Acute-Onset Retinal Conditions Mimicking Acute Optic Neuritis: Overview and Differential Diagnosis
Abstract
1. Introduction
2. Results
- Retinal phosphenes: more visible in the dark, are commonly caused by vitreous traction on the retina. However retinal inflammation may also produce flickering and shimmering. Phosphenes may rarely occur in optic neuritis [2] but pain upon eye movement is usually present. They are common in migraines (visual auras) but are homonymous and unaffected by light levels. They may respect a typical pattern (teichopsia) often easily recognized by patients themselves.
- Metamorphopsia: distortion of images is a clear indicator of a maculopathy as the cause of visual loss. Straight lines appear wavy and may be accompanied by central or paracentral scotoma and micropsia (objects look smaller if compared to an unaffected eye).
- Blind spot enlargement: if a blind spot enlargement is seen on the visual field test, a retinal disorder affecting the peripapillary retina should be suspected, especially in the absence of optic disc swelling. Optic disc edema usually does not cause such enlargement unless it is very severe.
- Photo stress test: after exposure to bright light, there is a slow recovery in cases of retinal disorder affecting the cone function.
- Absence of relative afferent pupillary defect (RAPD): optic neuropathy (such as optic neuritis) is associated with RAPD unless bilateral and symmetric. Thus, visual loss in the absence of RAPD usually points to the macula as the site of the lesion. However, one must remember that disorders of the peripapillary retina may be accompanied by mild disc swelling and a RAPD [3].
- Lack of pain with eye movement: the typical presentation for AON is a young female with acute loss of vision, evidence for an optic neuropathy (e.g., loss of visual acuity and visual field defect associated with a RAPD), pain with eye movement, and a normal fundus. The absence of pain with eye movement should raise suspicion for an alternative diagnosis (atypical optic neuritis, acute-onset retinopathy, ischemic/inflammatory/compressive optic neuropathy, among others).
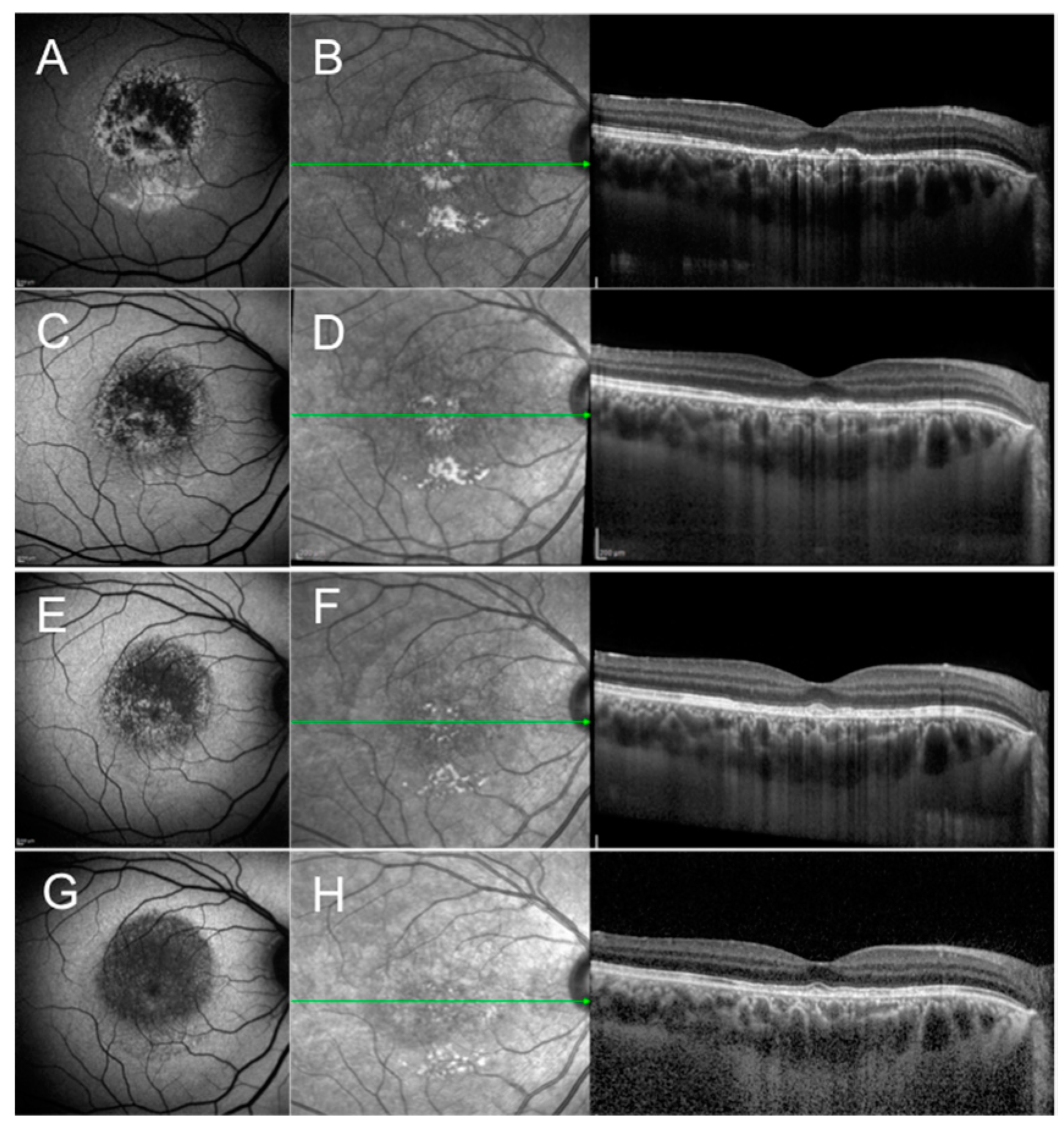
2.1. Acute Idiopathic Maculopathy
2.2. Acute Retinal Pigment Epitheliitis
2.3. Acute Macular Neuroretinopathy
2.4. Paracentral Acute Middle Maculopathy
2.5. Acute Zonal Occult Outer Retinopathy—Acute Annular Outer Retinopathy Variant
2.6. Acute Idiopathic Blind Spot Enlargement Syndrome
2.7. Multiple Evanescent White Dot Syndrome
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAOR | acute annular outer retinopathy |
| AIBSE | acute idiopathic blind spot enlargement |
| AIM | acute idiopathic maculopathy |
| AMN | acute macular neuroretinopathy |
| AON | acute optic neuritis |
| ARPE | acute retinal pigment epitheliitis |
| AZOOR | acute zonal occult outer retinopathy |
| DCP | deep capillary plexus |
| ELM | external limiting membrane |
| ERG | electroretinogram |
| EZ/IZ | ellipsoid zone/interdigitation zone |
| FA | fluorescein angiography |
| FAF | fundus autofluorescence |
| ICGA | indocyanine green angiography |
| INL | inner nuclear layer |
| IPL | inner plexiform layer |
| MEWDS | multiple evanescent white dot syndrome |
| MFC/PIC | multifocal choroiditis/punctate inner choroidopathy |
| MRI | magnetic resonance imaging |
| NIR | near-infrared |
| OCT | optical coherence tomography |
| ONL | outer nuclear layer |
| OPL | outer plexiform layer |
| PAMM | paracentral acute middle maculopathy |
| RAPD | relative afferent pupillary defect |
| RPE | retinal pigment epithelium |
References
- Peng, P.H.; Lee, T.S.; Cheng, C.K.; Peng, C.H.; Chan, W.C. Acute zonal occult outer retinopathy presenting as optic neuritis. Taiwan J. Ophthalmol. 2020, 10, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Shindo, A.; Kokubo, Y.; Taniguchi, A.; Kuze, M.; Kuzuhara, S. Case of acute zonal occult outer retinopathy (AZOOR): A 15 years’ mislabeling as retrobulbar optic neuritis. Rinsho Shinkeigaku 2007, 47, 116–118. [Google Scholar]
- Pellegrini, F.; Interlandi, E. A case of multiple evanescent white dot syndrome misdiagnosed as optic neuritis: Differential diagnosis for the neurologist. J. Neurosci. Rural Pract. 2016, 7, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Yannuzzi, L.A.; Jampol, L.M.; Rabb, M.F.; Sorenson, J.A.; Beyrer, C.; Wilcox, L.M. Unilateral acute idiopathic maculopathy. Arc. Ophthalmol. 1991, 109, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Freund, K.B.; Yannuzzi, L.A.; Barile, G.R.; Spaide, R.F.; Milewski, S.A.; Guyer, D.R. The expanding clinical spectrum of unilateral acute idiopathic maculopathy. Arch. Ophthalmol. 1996, 114, 555–559. [Google Scholar]
- Hasegawa, T.; Sannomiya, Y.; Toyoda, M.; Maruko, I.; Iida, T. Acute Idiopathic maculopathy after COVID-19 Vaccination. Am. J. Ophthalmol. Case Rep. 2022, 26, 101479. [Google Scholar] [CrossRef]
- Hughes, E.H.; Hunyoy, A.P.; Gorbatov, M.; Ho, I. Acute idiopathic maculopathy with coxsackievirus infection. Retin. Cases Brief Rep. 2012, 6, 19–21. [Google Scholar] [CrossRef]
- Meyerle, C.B.; Yannuzzi, L.A. Acute positive titer of antibody to coxsackievirus in acute idiopathic maculopathy. Retin. Cases Brief Rep. 2008, 2, 34–35. [Google Scholar] [CrossRef]
- Demirel, S.; Batioglu, F.; Ozmert, E. Unilateral acute maculopathy related to hand, foot and mouth disease: OCT and fluorescein angiography findings of a very rare disease. Eur. J. Ophthalmol. 2014, 24, 131–133. [Google Scholar] [CrossRef]
- Pajtler Rosar, A.; Casalino, G.; Cozzi, M.; Pellegrini, M.; Bottoni, F.; Dell’Arti, L.; Lavric, A.; Umek, L.; Globocnik Petrovic, M.; Pavesio, C.; et al. Acute idiopathic maculopathy: A Proposed Disease Staging Based on Multimodal Imaging. Retina 2021, 41, 2446–2455. [Google Scholar] [CrossRef]
- Xu, H.; Lin, P. Unilateral recurrent acute idiopathic maculopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 941–944. [Google Scholar] [CrossRef]
- Jung, C.S.; Payne, J.F.; Bergstrom, C.S.; Cribbs, B.E.; Yan, J.; Hubbard, G.B.; Olsen, T.W.; Yeh, S. Multimodality diagnostic imaging in Unilateral Acute Idiopathic Maculopathy. Arch. Ophthalmo. 2012, 130, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Haruta, H.; Sawa, M.; Saishin, Y.; Ohguro, N.; Tano, Y. Clinical findings in unilateral acute idiopathic maculopathy: New findings in acute idiopathic maculopathy. Int. Opthalmol. 2010, 30, 199–202. [Google Scholar] [CrossRef]
- Srour, M.; Querques, G.; Rostaqui, O.; Souied, E.H. Early spectral domain optical coherence tomography findings in unilateral acute idiopathic maculopathy. Retina 2013, 33, 2182–2184. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Saito, W.; Michiyuki, S.; Hirooka, K.; Mori, S.; Noda, K.; Ishida, S. Increased choroidal blood flow velocity with regression of unilateral acute idiopathic maculopathy. Jpn. J. Ophthalmol. 2015, 59, 252–260. [Google Scholar] [CrossRef]
- Fernandez-Avellaneda, P.; Breazzano, M.P.; Fragiotta, S.; Xu, X.; Zhang, Q.; Wang, R.K.; Freund, K.B. Bacillary layer detachment overlying reduced choriocapillaris flow in acute idiopathic maculopathy. Retin. Cases Brief Rep. 2022, 16, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Agarwal, A.K.; Erba, S.; Scialdone, A.; Miserocchi, E.; Bandello, F.; Introini, U.; Jampol, L.M.; Casalino, G. Placoid lesions of the retina: Progress in multimodal imaging and clinical perspective. Br. J. Ophthalmol. 2022, 106, 14–25. [Google Scholar] [CrossRef]
- Rosar, A.P.; Bochicchio, S.; Giani, A.; Bottoni, F.; Staurenghi, G. Acute Idiopathic maculopathy complicated by choroidal neovascularization: New insights into multimodal retinal imaging. Retin. Cases Brief Rep. 2019, 15, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Krill, A.E.; Deutman, A.F. Acute retinal pigment epitheliitus. Am. J. Ophthalmol. 1972, 74, 193–205. [Google Scholar] [CrossRef]
- Cho, H.J.; Han, S.Y.; Cho, S.W.; Lee, D.W.; Lee, T.G.; Kim, C.G.; Kim, J.W. Acute retinal pigment epitheliitis: Spectral-domain optical coherence tomography findings in 18 cases. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3314–3319. [Google Scholar] [CrossRef]
- Kılıç, R. Acute retinal pigment epitheliitis: A case presentation and literature review. Arq. Bras. Oftalmol. 2021, 84, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.W. Bilateral recurrent acute retinal pigment epitheliitis. Am. J. Ophthalmol. 1975, 79, 567–570. [Google Scholar] [CrossRef]
- Toledo, J.J.; García, J.R.; Asencio, M.; Schlincker, A.; López, A. Recurrent acute retinal pigment epitheliitis: Spectral domain optical coherence tomography and optical coherence tomography angiography features. Retin. Cases Brief Rep. 2021, 15, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Marano, F. A Long Term Recurrent Case of Acute Retinal Pigment Epithelitis. Ocul. Immunol. Inflamm. 2022, 1–6. [Google Scholar] [CrossRef]
- Aydoğan, T.; Güney, E.; Akçay, B.İ.; Bozkurt, T.K.; Ünlü, C.; Ergin, A. Acute retinal pigment epitheliitis: Spectral domain optical coherence tomography, fluorescein angiography, and autofluorescence findings. Case Rep. Med. 2015, 2015, 149497. [Google Scholar] [CrossRef]
- Gundogan, F.C.; Diner, O.; Tas, A.; Ilhan, A.; Yolcu, U. Macular function and morphology in acute retinal pigment epithelitis. Indian J. Ophthalmol. 2014, 62, 1156–1158. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Jang, S.Y.; Park, T.K.; Ohn, Y.H. Short-term clinical observation of acute retinal pigment epitheliitis using spectral-domain optical coherence tomography. Korean J. Ophthalmol. 2011, 25, 222–224. [Google Scholar] [CrossRef]
- Bennett, J.L. Optic Neuritis. Contin. (Minneap. Minn.) 2019, 25, 1236–1264. [Google Scholar] [CrossRef]
- Dell’Omo, R.; Pavesio, C.E. Multiple evanescent white dot syndrome (MEWDS). Int. Ophthalmol. Clin. 2012, 52, 221–228. [Google Scholar] [CrossRef]
- Bhavsar, K.V.; Lin, S.; Rahimy, E.; Joseph, A.; Freund, K.B.; Sarraf, D.; Cunningham, E.T., Jr. Acute macular neuroretinopathy: A comprehensive review of the literature. Surv. Ophthalmol. 2016, 61, 538–565. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Lee, D.W.; Kim, C.G.; Kim, J.W. Spectral domain optical coherence tomography findings in acute retinal pigment epitheliitis. Can. J Ophthalmol. 2011, 46, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Bos, P.J.; Deutman, A.F. Acute macular neuroretinopathy. Am. J. Ophthalmol. 1975, 80, 573–584. [Google Scholar] [CrossRef]
- Fekri, S.; Khorshidifar, M.; Dehghani, M.S.; Nouri, H.; Abtahi, S.H. Acute macular neuroretinopathy and COVID-19 vaccination: Case report and literature review. J. Fr. Ophtalmol. 2023, 46, 72–82. [Google Scholar] [CrossRef]
- Rahimy, E.; Sarraf, D. Paracentral acute middle maculopathy spectral-domain optical coherence tomography feature of deep capillary ischemia. Curr. Opin. Ophthalmol. 2014, 25, 207–212. [Google Scholar] [CrossRef]
- Gass, J.D.; Hamed, L.M. Acute macular neuroretinopathy and multiple evanescent white dot syndrome occurring in the same patients. Arch. Ophthalmol. 1989, 107, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Zamani, G.; Ataei Azimi, S.; Aminizadeh, A.; Shams Abadi, E.; Kamandi, M.; Mortazi, H.; Shariat, S.; Abrishami, M. Acute macular neuroretinopathy in a patient with acute myeloid leukemia and deceased by COVID-19: A case report. J. Ophthalmic. Inflamm. Infect. 2021, 10, 39. [Google Scholar] [CrossRef]
- Azar, G.; Bonnin, S.; Vasseur, V.; Faure, C.; Salviat, F.; Clermont, C.V.; Titah, C.; Farès, S.; Boulanger, E.; Derrien, S.; et al. Did the COVID-19 Pandemic Increase the Incidence of Acute Macular Neuroretinopathy? J. Clin. Med. 2021, 10, 5038. [Google Scholar] [CrossRef]
- Fawzi, A.A.; Pappuru, R.R.; Sarraf, D.; Le, P.P.; McCannel, C.A.; Sobrin, L.; Goldstein, D.A.; Honowitz, S.; Walsh, A.C.; Sadda, S.R.; et al. Acute macular neuroretinopathy: Long-term insights revealed by multimodal imaging. Retina 2012, 32, 1500–1513. [Google Scholar] [CrossRef]
- Casalino, G.; Arrigo, A.; Romano, F.; Munk, M.R.; Bandello, F.; Parodi, M.B. Acute macular neuroretinopathy: Pathogenetic insights from optical coherence tomography angiography. Br. J. Ophthalmol. 2019, 103, 410–414. [Google Scholar] [CrossRef]
- Yeh, S.; Hwang, T.S.; Weleber, R.G.; Watzke, R.C.; Francis, P.J. Acute macular outer retinopathy (AMOR): A reappraisal of acute macular neuroretinopathy using multimodality diagnostic testing. Arch. Ophthalmol. 2011, 129, 365–368. [Google Scholar] [CrossRef]
- Gelman, R.; Chen, R.; Blonska, A.; Barile, G.; Sparrow, J.R. Fundus autofluorescence imaging in a patient with rapidly developing scotoma. Retin. Cases Brief Rep. 2012, 6, 345–348. [Google Scholar] [CrossRef]
- Deschamps, R.; Vasseur, V.; Shor, N.; Vignal, C.; Salomon, L.; Gout, O.; Mauget-Faÿsse, M. A new association: Acute macular neuroretinopathy in acute optic neuritis. Acta Ophthalmol. 2019, 97, e753–e756. [Google Scholar] [CrossRef]
- Wyss, A.; Rüesch, R.; Schwizer, N.; Heckmann, J.; Zawinka, C.; Sturm, V. Akute makuläre Neuroretinopathie [Acute Macular Neuroretinopathy]. Klin. Monbl. Augenheilkd. 2019, 236, 555–561. [Google Scholar]
- Sarraf, D.; Rahimy, E.; Fawzi, A.A.; Sohn, E.; Barbazetto, I.; Zacks, D.N.; Mittra, R.A.; Klancnik, J.M., Jr.; Mrejen, S.; Goldberg, N.R.; et al. Paracentral acute middle maculopathy: A new variant of acute macular neuroretinopathy associated with retinal capillary ischemia. JAMA Ophthalmol. 2013, 131, 1275–1287. [Google Scholar] [CrossRef]
- Rahimy, E.; Kuehlewein, L.; Sadda, S.R.; Sarraf, D. Paracentral Acute Middle Maculopathy: What We Knew Then and What We Know Now. Retina 2015, 35, 1921–1930. [Google Scholar] [CrossRef]
- Moura-Coelho, N.; Gaspar, T.; Ferreira, J.T.; Dutra-Medeiros, M.; Cunha, J.P. Paracentral acute middle maculopathy-review of the literature. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 2583–2596. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Rahimy, E.; Sergott, R.C.; Nunes, R.P.; Souza, E.C.; Choudhry, N.; Cutler, N.E.; Houston, S.K.; Munk, M.R.; Fawzi, A.A.; et al. Spectrum of Retinal Vascular Diseases Associated With Paracentral Acute Middle Maculopathy. Am. J. Ophthalmol. 2015, 160, 26–34.e1. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Desai, S.J.; Baumal, C.R. Paracentral acute middle maculopathy in pregnancy. Retin. Cases Brief Rep. 2020, 14, 221–223. [Google Scholar] [CrossRef]
- Iovino, C.; Au, A.; Ramtohul, P.; Bacci, T.; AlBahlal, A.; Khan, A.M.; Al-Abdullah, A.A.; Wendel, R.; Chhablani, J.; Sadda, S.; et al. Coincident PAMM and AMN and Insights into a Common Pathophysiology. Am. J. Ophthalmol. 2022, 236, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.; Kim, H.; Lee, J.; Yi, J.; Chung, Y.R. Retinal vascular occlusions in COVID-19 infection and vaccination: A literature review. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 1793–1808. [Google Scholar] [CrossRef] [PubMed]
- Ramtohul, P.; Freund, K.B. Central Acute Middle Maculopathy: A Novel Variant of Paracentral Acute Middle Maculopathy in Foveal Hypoplasia. Ophthalmol. Retin. 2020, 4, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Rishi, P.; Chendilnathan, C.; Kumari, S. OCT angiography features of paracentral acute middle maculopathy. Indian J. Ophthalmol. 2019, 67, 417–419. [Google Scholar]
- Sridhar, J.; Shahlaee, A.; Rahimy, E.; Hong, B.K.; Khan, M.A.; Maguire, J.I.; Dunn, J.P.; Mehta, S.; Ho, A.C. Optical Coherence Tomography Angiography and En Face Optical Coherence Tomography Features of Paracentral Acute Middle Maculopathy. Am. J. Ophthalmol. 2015, 160, 1259–1268.e2. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.J.; Chen, M.H.; Frambach, C.R.; Rofagha, S.; Lee, S.S. Spectral domain versus swept source optical coherence tomography angiography of the retinal capillary plexuses in sickle cell maculopathy. Retin. Cases Brief Rep. 2018, 12, 87–92. [Google Scholar] [CrossRef]
- Nakamura, M.; Katagiri, S.; Hayashi, T.; Aoyagi, R.; Hasegawa, T.; Kogure, A.; Iida, T.; Nakano, T. Longitudinal follow-up of two patients with isolated paracentral acute middle maculopathy. Int. Med. Case Rep. J. 2019, 12, 143–149. [Google Scholar] [CrossRef]
- Gass, J.D.; Stern, C. Acute annular outer retinopathy as a variant of acute zonal occult outer retinopathy. Am. J. Ophthalmol. 1995, 119, 330–334. [Google Scholar] [PubMed]
- Fekrat, S.; Wilkinson, C.P.; Chang, B.; Yannuzzi, L.; Schatz, H.; Haller, J.A. Acute annular outer retinopathy: Report of four cases. Am. J. Ophthalmol. 2000, 130, 636–644. [Google Scholar] [CrossRef]
- Luckie, A.; Ai, E.; Del Piero, E. Progressive zonal outer retinitis. Am. J. Ophthalmol. 1994, 118, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Yasaka, Y.; Hasegawa, E.; Keino, H.; Usui, Y.; Maruyama, K.; Yamamoto, Y.; Kaburaki, T.; Iwata, D.; Takeuchi, M.; Kusuhara, S.; et al. A multicenter study of ocular inflammation after COVID-19 vaccination. Jpn. J. Ophthalmol. 2023, 67, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Mrejen, S.; Khan, S.; Gallego-Pinazo, R.; Jampol, L.M.; Yannuzzi, L.A. Acute zonal occult outer retinopathy: A classification based on multimodal imaging. JAMA Ophthalmol. 2014, 132, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Jiang, L.B.; Yan, W.Y.; Wang, Q.; Peng, X.Y. Clinical analysis of acute zonal occult outer retinopathy masquerading as optic neuropathy. Zhonghua Yan Ke Za Zhi 2013, 49, 495–499. [Google Scholar] [PubMed]
- Wang, J.C.; Finn, A.P.; Grotting, L.A.; Sobrin, L. Acute Zonal Occult Outer Retinopathy Associated with Retrobulbar Optic Neuritis. J. Neuroophthalmol. 2017, 37, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Zaslavsky, K.; Eshtiaghi, A.; Jeeva-Patel, T.; Christakis, P.G.; Margolin, E. Bitemporal Hemianopia Secondary to Acute Zonal Occult Outer Retinopathy. J. Neuroophthalmol. 2021, 41, e749–e751. [Google Scholar] [CrossRef] [PubMed]
- Gass, J.D.; Agarwal, A.; Scott, I.U. Acute zonal occult outer retinopathy: A long-term follow-up study. Am. J. Ophthalmol. 2002, 134, 329–339. [Google Scholar] [CrossRef]
- Hintzen, R.Q.; van den Born, L.I. Acute zonal occult outer retinopathy and multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1373–1375. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jacobson, D.M. Acute zonal occult outer retinopathy and central nervous system inflammation. J. Neuroophthalmol. 1996, 16, 172–177. [Google Scholar] [CrossRef]
- Fletcher, W.A.; Imes, R.K.; Goodman, D.; Hoyt, W.F. Acute idiopathic blind spot enlargement: A big blind spot syndrome without optic disc edema. Arch. Ophthalmol. 1988, 106, 44–49. [Google Scholar] [CrossRef]
- Jampol, L.M.; Sieving, P.A.; Pugh, D.; Fishman, G.A.; Gilbert, H. Multiple evanescent white dot syndrome, part I: Clinical findings. Arch. Ophthalmol. 1984, 102, 671–674. [Google Scholar] [CrossRef]
- Takeda, M.; Kimura, S.; Tamiya, M. Acute disseminated retinal pigment epitheliopathy. Folia Ophthalmol. Jpn. 1984, 35, 2613–2620. [Google Scholar]
- Hamed, L.M.; Glaser, J.S.; Gass, J.D.M.; Schatz, N.J. Protracted enlargement of the blind spot in multiple evanescent white dot syndrome. Arch. Ophthalmol. 1989, 107, 194. [Google Scholar] [CrossRef] [PubMed]
- Gass, J.D. Acute zonal occult outer retinopathy: Donders lecture: The Netherlands Ophthalmological Society. J. Clin. Neuroophthalmol. 1993, 13, 79–97. [Google Scholar]
- Gass, J.D. The acute zonal outer retinopathies. Am. J. Ophthalmol. 2000, 130, 655–657. [Google Scholar] [CrossRef]
- Volpe, N.J.; Rizzo, J.F., III; Lessell, S. Acute idiopathic blind spot enlargement syndrome: A review of 27 new cases. Arch. Ophthalmol. 2001, 119, 59–63. [Google Scholar]
- Watzke, R.C.; Shults, W.T. Clinical features and natural history of the acute idiopathic enlarged blind spot syndrome. Ophthalmology 2002, 109, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Campos-Baniak, M.G.; Colleaux, K. Acute idiopathic blind spot enlargement syndrome following measles, mumps and rubella vaccination. Can. J. Ophthalmol. 2019, 54, e199–e203. [Google Scholar] [CrossRef]
- Wang, M.; Sadaka, A.; Prager, T.; Lee, A.G.; Pellegrini, F.; Cirone, D.; De Simone, L.; Cimino, L. From A… to… Z(OOR): The clinical spectrum of acute zonal occult outer retinopathy. Neuro-Ophthalmology 2018, 42, 215–221. [Google Scholar] [CrossRef]
- Fletcher, W.A.; Imes, R.K. Acute Idiopathic Blind Spot Enlargement and Acute Zonal Occult Outer Retinopathy: Potential Mimics of Neuro-Ophthalmic Disease. J. Neuroophthalmol. 2020, 40, S43–S50. [Google Scholar] [CrossRef]
- Quinones, X.; Ortiz, J.; Santos, C.; Oliver, A.L.; Rodriguez, J. Acute idiopathic blind spot enlargement syndrome following influenza vaccination. Am. J. Ophthalmol. Case Rep. 2020, 20, 100949. [Google Scholar] [CrossRef]
- Shen, C.; Tennant, M. Unilateral Acute Idiopathic Blind Spot Enlargement following Second Dose of COVID-19 mRNA Vaccine. Ocul. Immunol. Inflamm. 2022, 31, 1219–1221. [Google Scholar] [CrossRef]
- Watzke, R.C.; Packer, A.J.; Folk, J.C.; Benson, W.E.; Burgess, D.; Ober, R.R. Punctate inner choroidopathy. Am. J. Ophthalmol. 1984, 98, 572–584. [Google Scholar] [CrossRef]
- Newcomb, R.D. Acute, idiopathic blind spot enlargement syndrome. Optom. Vis Sci. 2000, 77, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Zicarelli, F.; Mantovani, A.; Preziosa, C.; Staurenghi, G. Multimodal Imaging of Multiple Evanescent White Dot Syndrome: A New Interpretation. Ocul. Immunol. Inflamm. 2020, 28, 814–820. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, W. Presumed Recurrent MEWDS following COVID-19 Vaccination. Ocul. Immunol. Inflamm. 2021, 29, 1234–1237. [Google Scholar] [CrossRef]
- Soifer, M.; Nguyen, N.V.; Leite, R.; Fernandes, J.; Kodati, S. Recurrent Multiple Evanescent White Dot Syndrome (MEWDS) Following First Dose and Booster of the mRNA-1273 COVID-19 Vaccine: Case Report and Review of Literature. Vaccines 2022, 10, 1776. [Google Scholar] [CrossRef] [PubMed]
- Bosello, F.; Westcott, M.; Casalino, G.; Agorogiannis, G.; Micciolo, R.; Rees, A.; Pavesio, C. Multiple evanescent white dot syndrome: Clinical course and factors influencing visual acuity recovery. Br. J. Ophthalmol. 2022, 106, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Marsiglia, M.; Gallego-Pinazo, R.; Cunha de Souza, E.; Munk, M.R.; Yu, S.; Mrejen, S.; Cunningham, E.T., Jr.; Lujan, B.J.; Goldberg, N.R.; Albini, T.A.; et al. Expanded Clinical Spectrum of Multiple Evanescent White Dot Syndrome With Multimodal Imaging. Retina 2016, 36, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Shelsta, H.N.; Rao, R.R.; Bhatt, H.K.; Jampol, L.M. Atypical presentations of multiple evanescent white dot syndrome without white dots: A case series. Retina 2011, 31, 973–976. [Google Scholar] [CrossRef]
- Pichi, F. Optical coherence tomography angiography shows an intact choriocapillaris flow in multiple evanescent white dot syndrome. Int. Ophthalmol. 2018, 38, 2271–2272. [Google Scholar] [CrossRef]
- Oh, K.T.; Folk, J.C.; Maturi, R.K.; Moore, P.; Kardon, R.H. Multifocal electroretinography in multifocal choroiditis and the multiple evanescent white dot syndrome. Retina 2001, 21, 581–589. [Google Scholar] [CrossRef]
- Horiguchi, M.; Miyake, Y.; Nakamura, M.; Fujii, Y. Focal electroretinogram and visual field defect in multiple evanescent white dot syndrome. Br. J. Ophthalmol. 1993, 77, 452–455. [Google Scholar] [CrossRef]
- Cicinelli, M.V.; Ramtohul, P.; Marchese, A.; Bandello, F.; Bailey Freund, K.; Miserocchi, E.; Jampol, L.M. Latest advances in white spot syndromes: New findings and interpretations. Prog. Retin Eye Res. 2023, 97, 101207. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.G.; Kim, T.Y.; Kim, M.; Byeon, S.H.; Kim, S.S.; Koh, H.J.; Lee, S.C.; Lee, C.S. Expanding the Clinical Spectrum of Multiple Evanescent White Dot Syndrome with Overlapping Multifocal Choroiditis. Ocul. Immunol. Inflamm. 2020, 30, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Essilfie, J.; Bacci, T.; Abdelhakim, A.H.; Ramtohul, P.; Turchi, F.; Freund, K.B.; Yannuzzi, L.A. Are There Two Forms of Multiple Evanescent White Dot Syndrome? Retina 2022, 42, 227–235. [Google Scholar] [CrossRef]
- Russell, J.F.; Pichi, F.; Scott, N.L.; Hartley, M.J.; Bell, D.; Agarwal, A.; Leong, B.; Holland, G.N.; Freund, K.B.; Sarraf, D. Masqueraders of multiple evanescent white dot syndrome (MEWDS). Int. Ophthalmol. 2020, 40, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Dodwell, D.G.; Jampol, L.M.; Rosenberg, M.; Berman, A.; Zaret, C.R. Optic nerve involvement associated with the multiple evanescent white-dot syndrome. Ophthalmology 1990, 97, 862–868. [Google Scholar] [CrossRef]
- Khaleeli, Z.; Tucker, W.R.; Del Porto, L.; Virgo, J.D.; Plant, G.T. Remember the retina: Retinal disorders presenting to neurologists. Pract. Neurol. 2018, 18, 84–96. [Google Scholar] [CrossRef]
- Querques, G.; Bux, A.V.; Forte, R.; Francesco, P.; Cristiana, I.; Noci, N.D. Syndrome des taches blanches évanescentes et sclérose en plaques [Multiple evanescent white dot syndrome and multiple sclerosis]. J. Fr. Ophtalmol. 2011, 34, 252–255. (In French) [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Interlandi, E.; Pellegrini, F.; Giuffrè, C.; Cirone, D.; Brocca, D.; Lee, A.G.; Casalino, G. Acute-Onset Retinal Conditions Mimicking Acute Optic Neuritis: Overview and Differential Diagnosis. J. Clin. Med. 2023, 12, 5720. https://doi.org/10.3390/jcm12175720
Interlandi E, Pellegrini F, Giuffrè C, Cirone D, Brocca D, Lee AG, Casalino G. Acute-Onset Retinal Conditions Mimicking Acute Optic Neuritis: Overview and Differential Diagnosis. Journal of Clinical Medicine. 2023; 12(17):5720. https://doi.org/10.3390/jcm12175720
Chicago/Turabian StyleInterlandi, Emanuela, Francesco Pellegrini, Chiara Giuffrè, Daniele Cirone, Daniele Brocca, Andrew G. Lee, and Giuseppe Casalino. 2023. "Acute-Onset Retinal Conditions Mimicking Acute Optic Neuritis: Overview and Differential Diagnosis" Journal of Clinical Medicine 12, no. 17: 5720. https://doi.org/10.3390/jcm12175720
APA StyleInterlandi, E., Pellegrini, F., Giuffrè, C., Cirone, D., Brocca, D., Lee, A. G., & Casalino, G. (2023). Acute-Onset Retinal Conditions Mimicking Acute Optic Neuritis: Overview and Differential Diagnosis. Journal of Clinical Medicine, 12(17), 5720. https://doi.org/10.3390/jcm12175720





