moroni, L.; Battezzati, P.M.; Podda, M.; Mackay, I.R.; Gershwin, M.E.; Invernizzi, P. Evolving Trends in Female to Male Incidence and Male Mortality of Primary Biliary Cholangitis. Sci. Rep. 2016, 6, 25906. [Google Scholar] [CrossRef]
- Natarajan, Y.; Tansel, A.; Patel, P.; Emologu, K.; Shukla, R.; Qureshi, Z.; El-Serag, H.B.; Thrift, A.P.; Kanwal, F. Incidence of Hepatocellular Carcinoma in Primary Biliary Cholangitis: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2021, 66, 2439–2451. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.J.; Lammers, W.J.; van Buuren, H.R.; Parés, A.; Floreani, A.; Janssen, H.L.A.; Invernizzi, P.; Battezzati, P.M.; Ponsioen, C.Y.; Corpechot, C.; et al. Stratification of Hepatocellular Carcinoma Risk in Primary Biliary Cirrhosis: A Multicentre International Study. Gut 2016, 65, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Hirohara, J.; Ueno, Y.; Nakano, T.; Kakuda, Y.; Tsubouchi, H.; Ichida, T.; Nakanuma, Y. Incidence of and Risk Factors for Hepatocellular Carcinoma in Primary Biliary Cirrhosis: National Data from Japan. Hepatology 2013, 57, 1942–1949. [Google Scholar] [CrossRef]
- Lammert, C.; Juran, B.D.; Schlicht, E.; Chan, L.L.; Atkinson, E.J.; de Andrade, M.; Lazaridis, K.N. Biochemical Response to Ursodeoxycholic Acid Predicts Survival in a North American Cohort of Primary Biliary Cirrhosis Patients. J. Gastroenterol. 2014, 49, 1414–1420. [Google Scholar] [CrossRef]
- Tian, S.; Liu, Y.; Sun, K.; Zhou, X.; Ma, S.; Zhang, M.; Zhou, X.; Wang, L.; Han, Y. A Nomogram Based on Pretreatment Clinical Parameters for the Prediction of Inadequate Biochemical Response in Primary Biliary Cholangitis. J. Clin. Lab. Anal. 2020, 34, e23501. [Google Scholar] [CrossRef]
- Lammers, W.J.; Hirschfield, G.M.; Corpechot, C.; Nevens, F.; Lindor, K.D.; Janssen, H.L.A.; Floreani, A.; Ponsioen, C.Y.; Mayo, M.J.; Invernizzi, P.; et al. Development and Validation of a Scoring System to Predict Outcomes of Patients With Primary Biliary Cirrhosis Receiving Ursodeoxycholic Acid Therapy. Gastroenterology 2015, 149, 1804–1812.e4. [Google Scholar] [CrossRef]
- Delgado, J.-S.; Vodonos, A.; Delgado, B.; Jotkowitz, A.; Rosenthal, A.; Fich, A.; Novack, V. Primary Biliary Cirrhosis in Southern Israel: A 20year Follow up Study. Eur. J. Intern. Med. 2012, 23, e193–e198. [Google Scholar] [CrossRef]
- Chen, J.; Xue, D.; Gao, F.; Tao, L.; Li, Y.; Zhang, Q.; Wang, R.; Sun, L.; Yang, X.; Liu, Y.; et al. Influence Factors and a Predictive Scoring Model for Measuring the Biochemical Response of Primary Biliary Cholangitis to Ursodeoxycholic Acid Treatment. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Madir, A.; Božin, T.; Mikolašević, I.; Milić, S.; Štimac, D.; Mijić, M.; Filipec Kanižaj, T.; Biloglav, Z.; Lucijanić, M.; Lucijanić, I.; et al. Epidemiological and Clinical Features of Primary Biliary Cholangitis in Two Croatian Regions: A Retrospective Study. Croat. Med. J. 2019, 60, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Pinto, H.; Liberal, R.; Lopes, S.; Machado, M.V.; Carvalho, J.; Dias, T.; Santos, A.; Agostinho, C.; Figueiredo, P.; Loureiro, R.; et al. Predictors for Incomplete Response to Ursodeoxycholic Acid in Primary Biliary Cholangitis. Data from a National Registry of Liver Disease. United Eur. Gastroenterol. J. 2021, 9, 699–706. [Google Scholar] [CrossRef]
- Invernizzi, F.; Cilla, M.; Trapani, S.; Guarino, M.; Cossiga, V.; Gambato, M.; Morelli, M.C.; Morisco, F.; Burra, P.; Floreani, A. Gender and Autoimmune Liver Diseases: Relevant Aspects in Clinical Practice. J. Pers. Med. 2022, 12, 925. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, D.; De Vincentis, A.; Malinverno, F.; Viganò, M.; Alvaro, D.; Pompili, M.; Picciotto, A.; Palitti, V.P.; Russello, M.; Storato, S.; et al. Real-World Experience with Obeticholic Acid in Patients with Primary Biliary Cholangitis. JHEP Rep. 2021, 3, 100248. [Google Scholar] [CrossRef]
- Mells, G.F.; Pells, G.; Newton, J.L.; Bathgate, A.J.; Burroughs, A.K.; Heneghan, M.A.; Neuberger, J.M.; Day, D.B.; Ducker, S.J.; Sandford, R.N.; et al. Impact of Primary Biliary Cirrhosis on Perceived Quality of Life: The UK-PBC National Study. Hepatology 2013, 58, 273–283. [Google Scholar] [CrossRef]
- Hegade, V.S.; Mells, G.F.; Fisher, H.; Kendrick, S.; DiBello, J.; Gilchrist, K.; Alexander, G.J.; Hirschfield, G.M.; Sandford, R.N.; Jones, D.E.J. Pruritus Is Common and Undertreated in Patients With Primary Biliary Cholangitis in the United Kingdom. Clin. Gastroenterol. And. Hepatol. 2019, 17, 1379–1387.e3. [Google Scholar] [CrossRef]
- Poupon, R.E.; Chrétien, Y.; Chazouillères, O.; Poupon, R.; Chwalow, J. Quality of Life in Patients with Primary Biliary Cirrhosis. Hepatology 2004, 40, 489–494. [Google Scholar] [CrossRef]
- Quarneti, C.; Muratori, P.; Lalanne, C.; Fabbri, A.; Menichella, R.; Granito, A.; Masi, C.; Lenzi, M.; Cassani, F.; Pappas, G.; et al. Fatigue and Pruritus at Onset Identify a More Aggressive Subset of Primary Biliary Cirrhosis. Liver. Int. 2015, 35, 636–641. [Google Scholar] [CrossRef]
- Jones, D.E.; Al-Rifai, A.; Frith, J.; Patanwala, I.; Newton, J.L. The Independent Effects of Fatigue and UDCA Therapy on Mortality in Primary Biliary Cirrhosis: Results of a 9year Follow-Up. J. Hepatol 2010, 53, 911–917. [Google Scholar] [CrossRef]
- Prince, M.I. Asymptomatic Primary Biliary Cirrhosis: Clinical Features, Prognosis, and Symptom Progression in a Large Population Based Cohort. Gut 2004, 53, 865–870. [Google Scholar] [CrossRef]
- Mitchison, H.C.; Lucey, M.R.; Kelly, P.J.; Neuberger, J.M.; Williams, R.; James, O.F.W. Symptom Development and Prognosis in Primary Biliary Cirrhosis: A Study in Two Centers. Gastroenterology 1990, 99, 778–784. [Google Scholar] [CrossRef]
- Mahl, T.C.; Shockcor, W.; Boyer, J.L. Primary Biliary Cirrhosis: Survival of a Large Cohort of Symptomatic and Asymptomatic Patients Followed for 24 Years. J. Hepatol 1994, 20, 707–713. [Google Scholar] [CrossRef]
- Marzioni, M.; Bassanelli, C.; Ripellino, C.; Urbinati, D.; Alvaro, D. Epidemiology of Primary Biliary Cholangitis in Italy: Evidence from a Real-World Database. Dig. Liver Dis. 2019, 51, 724–729. [Google Scholar] [CrossRef]
- Efe, C.; Torgutalp, M.; Henriksson, I.; Alalkim, F.; Lytvyak, E.; Trivedi, H.; Eren, F.; Fischer, J.; Chayanupatkul, M.; Coppo, C.; et al. Extrahepatic Autoimmune Diseases in Primary Biliary Cholangitis: Prevalence and Significance for Clinical Presentation and Disease Outcome. J. Gastroenterol. Hepatol 2021, 36, 936–942. [Google Scholar] [CrossRef]
- Boberg, K.M.; Chapman, R.W.; Hirschfield, G.M.; Lohse, A.W.; Manns, M.P.; Schrumpf, E. Overlap Syndromes: The International Autoimmune Hepatitis Group (IAIHG) Position Statement on a Controversial Issue. J. Hepatol. 2011, 54, 374–385. [Google Scholar] [CrossRef]
- Chazouillères, O.; Wendum, D.; Serfaty, L.; Montembault, S.; Rosmorduc, O.; Poupon, R. Primary Biliary Cirrhosis-Autoimmune Hepatitis Overlap Syndrome: Clinical Features and Response to Therapy. Hepatology 1998, 28, 296–301. [Google Scholar] [CrossRef]
- Chazouillères, O. Overlap Syndromes. Dig. Dis. 2015, 33, 181–187. [Google Scholar] [CrossRef]
- Neuhauser, M.; Bjornsson, E.; Treeprasertsuk, S.; Enders, F.; Silveira, M.; Talwalkar, J.; Lindor, K. Autoimmune Hepatitis–PBC Overlap Syndrome: A Simplified Scoring System May Assist in the Diagnosis. Am. J. Gastroenterol. 2010, 105, 345–353. [Google Scholar] [CrossRef]
- Chazouillères, O.; Wendum, D.; Serfaty, L.; Rosmorduc, O.; Poupon, R. Long Term Outcome and Response to Therapy of Primary Biliary Cirrhosis—Autoimmune Hepatitis Overlap Syndrome. J. Hepatol. 2006, 44, 400–406. [Google Scholar] [CrossRef]
- Vleggaar, F.P.; van Buuren, H.R.; Zondervan, P.E.; ten Kate, F.J.W.; Hop, W.C.J. Jaundice in Non-Cirrhotic Primary Biliary Cirrhosis: The Premature Ductopenic Variant. Gut 2001, 49, 276–281. [Google Scholar] [CrossRef]
- Kumagi, T.; Guindi, M.; Fischer, S.E.; Arenovich, T.; Abdalian, R.; Coltescu, C.; Heathcote, J.E.; Hirschfield, G.M. Baseline Ductopenia and Treatment Response Predict Long-Term Histological Progression in Primary Biliary Cirrhosis. Am. J. Gastroenterol. 2010, 105, 2186–2194. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Ronca, V.; Bruno, S.; Invernizzi, P.; Mells, G.F. Toward Precision Medicine in Primary Biliary Cholangitis. Dig. Liver Dis. 2016, 48, 843–850. [Google Scholar] [CrossRef]
- Invernizzi, P.; Lleo, A.; Podda, M. Interpreting Serological Tests in Diagnosing Autoimmune Liver Diseases. Semin. Liver. Dis. 2007, 27, 161–172. [Google Scholar] [CrossRef]
- Marzorati, S.; Invernizzi, P.; Lleo, A. Making Sense of Autoantibodies in Cholestatic Liver Diseases. Clin. Liver. Dis. 2016, 20, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Cauch-Dudek, K.; Heathcote, E.J.; Lindor, K.; Jorgensen, R.; Klein, R. Antimitochondrial Antibody Profiles: Are They Valid Prognostic Indicators in Primary Biliary Cirrhosis? Am. J. Gastroenterol. 2002, 97, 999–1002. [Google Scholar] [CrossRef]
- Yamagiwa, S. Autoantibodies in Primary Biliary Cirrhosis: Recent Progress in Research on the Pathogenetic and Clinical Significance. World. J. Gastroenterol. 2014, 20, 2606. [Google Scholar] [CrossRef]
- Juliusson, G.; Imam, M.; Björnsson, E.S.; Talwalkar, J.A.; Lindor, K.D. Long-Term Outcomes in Antimitochondrial Antibody Negative Primary Biliary Cirrhosis. Scand. J. Gastroenterol. 2016, 51, 745–752. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of Cholestatic Liver Diseases. J. Hepatol. 2009, 51, 237–267. [Google Scholar] [CrossRef]
- Vergani, D.; Alvarez, F.; Bianchi, F.B.; Cançado, E.L.R.; Mackay, I.R.; Manns, M.P.; Nishioka, M.; Penner, E. Liver Autoimmune Serology: A Consensus Statement from the Committee for Autoimmune Serology of the International Autoimmune Hepatitis Group. J. Hepatol. 2004, 41, 677–683. [Google Scholar] [CrossRef]
- Hirschfield, G.M.; Heathcote, E.J. Antimitochondrial Antibody-Negative Primary Biliary Cirrhosis. Clin. Liver. Dis. 2008, 12, 323–331. [Google Scholar] [CrossRef]
- Ronca, V.; Gerussi, A.; Cristoferi, L.; Carbone, M.; Invernizzi, P. Precision Medicine in Primary Biliary Cholangitis. J. Dig. Dis. 2019, 20, 338–345. [Google Scholar] [CrossRef]
- Wesierska-Gadek, J.; Penner, E.; Battezzati, P.M.; Selmi, C.; Zuin, M.; Hitchman, E.; Worman, H.J.; Gershwin, M.E.; Podda, M.; Invernizzi, P. Correlation of Initial Autoantibody Profile and Clinical Outcome in Primary Biliary Cirrhosis. Hepatology 2006, 43, 1135–1144. [Google Scholar] [CrossRef]
- Huang, C.; Han, W.; Wang, C.; Liu, Y.; Chen, Y.; Duan, Z. Early Prognostic Utility of Gp210 Antibody-Positive Rate in Primary Biliary Cholangitis: A Meta-Analysis. Dis. Markers 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Haldar, D.; Janmohamed, A.; Plant, T.; Davidson, M.; Norman, H.; Russell, E.; Serevina, O.; Chung, K.; Qamar, K.; Gunson, B.; et al. Antibodies to Gp210 and Understanding Risk in Patients with Primary Biliary Cholangitis. Liver. Int. 2021, 41, 535–544. [Google Scholar] [CrossRef]
- Yang, F.; Yang, Y.; Wang, Q.; Wang, Z.; Miao, Q.; Xiao, X.; Wei, Y.; Bian, Z.; Sheng, L.; Chen, X.; et al. The Risk Predictive Values of UK-PBC and GLOBE Scoring System in Chinese Patients with Primary Biliary Cholangitis: The Additional Effect of Anti-Gp210. Aliment. Pharmacol. Ther. 2017, 45, 733–743. [Google Scholar] [CrossRef]
- Zhao, D.-T.; Yan, H.-P.; Liao, H.-Y.; Liu, Y.-M.; Han, Y.; Zhang, H.-P.; Zhang, W.-M.; Huang, C.-Y.; Liu, X.-H.; Lou, J.-L.; et al. Using Two-Step Cluster Analysis to Classify Inpatients with Primary Biliary Cholangitis Based on Autoantibodies: A Real-World Retrospective Study of 537 Patients in China. Front. Immunol. 2023, 13, 98076. [Google Scholar] [CrossRef]
- Gatselis, N.K.; Zachou, K.; Norman, G.L.; Gabeta, S.; Papamichalis, P.; Koukoulis, G.K.; Dalekos, G.N. Clinical Significance of the Fluctuation of Primary Biliary Cirrhosis-Related Autoantibodies during the Course of the Disease. Autoimmunity 2013, 46, 471–479. [Google Scholar] [CrossRef]
- Reig, A.; Norman, G.L.; Garcia, M.; Shums, Z.; Ruiz-Gaspà, S.; Bentow, C.; Mahler, M.; Romera, M.A.; Vinas, O.; Pares, A. Novel Anti–Hexokinase 1 Antibodies Are Associated With Poor Prognosis in Patients With Primary Biliary Cholangitis. Am. J. Gastroenterol. 2020, 115, 1634–1641. [Google Scholar] [CrossRef]
- Tana, M.M.; Shums, Z.; Milo, J.; Norman, G.L.; Leung, P.S.; Gershwin, M.E.; Noureddin, M.; Kleiner, D.E.; Zhao, X.; Heller, T.; et al. The Significance of Autoantibody Changes Over Time in Primary Biliary Cirrhosis. Am. J. Clin. Pathol. 2015, 144, 601–606. [Google Scholar] [CrossRef]
- Liberal, R.; Grant, C.R.; Sakkas, L.; Bizzaro, N.; Bogdanos, D.P. Diagnostic and Clinical Significance of Anti-Centromere Antibodies in Primary Biliary Cirrhosis. Clin. Res. Hepatol. Gastroenterol. 2013, 37, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Kondo, H.; Mori, T.; Komori, A.; Matsuyama, M.; Ito, M.; Takii, Y.; Koyabu, M.; Yokoyama, T.; Migita, K.; et al. Anti-Gp210 and Anti-Centromere Antibodies Are Different Risk Factors for the Progression of Primary Biliary Cirrhosis. Hepatology 2007, 45, 118–127. [Google Scholar] [CrossRef]
- Gao, L.; Tian, X.; Liu, B.; Zhang, F. The Value of Antinuclear Antibodies in Primary Biliary Cirrhosis. Clin. Exp. Med. 2008, 8, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Norman, G.L.; Yang, C.-Y.; Ostendorff, H.P.; Shums, Z.; Lim, M.J.; Wang, J.; Awad, A.; Hirschfield, G.M.; Milkiewicz, P.; Bloch, D.B.; et al. Anti-Kelch-like 12 and Anti-Hexokinase 1: Novel Autoantibodies in Primary Biliary Cirrhosis. Liver. Int. 2015, 35, 642–651. [Google Scholar] [CrossRef]
- Rigopoulou, E.I.; Bogdanos, D.P. Role of Autoantibodies in the Clinical Management of Primary Biliary Cholangitis. World. J. Gastroenterol. 2023, 29, 1795–1810. [Google Scholar] [CrossRef]
- Levy, C.; Bowlus, C.L. Role of Antinuclear Antibodies in Primary Biliary Cholangitis. Am. J. Gastroenterol. 2020, 115, 1604–1606. [Google Scholar] [CrossRef]
- Bauer, A.; Habior, A.; Gawel, D. Diagnostic and Clinical Value of Specific Autoantibodies against Kelch-like 12 Peptide and Nuclear Envelope Proteins in Patients with Primary Biliary Cholangitis. Biomedicines 2022, 10, 801. [Google Scholar] [CrossRef]
- Murillo Perez, C.F.; Hirschfield, G.M.; Corpechot, C.; Floreani, A.; Mayo, M.J.; van der Meer, A.; Ponsioen, C.Y.; Lammers, W.J.; Parés, A.; Invernizzi, P.; et al. Fibrosis Stage Is an Independent Predictor of Outcome in Primary Biliary Cholangitis despite Biochemical Treatment Response. Aliment. Pharmacol. Ther. 2019, 50, 1127–1136. [Google Scholar] [CrossRef]
- Rubin, E.; Schaffner, F.; Popper, H. Primary Biliary Cirrhosis. Chronic non-suppurative destructive cholangitis. Am. J. Pathol. 1965, 46, 387–407. [Google Scholar]
- Tsuneyama, K.; Baba, H.; Morimoto, Y.; Tsunematsu, T.; Ogawa, H. Primary Biliary Cholangitis: Its Pathological Characteristics and Immunopathological Mechanisms. J. Med. Investig. 2017, 64, 7–13. [Google Scholar] [CrossRef]
- Carey, E.J.; Ali, A.H.; Lindor, K.D. Primary Biliary Cirrhosis. Lancet 2015, 386, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, J.; Dickson, E.R.; McDonald, G.S.A. Staging of Chronic Nonsuppurative Destructive Cholangitis (Syndrome of Primary Biliary Cirrhosis). Virchows Arch. A. Pathol. Anat. Histol. 1978, 379, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Scheuer, P. Primary Biliary Cirrhosis. Proc. R. Soc. Med. 1967, 60, 1257–1260. [Google Scholar] [PubMed]
- Degott, C.; Zafrani, E.S.; Callard, P.; Balkau, B.; Poupon, R.E.; Poupon, R. Histopathological Study of Primary Biliary Cirrhosis and the Effect of Ursodeoxycholic Acid Treatment on Histology Progression. Hepatology 1999, 29, 1007–1012. [Google Scholar] [CrossRef]
- Harada, K.; Hsu, M.; Ikeda, H.; Zeniya, M.; Nakanuma, Y. Application and Validation of a New Histologic Staging and Grading System for Primary Biliary Cirrhosis. J. Clin. Gastroenterol. 2013, 47, 174–181. [Google Scholar] [CrossRef]
- Kakuda, Y.; Harada, K.; Sawada-Kitamura, S.; Ikeda, H.; Sato, Y.; Sasaki, M.; Okafuji, H.; Mizukoshi, E.; Terasaki, S.; Ohta, H.; et al. Evaluation of a New Histologic Staging and Grading System for Primary Biliary Cirrhosis in Comparison with Classical Systems. Hum. Pathol. 2013, 44, 1107–1117. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Zen, Y.; Harada, K.; Sasaki, M.; Nonomura, A.; Uehara, T.; Sano, K.; Kondo, F.; Fukusato, T.; Tsuneyama, K.; et al. Application of a New Histological Staging and Grading System for Primary Biliary Cirrhosis to Liver Biopsy Specimens: Interobserver Agreement. Pathol. Int. 2010, 60, 167–174. [Google Scholar] [CrossRef]
- Wendum, D.; Boëlle, P.-Y.; Bedossa, P.; Zafrani, E.-S.; Charlotte, F.; Saint-Paul, M.-C.; Michalak, S.; Chazouillères, O.; Corpechot, C. Primary Biliary Cirrhosis: Proposal for a New Simple Histological Scoring System. Liver. Int. 2015, 35, 652–659. [Google Scholar] [CrossRef]
- Namisaki, T.; Moriya, K.; Noguchi, R.; Kitade, M.; Kawaratani, H.; Yamao, J.; Mitoro, A.; Yoshida, M.; Sawai, M.; Uejima, M.; et al. Liver Fibrosis Progression Predicts Survival in Patients with Primary Biliary Cirrhosis. Hepatol. Res. 2017, 47, E178–E186. [Google Scholar] [CrossRef]
- Rockey, D.C.; Caldwell, S.H.; Goodman, Z.D.; Nelson, R.C.; Smith, A.D. Liver Biopsy. Hepatology 2009, 49, 1017–1044. [Google Scholar] [CrossRef]
- Fujinaga, Y.; Namisaki, T.; Moriya, K.; Kitade, M.; Kawaratani, H.; Shimozato, N.; Kaji, K.; Takaya, H.; Sawada, Y.; Seki, K.; et al. Identification of Clinical Risk Factors for Histological Progression of Primary Biliary Cholangitis. Hepatol. Res. 2019, 49, 1015–1025. [Google Scholar] [CrossRef]
- Sekiguchi, T.; Umemura, T.; Fujimori, N.; Shibata, S.; Ichikawa, Y.; Kimura, T.; Joshita, S.; Komatsu, M.; Matsumoto, A.; Tanaka, E.; et al. Serum Cell Death Biomarkers for Prediction of Liver Fibrosis and Poor Prognosis in Primary Biliary Cirrhosis. PLoS ONE 2015, 10, e0131658. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Bruns, T.; Cheung, A.; Li, K.-K.; Kittler, C.; Kumagi, T.; Shah, H.; Corbett, C.; Al-Harthy, N.; Acarsu, U.; et al. Optimising Risk Stratification in Primary Biliary Cirrhosis: AST/Platelet Ratio Index Predicts Outcome Independent of Ursodeoxycholic Acid Response. J. Hepatol. 2014, 60, 1249–1258. [Google Scholar] [CrossRef]
- Fujinaga, Y.; Namisaki, T.; Takaya, H.; Tsuji, Y.; Suzuki, J.; Shibamoto, A.; Kubo, T.; Iwai, S.; Tomooka, F.; Takeda, S.; et al. Enhanced Liver Fibrosis Score as a Surrogate of Liver-Related Complications and Mortality in Primary Biliary Cholangitis. Medicine 2021, 100, e27403. [Google Scholar] [CrossRef]
- Umemura, T.; Joshita, S.; Sekiguchi, T.; Usami, Y.; Shibata, S.; Kimura, T.; Komatsu, M.; Matsumoto, A.; Ota, M.; Tanaka, E. Serum Wisteria Floribunda Agglutinin-Positive Mac-2-Binding Protein Level Predicts Liver Fibrosis and Prognosis in Primary Biliary Cirrhosis. Am. J. Gastroenterol. 2015, 110, 857–864. [Google Scholar] [CrossRef]
- Nishikawa, H.; Enomoto, H.; Iwata, Y.; Hasegawa, K.; Nakano, C.; Takata, R.; Nishimura, T.; Yoh, K.; Aizawa, N.; Sakai, Y.; et al. Impact of Serum Wisteria Floribunda Agglutinin Positive Mac-2-Binding Protein and Serum Interferon-γ-Inducible Protein-10 in Primary Biliary Cirrhosis. Hepatol. Res. 2016, 46, 575–583. [Google Scholar] [CrossRef]
- Osman, K.T.; Maselli, D.B.; Idilman, I.S.; Rowan, D.J.; Viehman, J.K.; Harmsen, W.S.; Harnois, D.M.; Carey, E.J.; Gossard, A.A.; LaRusso, N.F.; et al. Liver Stiffness Measured by Either Magnetic Resonance or Transient Elastography Is Associated With Liver Fibrosis and Is an Independent Predictor of Outcomes Among Patients With Primary Biliary Cholangitis. J. Clin. Gastroenterol. 2021, 55, 449–457. [Google Scholar] [CrossRef]
- Sandrin, L.; Fourquet, B.; Hasquenoph, J.-M.; Yon, S.; Fournier, C.; Mal, F.; Christidis, C.; Ziol, M.; Poulet, B.; Kazemi, F.; et al. Transient Elastography: A New Noninvasive Method for Assessment of Hepatic Fibrosis. Ultrasound. Med. Biol. 2003, 29, 1705–1713. [Google Scholar] [CrossRef]
- Castéra, L.; Foucher, J.; Bernard, P.-H.; Carvalho, F.; Allaix, D.; Merrouche, W.; Couzigou, P.; de Lédinghen, V. Pitfalls of Liver Stiffness Measurement: A 5-Year Prospective Study of 13,369 Examinations. Hepatology 2010, 5, 828–835. [Google Scholar] [CrossRef]
- Myers, R.P.; Pomier-Layrargues, G.; Kirsch, R.; Pollett, A.; Duarte-Rojo, A.; Wong, D.; Beaton, M.; Levstik, M.; Crotty, P.; Elkashab, M. Feasibility and Diagnostic Performance of the FibroScan XL Probe for Liver Stiffness Measurement in Overweight and Obese Patients. Hepatology 2012, 55, 199–208. [Google Scholar] [CrossRef]
- Stebbing, J.; Farouk, L.; Panos, G.; Anderson, M.; Jiao, L.R.; Mandalia, S.; Bower, M.; Gazzard, B.; Nelson, M. A Meta-Analysis of Transient Elastography for the Detection of Hepatic Fibrosis. J. Clin. Gastroenterol. 2010, 44, 214–219. [Google Scholar] [CrossRef]
- Castera, L.; Forns, X.; Alberti, A. Non-Invasive Evaluation of Liver Fibrosis Using Transient Elastography. J. Hepatol. 2008, 48, 835–847. [Google Scholar] [CrossRef]
- Corpechot, C.; Carrat, F.; Poujol-Robert, A.; Gaouar, F.; Wendum, D.; Chazouillères, O.; Poupon, R. Noninvasive Elastography-Based Assessment of Liver Fibrosis Progression and Prognosis in Primary Biliary Cirrhosis. Hepatology 2012, 56, 198–208. [Google Scholar] [CrossRef]
- Cristoferi, L.; Calvaruso, V.; Overi, D.; Viganò, M.; Rigamonti, C.; Degasperi, E.; Cardinale, V.; Labanca, S.; Zucchini, N.; Fichera, A.; et al. Accuracy of Transient Elastography in Assessing Fibrosis at Diagnosis in Naïve Patients With Primary Biliary Cholangitis: A Dual Cut-Off Approach. Hepatology 2021, 74, 1496–1508. [Google Scholar] [CrossRef]
- Corpechot, C.; Carrat, F.; Gaouar, F.; Chau, F.; Hirschfield, G.; Gulamhusein, A.; Montano-Loza, A.J.; Lytvyak, E.; Schramm, C.; Pares, A.; et al. Liver Stiffness Measurement by Vibration-Controlled Transient Elastography Improves Outcome Prediction in Primary Biliary Cholangitis. J. Hepatol. 2022, 77, 1545–1553. [Google Scholar] [CrossRef]
- Muller, M.; Gennisson, J.-L.; Deffieux, T.; Tanter, M.; Fink, M. Quantitative Viscoelasticity Mapping of Human Liver Using Supersonic Shear Imaging: Preliminary In Vivo Feasability Study. Ultrasound Med. Biol. 2009, 35, 219–229. [Google Scholar] [CrossRef]
- Yan, Y.; Xing, X.; Lu, Q.; Wang, X.; Luo, X.; Yang, L. Assessment of Biopsy Proven Liver Fibrosis by Two-Dimensional Shear Wave Elastography in Patients with Primary Biliary Cholangitis. Dig. Liver Dis. 2020, 52, 555–560. [Google Scholar] [CrossRef]
- Schulz, M.; Wilde, A.-C.B.; Demir, M.; Müller, T.; Tacke, F.; Wree, A. Shear Wave Elastography and Shear Wave Dispersion Imaging in Primary Biliary Cholangitis—A Pilot Study. Quant. Imaging Med. Surg. 2022, 12, 1235–1242. [Google Scholar] [CrossRef]
- Nightingale, K.; Soo, M.S.; Nightingale, R.; Trahey, G. Acoustic Radiation Force Impulse Imaging: In Vivo Demonstration of Clinical Feasibility. Ultrasound Med. Biol. 2002, 28, 227–235. [Google Scholar] [CrossRef]
- Goertz, R.S.; GaBmann, L.; Strobel, D.; Wildner, D.; Schellhaas, B.; Neurath, M.F.; Pfeifer, L. Acoustic Radiation Force Impulse (ARFI) Elastography in Autoimmune and Cholestatic Liver Diseases. Ann. Hepatol. 2019, 18, 23–29. [Google Scholar] [CrossRef]
- Venkatesh, S.K.; Yin, M.; Ehman, R.L. Magnetic Resonance Elastography of Liver: Technique, Analysis, and Clinical Applications. J. Magn. Reson. Imaging 2013, 37, 544–555. [Google Scholar] [CrossRef]
- Ichikawa, S.; Motosugi, U.; Morisaka, H.; Sano, K.; Ichikawa, T.; Tatsumi, A.; Enomoto, N.; Matsuda, M.; Fujii, H.; Onishi, H. Comparison of the Diagnostic Accuracies of Magnetic Resonance Elastography and Transient Elastography for Hepatic Fibrosis. Magn. Reson. Imaging 2015, 33, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Venkatesh, S.K.; Wang, Z.; Miller, F.H.; Motosugi, U.; Low, R.N.; Hassanein, T.; Asbach, P.; Godfrey, E.M.; Yin, M.; et al. Diagnostic Performance of Magnetic Resonance Elastography in Staging Liver Fibrosis: A Systematic Review and Meta-Analysis of Individual Participant Data. Clin. Gastroenterol. Hepatol. 2015, 13, 440–451.e6. [Google Scholar] [CrossRef]
- Lammers, W.J.; van Buuren, H.R.; Hirschfield, G.M.; Janssen, H.L.A.; Invernizzi, P.; Mason, A.L.; Ponsioen, C.Y.; Floreani, A.; Corpechot, C.; Mayo, M.J.; et al. Levels of Alkaline Phosphatase and Bilirubin Are Surrogate End Points of Outcomes of Patients With Primary Biliary Cirrhosis: An International Follow-up Study. Gastroenterology 2014, 147, 1338–1349.e5. [Google Scholar] [CrossRef]
- Corpechot, C.; Abenavoli, L.; Rabahi, N.; Chrétien, Y.; Andréani, T.; Johanet, C.; Chazouillères, O.; Poupon, R. Biochemical Response to Ursodeoxycholic Acid and Long-Term Prognosis in Primary Biliary Cirrhosis. Hepatology 2008, 48, 871–877. [Google Scholar] [CrossRef]
- Gerussi, A.; Bernasconi, D.P.; O’Donnell, S.E.; Lammers, W.J.; Van Buuren, H.; Hirschfield, G.; Janssen, H.; Corpechot, C.; Reig, A.; Pares, A.; et al. Measurement of Gamma Glutamyl Transferase to Determine Risk of Liver Transplantation or Death in Patients with Primary Biliary Cholangitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1688–1697.e14. [Google Scholar] [CrossRef]
- Trivella, J.; John, B.V.; Levy, C. Primary Biliary Cholangitis: Epidemiology, Prognosis, and Treatment. Hepatol. Commun. 2023, 7, e179. [Google Scholar] [CrossRef]
- Dickson, E.R.; Grambsch, P.M.; Fleming, T.R.; Fisher, L.D.; Langworthy, A. Prognosis in Primary Biliary Cirrhosis: Model for Decision Making. Hepatology 1989, 10, 1–7. [Google Scholar] [CrossRef]
- Goet, J.C.; Harms, M.H.; Carbone, M.; Hansen, B.E. Risk Stratification and Prognostic Modelling in Primary Biliary Cholangitis. Best. Pract. Res. Clin. Gastroenterol. 2018, 34–35, 95–106. [Google Scholar] [CrossRef]
- Murillo Perez, C.F.; Harms, M.H.; Lindor, K.D.; van Buuren, H.R.; Hirschfield, G.M.; Corpechot, C.; van der Meer, A.J.; Feld, J.J.; Gulamhusein, A.; Lammers, W.J.; et al. Goals of Treatment for Improved Survival in Primary Biliary Cholangitis: Treatment Target Should Be Bilirubin Within the Normal Range and Normalization of Alkaline Phosphatase. Am. J. Gastroenterol. 2020, 115, 1066–1074. [Google Scholar] [CrossRef]
- Parés, A.; Caballería, L.; Rodés, J. Excellent Long-Term Survival in Patients With Primary Biliary Cirrhosis and Biochemical Response to Ursodeoxycholic Acid. Gastroenterology 2006, 130, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-N.; Shi, T.-Y.; Shi, X.-H.; Wang, L.; Yang, Y.-J.; Liu, B.; Gao, L.-X.; Shuai, Z.-W.; Kong, F.; Chen, H.; et al. Early Biochemical Response to Ursodeoxycholic Acid and Long-Term Prognosis of Primary Biliary Cirrhosis: Results of a 14-Year Cohort Study. Hepatology 2013, 58, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Murillo Perez, C.F.; Ioannou, S.; Hassanally, I.; Trivedi, P.J.; Corpechot, C.; van der Meer, A.J.; Lammers, W.J.; Battezzati, P.M.; Lindor, K.D.; Nevens, F.; et al. Optimizing Therapy in Primary Biliary Cholangitis: Alkaline Phosphatase at Six Months Identifies One-year Non-responders and Predicts Survival. Liver Int. 2023, 43, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, E.M.M.; Hansen, B.E.; de Vries, R.A.; den Ouden–Muller, J.W.; van Ditzhuijsen, T.J.M.; Haagsma, E.B.; Houben, M.H.M.G.; Witteman, B.J.M.; van Erpecum, K.J.; van Buuren, H.R. Improved Prognosis of Patients With Primary Biliary Cirrhosis That Have a Biochemical Response to Ursodeoxycholic Acid. Gastroenterology 2009, 136, 1281–1287. [Google Scholar] [CrossRef]
- Azemoto, N.; Abe, M.; Murata, Y.; Hiasa, Y.; Hamada, M.; Matsuura, B.; Onji, M. Early Biochemical Response to Ursodeoxycholic Acid Predicts Symptom Development in Patients with Asymptomatic Primary Biliary Cirrhosis. J. Gastroenterol. 2009, 44, 630–634. [Google Scholar] [CrossRef]
- Corpechot, C.; Chazouillères, O.; Poupon, R. Early Primary Biliary Cirrhosis: Biochemical Response to Treatment and Prediction of Long-Term Outcome. J. Hepatol. 2011, 55, 1361–1367. [Google Scholar] [CrossRef]
- Carbone, M.; Sharp, S.J.; Flack, S.; Paximadas, D.; Spiess, K.; Adgey, C.; Griffiths, L.; Lim, R.; Trembling, P.; Williamson, K.; et al. The UK-PBC Risk Scores: Derivation and Validation of a Scoring System for Long-term Prediction of End-stage Liver Disease in Primary Biliary Cholangitis. Hepatology 2016, 63, 930–950. [Google Scholar] [CrossRef]
- Carbone, M.; Harms, M.H.; Lammers, W.J.; Marmon, T.; Pencek, R.; MacConell, L.; Shapiro, D.; Jones, D.E.; Mells, G.F.; Hansen, B.E. Clinical Application of the GLOBE and United Kingdom-primary Biliary Cholangitis Risk Scores in a Trial Cohort of Patients with Primary Biliary Cholangitis. Hepatol. Commun. 2018, 2, 683–692. [Google Scholar] [CrossRef]
- Carbone, M.; Nardi, A.; Flack, S.; Carpino, G.; Varvaropoulou, N.; Gavrila, C.; Spicer, A.; Badrock, J.; Bernuzzi, F.; Cardinale, V.; et al. Pretreatment Prediction of Response to Ursodeoxycholic Acid in Primary Biliary Cholangitis: Development and Validation of the UDCA Response Score. Lancet Gastroenterol. Hepatol. 2018, 3, 626–634. [Google Scholar] [CrossRef]
- Yagi, M.; Matsumoto, K.; Komori, A.; Abe, M.; Hashimoto, N.; Inao, M.; Namisaki, T.; Kawata, K.; Ninomiya, M.; Fujii, H.; et al. A Validation Study of the Ursodeoxycholic Acid Response Score in Japanese Patients with Primary Biliary Cholangitis. Liver Int. 2020, 40, 1926–1933. [Google Scholar] [CrossRef]
- Levy, C.; Manns, M.; Hirschfield, G. New Treatment Paradigms in Primary Biliary Cholangitis. Clin. Gastroenterol. Hepatol. 2023, 21, 2076–2087. [Google Scholar] [CrossRef] [PubMed]
- Cristoferi, L.; Nardi, A.; Ronca, V.; Invernizzi, P.; Mells, G.; Carbone, M. Prognostic Models in Primary Biliary Cholangitis. J. Autoimmun. 2018, 95, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.N.; Wulff, J.; Comerford, M.; Vuppalanchi, R.; Chalasani, N. Serum Metabolic Signatures of Primary Biliary Cirrhosis and Primary Sclerosing Cholangitis. Liver Int. 2015, 35, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Mindikoglu, A.L.; Coarfa, C.; Opekun, A.R.; Shah, V.H.; Arab, J.P.; Lazaridis, K.N.; Putluri, N.; Ambati, C.R.; Robertson, M.J.; Devaraj, S.; et al. Metabolomic Biomarkers Are Associated with Mortality in Patients with Cirrhosis Caused by Primary Biliary Cholangitis or Primary Sclerosing Cholangitis. Future Sci. OA 2020, 6, e124. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Yang, T.; Zhou, Y.; Gao, G.-Y.; Xing, F.; Peng, Y.; Tao, Y.-Y.; Liu, C.-H. Serum Metabolomics Analysis Reveals a Distinct Metabolic Profile of Patients with Primary Biliary Cholangitis. Sci. Rep. 2017, 7, 784. [Google Scholar] [CrossRef]
- Walker, D.I.; Juran, B.D.; Cheung, A.C.; Schlicht, E.M.; Liang, Y.; Niedzwiecki, M.; LaRusso, N.F.; Gores, G.J.; Jones, D.P.; Miller, G.W.; et al. High-Resolution Exposomics and Metabolomics Reveals Specific Associations in Cholestatic Liver Diseases. Hepatol. Commun. 2022, 6, 965–979. [Google Scholar] [CrossRef]
- Zheng, Y.; Ran, Y.; Zhang, H.; Wang, B.; Zhou, L. The Microbiome in Autoimmune Liver Diseases: Metagenomic and Metabolomic Changes. Front. Physiol. 2021, 12, e715852. [Google Scholar] [CrossRef]
- Lammert, C.; Shin, A.S.; Xu, H.; Hemmerich, C.; O’Connell, T.M.; Chalasani, N. Short-Chain Fatty Acid and Fecal Microbiota Profiles Are Linked to Fibrosis in Primary Biliary Cholangitis. FEMS Microbiol. Lett. 2021, 368, e38. [Google Scholar] [CrossRef]
- Gerussi, A.; Verda, D.; Bernasconi, D.P.; Carbone, M.; Komori, A.; Abe, M.; Inao, M.; Namisaki, T.; Mochida, S.; Yoshiji, H.; et al. Machine Learning in Primary Biliary Cholangitis: A Novel Approach for Risk Stratification. Liver Int. 2022, 42, 615–627. [Google Scholar] [CrossRef]
- Kalapala, R.; Rughwani, H.; Reddy, D.N. Artificial Intelligence in Hepatology- Ready for the Primetime. J. Clin. Exp. Hepatol. 2023, 13, 149–161. [Google Scholar] [CrossRef]
- Gerussi, A.; Scaravaglio, M.; Cristoferi, L.; Verda, D.; Milani, C.; De Bernardi, E.; Ippolito, D.; Asselta, R.; Invernizzi, P.; Kather, J.N.; et al. Artificial Intelligence for Precision Medicine in Autoimmune Liver Disease. Front. Immunol. 2022, 13, e966329. [Google Scholar] [CrossRef] [PubMed]
| Low Risk | High Risk | |
|---|---|---|
| Age | >55 years | <55 years |
| Sex | Female | Male |
| Clinical pattern | No symptoms | Symptomatic disease AIH/PBC OS Premature Ductopenic Variant |
| Antibody profile | AMA- | Anti-gp210+ ACA+ Anti-HK1+ Anti-KLHL12+ |
| Biochemical panel | Normal bilirubin ALP < 2× ULN | ↑ bilirubin ALP ≥ 2× ULN APRI score > 0.54 |
| Histology | No/Mild fibrosis | Advanced fibrosis/cirrhosis Interface hepatitis Ductopenia at the diagnosis |
| Noninvasive markers of fibrosis | LSM < 8 kPa/↑ < 2.1 kPa/y ELF score < 10.0 MRE < 4.6 kPa | LSM > 15 kPa/↑ > 2.1 kPa/y ELF score ≥ 10.0 MRE > 4.6 kPa |
| Score | Evaluation Time (mo) | Outcomes | Treatment Failure If | c-Statistics 5, 10, 15 Years |
|---|---|---|---|---|
| Barcelona, 2006 [121] | 12 | LTF survival | Decrease in ALP ≤ 40% and ALP ≥ 1× ULN | 0.56, 0.61, 0.61 |
| Paris I, 2008 [115] | 12 | LTF survival | ALP ≥ 3× ULN or AST ≥ 2× ULN or bilirubin > 1 mg/dL | 0.81, 0.81, 0.80 |
| Rotterdam, 2009 [124] | 12 | LTF survival | Bilirubin > 1 mg/dL and/or albumin < 1× ULN | NA |
| Toronto, 2010 [52] | 24 | LTF survival, histological progression | ALP ≥ 1.67× ULN | 0.65, 0.70, 0.70 |
| Paris II, 2011 [126] | 12 | LTF survival, ascites, variceal bleeding, encephalopathy | ALP ≥ 1.5× ULN or AST ≥ 1.5× ULN or bilirubin > 1 mg/dL | 0.75, 0.75, 0.74 |
| Ehim, 2011 [125] | 6 | LTF survival | Decrease in GGT ≤ 70% and GGT ≥ 1× ULN | NA |
| Score | Evaluation Time (mo) | Outcomes | Variables | c-Statistics |
|---|---|---|---|---|
| UK-PBC [127] | 12 | Risk of LT or liver-related death within 5, 10, 15 years |
| 0.96, 0.95, 0.94 (at 5, 10, 15 years) |
| GLOBE [29] | 12 | LTF survival at 3, 5, 10 years |
| 0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martini, F.; Balducci, D.; Mancinelli, M.; Buzzanca, V.; Fracchia, E.; Tarantino, G.; Benedetti, A.; Marzioni, M.; Maroni, L. Risk Stratification in Primary Biliary Cholangitis. J. Clin. Med. 2023, 12, 5713. https://doi.org/10.3390/jcm12175713
Martini F, Balducci D, Mancinelli M, Buzzanca V, Fracchia E, Tarantino G, Benedetti A, Marzioni M, Maroni L. Risk Stratification in Primary Biliary Cholangitis. Journal of Clinical Medicine. 2023; 12(17):5713. https://doi.org/10.3390/jcm12175713
Chicago/Turabian StyleMartini, Francesco, Daniele Balducci, Martina Mancinelli, Valerio Buzzanca, Elena Fracchia, Giuseppe Tarantino, Antonio Benedetti, Marco Marzioni, and Luca Maroni. 2023. "Risk Stratification in Primary Biliary Cholangitis" Journal of Clinical Medicine 12, no. 17: 5713. https://doi.org/10.3390/jcm12175713





