Circadian Urinary Excretion of Water, and Not Salt, Is Affected by the White Coat Effect
Abstract
1. Introduction
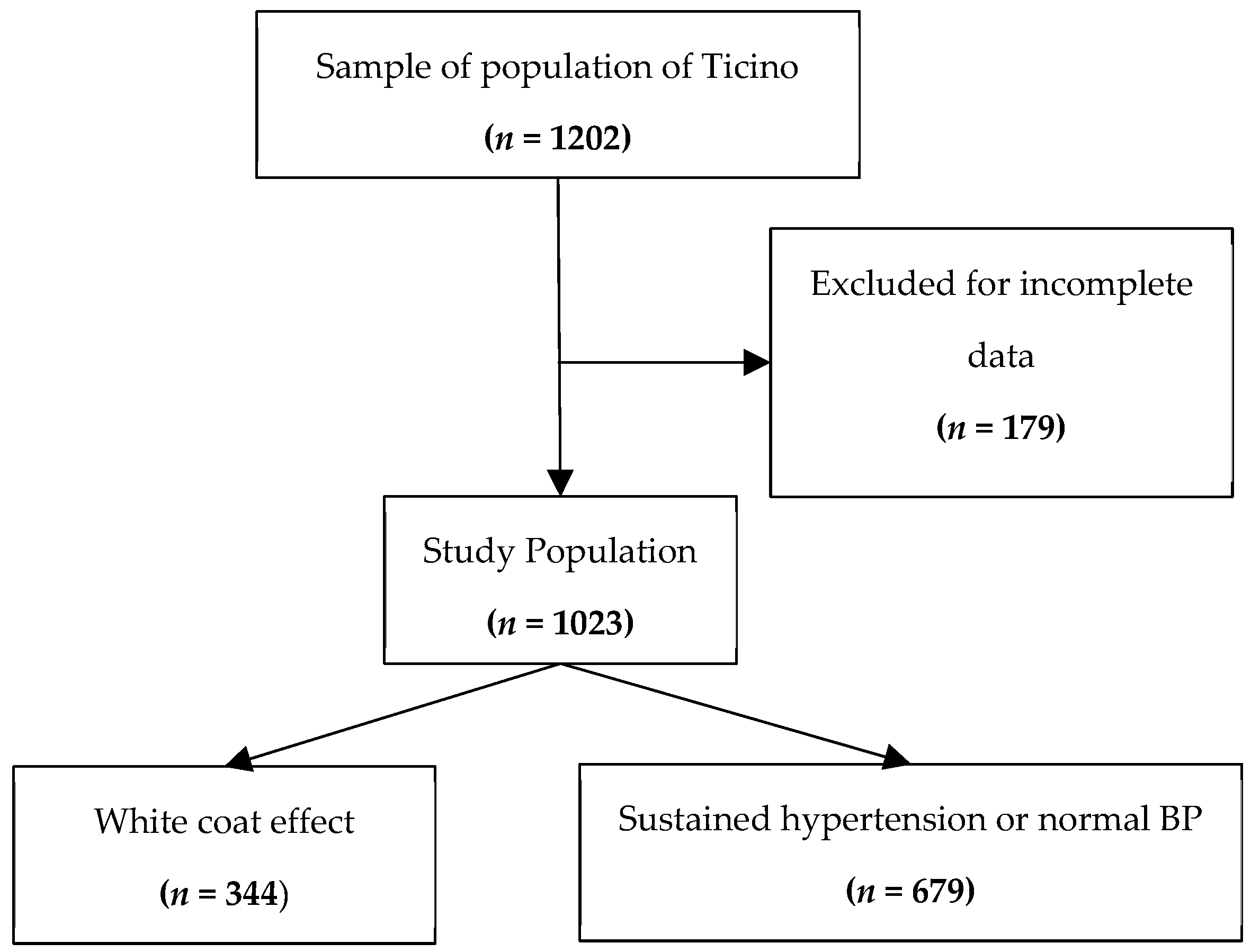
2. Materials and Methods
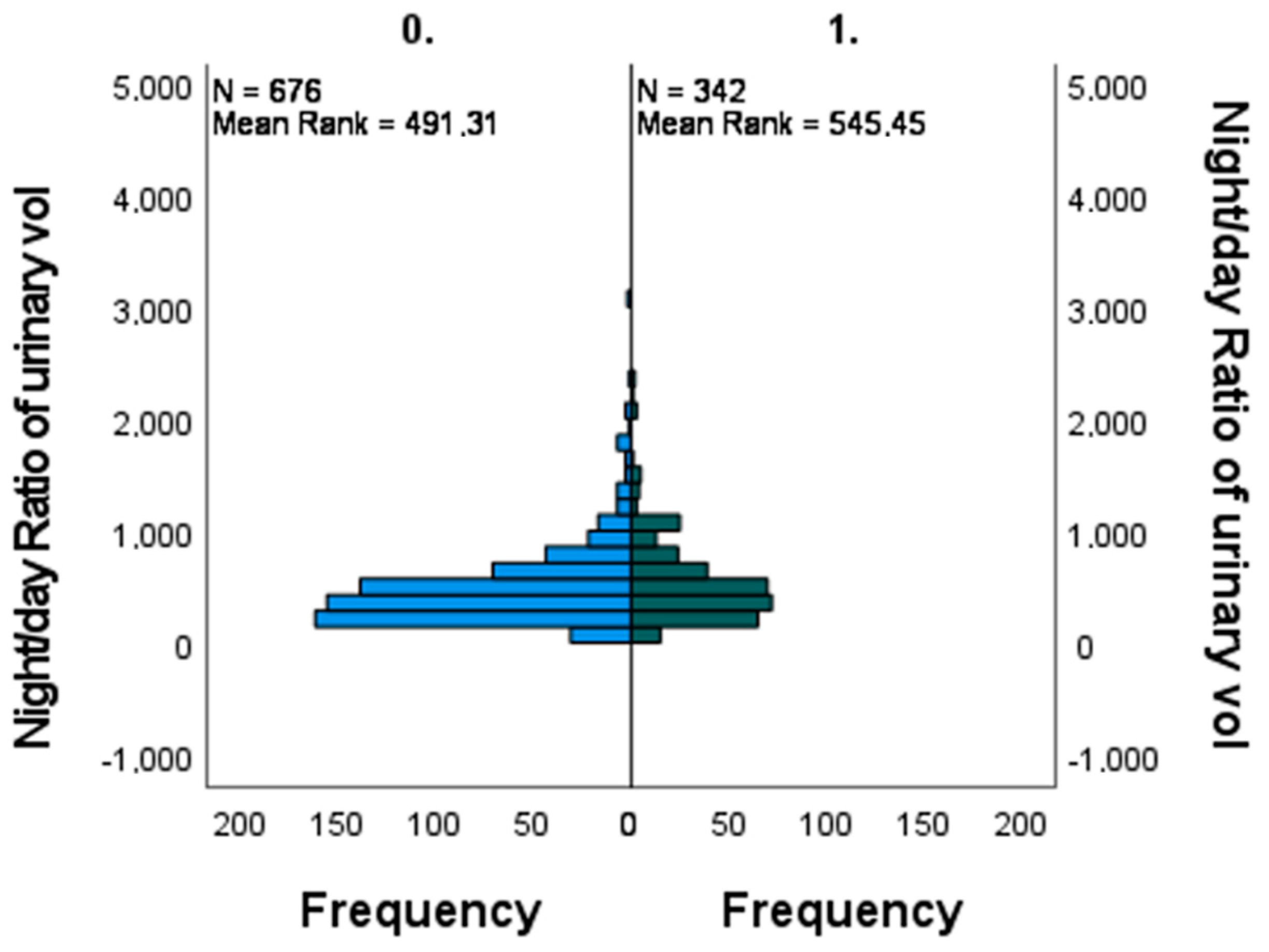
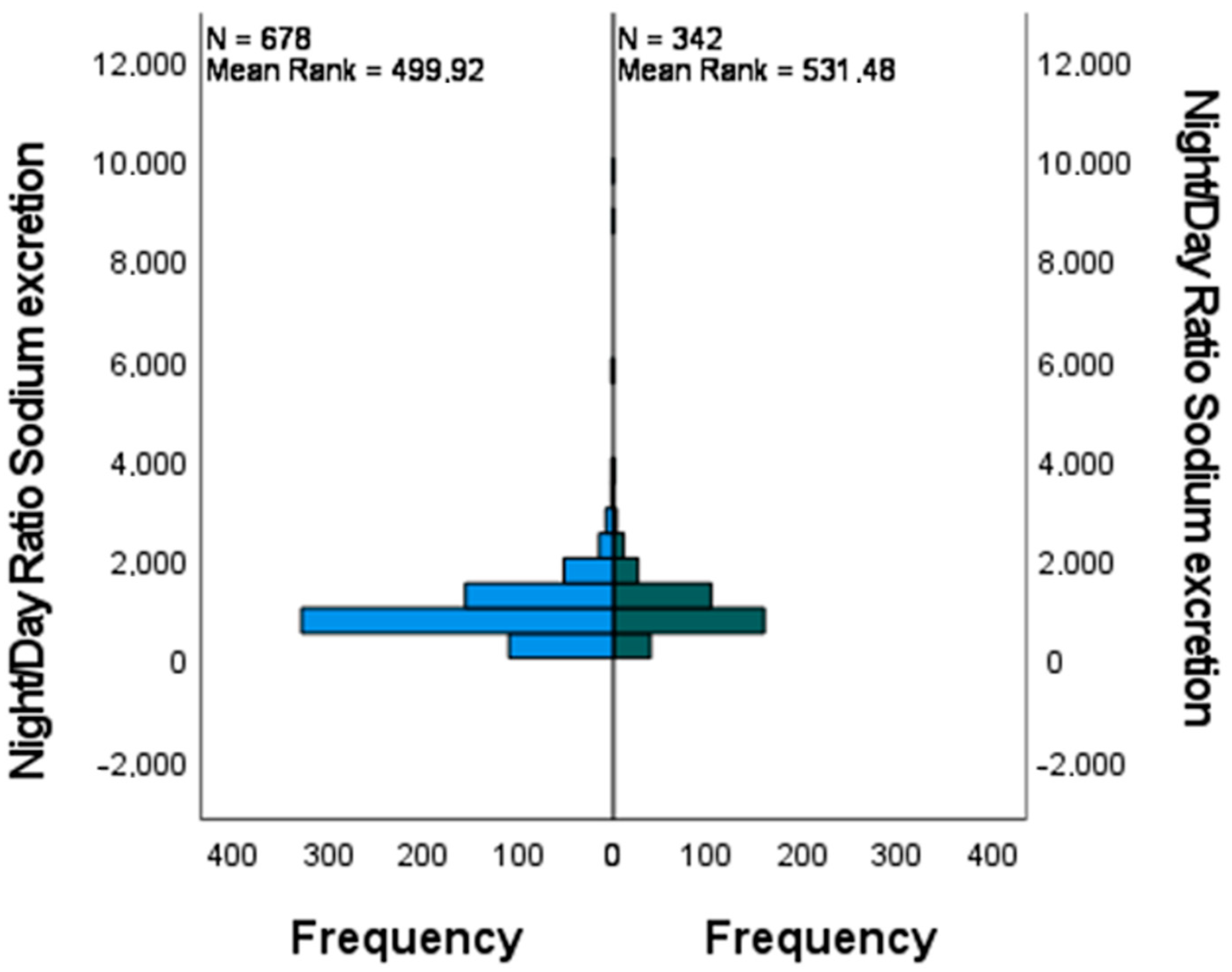
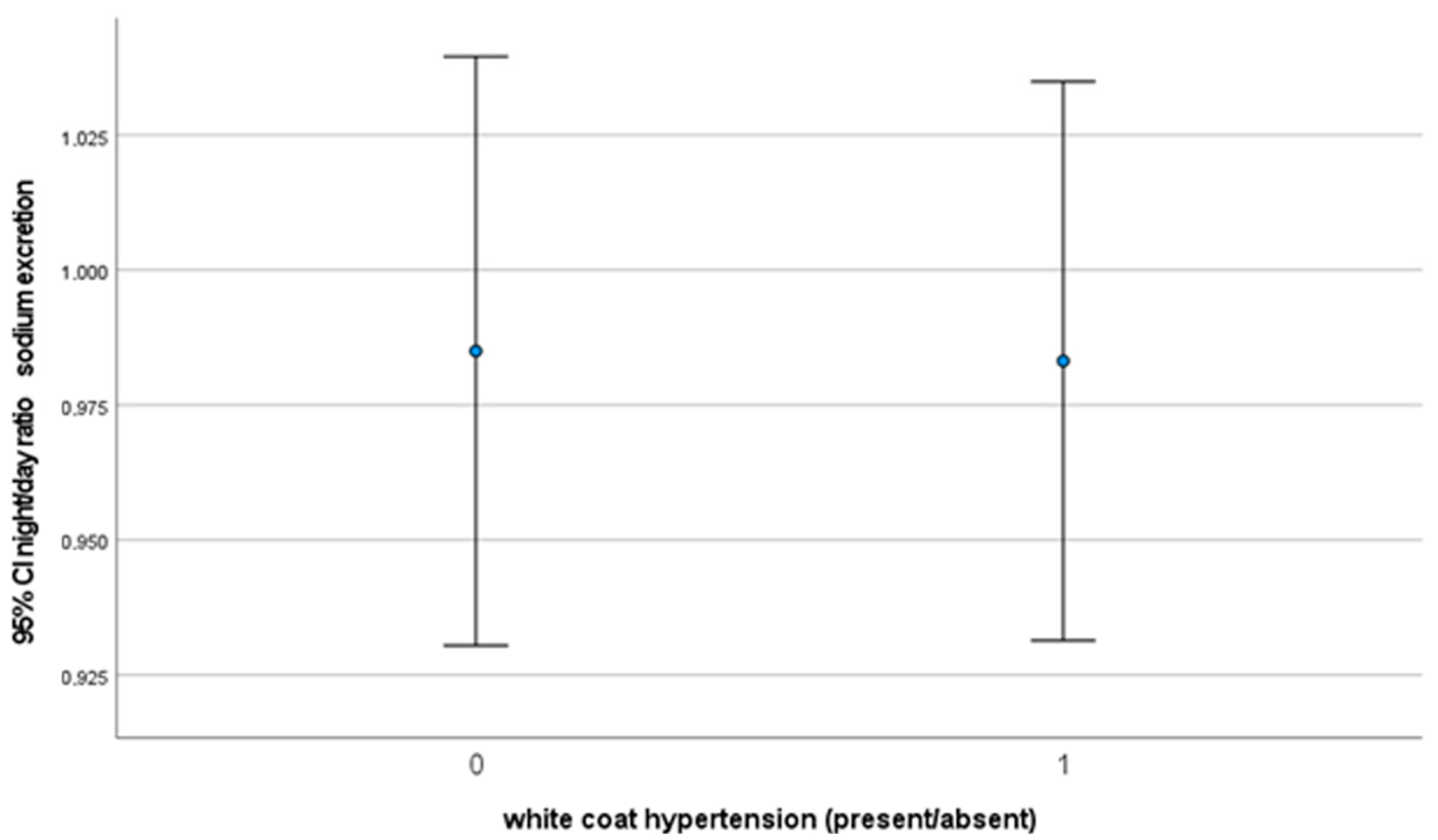
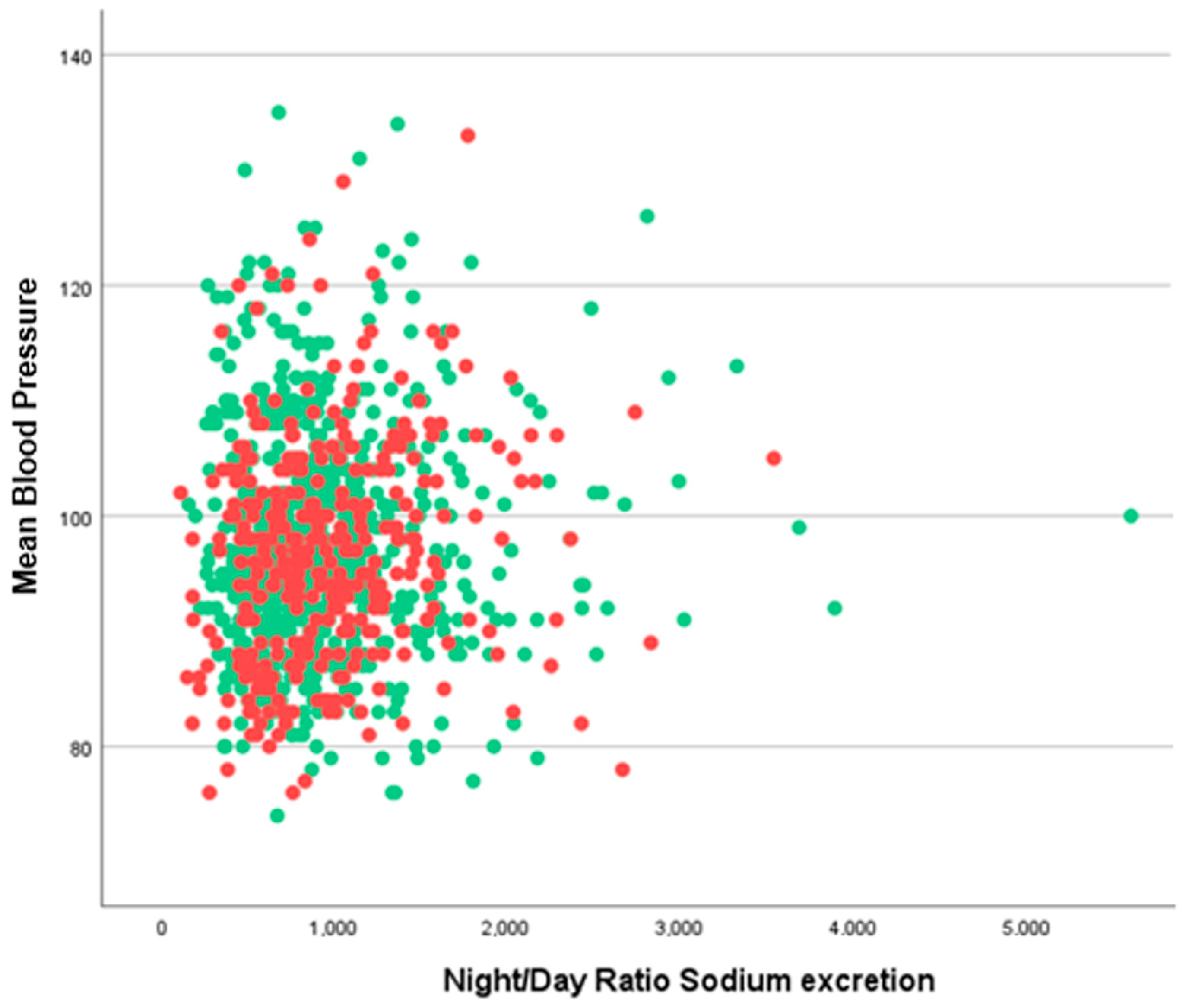
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pavan, L.; Casiglia, E.; Braga, L.M.; Winnicki, M.; Puato, M.; Pauletto, P.; Pessina, A.C. Effects of a traditional lifestyle on the cardiovascular risk profile: The Amondava population of the Brazilian Amazon. Comparison with matched African, Italian and Polish populations. J. Hypertens. 1999, 17, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Rust, P.; Ekmekcioglu, C. Impact of Salt Intake on the Pathogenesis and Treatment of Hypertension. Adv. Exp. Med. Biol. 2017, 956, 61–84. [Google Scholar] [CrossRef]
- Montani, J.P.; Antic, V.; Yang, Z.; Dulloo, A. Pathways from obesity to hypertension: From the perspective of a vicious triangle. Int. J. Obes. 2002, 26 (Suppl. S2), S28–S38. [Google Scholar] [CrossRef]
- Virdis, A.; Giannarelli, C.; Neves, M.F.; Taddei, S.; Ghiadoni, L. Cigarette smoking and hypertension. Curr. Pharm. Des. 2010, 16, 2518–2525. [Google Scholar] [CrossRef] [PubMed]
- Prevalence of Tobacco Smoking in Switzerland: Do Reported Numbers Underestimate Reality? Swiss Medical Weekly. 11 May 2017. 147(1920). Available online: https://smw.ch/index.php/smw/article/view/2298 (accessed on 3 April 2023).
- Eckel, N.; Li, Y.; Kuxhaus, O.; Stefan, N.; Hu, F.B.; Schulze, M.B. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90 257 women (the Nurses’ Health Study): 30 year follow-up from a prospective cohort study. Lancet Diabetes Endocrinol. 2018, 6, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Grillo, A.; Salvi, L.; Coruzzi, P.; Salvi, P.; Parati, G. Sodium Intake and Hypertension. Nutrients 2019, 11, 1970. [Google Scholar] [CrossRef]
- Glatz, N.; Chappuis, A.; Conen, D.; Erne, P.; Péchère-Bertschi, A.; Guessous, I.; Forni, V.; Gabutti, L.; Muggli, F.; Gallino, A.; et al. Associations of sodium, potassium and protein intake with blood pressure and hypertension in Switzerland. Swiss Med. Wkly. 2017, 147, w14411. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104, Erratum in Eur. Heart J. 2019, 40, 475. [Google Scholar] [CrossRef]
- Bonny, O.; Firsov, D. Circadian regulation of renal function and potential role in hypertension. Curr. Opin. Nephrol. Hypertens. 2013, 22, 439–444. [Google Scholar] [CrossRef]
- Susa, K.; Sohara, E.; Isobe, K.; Chiga, M.; Rai, T.; Sasaki, S.; Uchida, S. WNK-OSR1/SPAK-NCC signal cascade has circadian rhythm dependent on aldosterone. Biochem. Biophys. Res. Commun. 2012, 427, 743–747. [Google Scholar] [CrossRef]
- Doi, M.; Takahashi, Y.; Komatsu, R.; Yamazaki, F.; Yamada, H.; Haraguchi, S.; Emoto, N.; Okuno, Y.; Tsujimoto, G.; Kanematsu, A.; et al. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat. Med. 2010, 16, 67–74. [Google Scholar] [CrossRef]
- Soliman, R.H.; Pollock, D.M. Circadian Control of Sodium and Blood Pressure Regulation. Am. J. Hypertens. 2021, 34, 1130–1142. [Google Scholar] [CrossRef]
- Zhang, J.; Rao, J.; Liu, M.; Zhou, W.; Li, Y.; Wu, J.; Peng, H.; Lou, T. Abnormal circadian rhythm of urinary sodium excretion correlates closely with hypertension and target organ damage in Chinese patients with CKD. Int. J. Med. Sci. 2020, 17, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Kawasaki, T.; Kawazoe, N.; Abe, I.; Uezono, K.; Ueno, M.; Fukiyama, K.; Omae, T. Circadian variations of urinary dopamine, norepinephrine, epinephrine and sodium in normotensive and hypertensive subjects. Nephron 1990, 55, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Del Giorno, R.; Troiani, C.; Gabutti, S.; Stefanelli, K.; Puggelli, S.; Gabutti, L. Impaired Daytime Urinary Sodium Excretion Impacts Nighttime Blood Pressure and Nocturnal Dipping at Older Ages in the General Population. Nutrients 2020, 12, 2013. [Google Scholar] [CrossRef]
- Dyer, A.R.; Stamler, R.; Grimm, R.; Stamler, J.; Berman, R.; Gosch, F.C.; A Emidy, L.; Elmer, P.; Fishman, J.; Van Heel, N. Do hypertensive patients have a different diurnal pattern of electrolyte excretion? Hypertension 1987, 10, 417–424. [Google Scholar] [CrossRef]
- Bankir, L.; Bochud, M.; Maillard, M.; Bovet, P.; Gabriel, A.; Burnier, M. Nighttime blood pressure and nocturnal dipping are associated with daytime urinary sodium excretion in African subjects. Hypertension 2008, 51, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Uzu, T.; Nishimura, M.; Takeji, M.; Kuroda, S.; Nakamura, S.; Inenaga, T.; Kimura, G. Circadian rhythm of natriuresis is disturbed in nondipper type of essential hypertension. Am. J. Kidney Dis. 1999, 33, 29–35. [Google Scholar] [CrossRef]
- Guerrot, D.; Hansel, B.; Perucca, J.; Roussel, R.; Bouby, N.; Girerd, X.; Bankir, L. Reduced insulin secretion and nocturnal dipping of blood pressure are associated with a disturbed circadian pattern of urine excretion in metabolic syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E929–E933. [Google Scholar] [CrossRef] [PubMed]
- Staessen, J.A.; Birkenhäger, W.; Bulpitt, C.J.; Fagard, R.; Fletcher, A.E.; Lijnen, P.; Thijs, L.; Amery, A. The relationship between blood pressure and sodium and potassium excretion during the day and at night. J. Hypertens. 1993, 11, 443–447. [Google Scholar] [CrossRef]
- .DiBona, G.F. Physiology in perspective: The Wisdom of the Body. Neural control of the kidney. Am. J. Physiol. Integr. Comp. Physiol. 2005, 289, R633–R641. [Google Scholar] [CrossRef]
- Ahmad, Y.; Francis, D.P.; Bhatt, D.L.; Howard, J.P. Renal Denervation for Hypertension: A Systematic Review and Meta-Analysis of Randomized, Blinded, Placebo-Controlled Trials. JACC Cardiovasc. Interv. 2021, 14, 2614–2624. [Google Scholar] [CrossRef]
- Esler, M. Sympathetic nervous system moves toward center stage in cardiovascular medicine: From Thomas Willis to resistant hypertension. Hypertension 2014, 63, e25–e32. [Google Scholar] [CrossRef]
- Grassi, G.; Seravalle, G.; Dell’Oro, R.; Turri, C.; Bolla, G.B.; Mancia, G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension 2000, 36, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Seravalle, G.; Colombo, M.; Perego, P.; Giardini, V.; Volpe, M.; Dell’Oro, R.; Mancia, G.; Grassi, G. Long-term sympathoinhibitory effects of surgically induced weight loss in severe obese patients. Hypertension 2014, 64, 431–437. [Google Scholar] [CrossRef]
- Stevens, V.J.; Obarzanek, E.; Cook, N.R.; Lee, I.-M.; Appel, L.J.; West, D.S.; Milas, N.C.; Mattfeldt-Beman, M.; Belden, L.; Bragg, C.; et al. Long-term weight loss and changes in blood pressure: Results of the Trials of Hypertension Prevention, phase II. Ann. Intern. Med. 2001, 134, 1–11. [Google Scholar] [CrossRef]
- Grassi, G.; Seravalle, G.; Dell’Oro, R.; Turri, C.; Pasqualinotto, L.; Colombo, M.; Mancia, G. Participation of the hypothalamus-hypophysis axis in the sympathetic activation of human obesity. Hypertension 2001, 38, 1316–1320. [Google Scholar] [CrossRef][Green Version]
- Pioli, M.R.; Ritter, A.M.; de Faria, A.P.; Modolo, R. White coat syndrome and its variations: Differences and clinical impact. Integr. Blood Press. Control. 2018, 11, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Sala, C.; Grassi, G.; Mancia, G. White Coat Hypertension: To Treat or Not to Treat? Curr. Hypertens. Rep. 2016, 18, 80. [Google Scholar] [CrossRef]
- Mancia, G.; Facchetti, R.; Parati, G.; Zanchetti, A. Effect of long-term antihypertensive treatment on white-coat hypertension. Hypertension 2014, 64, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Fagard, R.H.; Staessen, J.A.; Thijs, L.; Gasowski, J.; Bulpitt, C.J.; Clement, D.; de Leeuw, P.W.; Dobovisek, J.; Jääskivi, M.; Leonetti, G.; et al. Response to antihypertensive therapy in older patients with sustained and nonsustained systolic hypertension. Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Circulation 2000, 102, 1139–1144. [Google Scholar] [CrossRef]
- Sander, D.; Kukla, C.; Klingelhofer, J.; Winbeck, K.; Conrad, B. Relationship between circadian blood pressure patterns and progression of early carotid atherosclerosis: A 3-year follow-up study. Circulation 2000, 102, 1536–1541. [Google Scholar] [CrossRef]
- Mancia, G.; Bombelli, M.; Facchetti, R.; Madotto, F.; Corrao, G.; Trevano, F.Q.; Grassi, G.; Sega, R. Long-term prognostic value of blood pressure variability in the general population: Results of the Pressioni Arteriose Monitorate e Loro Associazioni Study. Hypertension 2007, 49, 1265–1270. [Google Scholar] [CrossRef]
- Kikuya, M.; Hozawa, A.; Ohokubo, T.; Tsuji, I.; Michimata, M.; Matsubara, M.; Ota, M.; Nagai, K.; Araki, T.; Satoh, H.; et al. Prognostic significance of blood pressure and heart rate variabilities: The Ohasama study. Hypertension 2000, 36, 901–906. [Google Scholar] [CrossRef]
- Del Giorno, R.; Ceresa, C.; Gabutti, S.; Troiani, C.; Gabutti, L. Arterial Stiffness and Central Hemodynamics are Associated with Low Diurnal Urinary Sodium Excretion. Diabetes Metab. Syndr. Obes. 2020, 13, 3289–3299. [Google Scholar] [CrossRef]
- Del Giorno, R.; Troiani, C.; Gabutti, S.; Stefanelli, K.; Gabutti, L. Comparing oscillometric and tonometric methods to assess pulse wave velocity: A population-based study. Ann. Med. 2021, 53, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ufficio di statistica del Cantone Ticino. Panoramica del Tema. Available online: https://m3.ti.ch/DFE/DR/USTAT/allegati/prodima/3201_popolazione.pdf (accessed on 28 August 2023).
- Consortium, T. Recommended standards for assessing blood pressure in human research where blood pressure or hypertension is a major focus. Clin. Exp. Hypertens. 2018, 40, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Staessen, J.; Broughton, P.M.; Fletcher, A.E.; Markowe, H.L.; Marmot, M.G.; Rose, G.; Semmence, A.; Shipley, M.J.; Bulpitt, C.J. The assessment of the relationship between blood pressure and sodium intake using whole-day, daytime and overnight urine collections. J. Hypertens. 1991, 9, 1035–1040. [Google Scholar] [CrossRef]
- Joyner, M.J.; Charkoudian, N.; Wallin, B.G. A sympathetic view of the sympathetic nervous system and human blood pressure regulation. Exp. Physiol. 2008, 93, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dov, I.Z.; Mekler, J.; Ben-Arie, L.; Bursztyn, M. Lack of association between body-mass index and white-coat hypertension among referred patients. Blood Press. Monit. 2007, 12, 95–99. [Google Scholar] [CrossRef]
- Kotsis, V.; Stabouli, S.; Bouldin, M.; Low, A.; Toumanidis, S.; Zakopoulos, N. Impact of obesity on 24-hour ambulatory blood pressure and hypertension. Hypertension 2005, 45, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Den Hond, E.; Celis, H.; Vandenhoven, G.; O’Brien, E.; Staessen, J.A.; THOP investigators. Determinants of white-coat syndrome assessed by ambulatory blood pressure or self-measured home blood pressure. Blood Press. Monit. 2003, 8, 37–40. [Google Scholar] [CrossRef]
- Zanchetti, A. Hyperlipidemia in the hypertensive patient. Am. J. Med. 1994, 96, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Sivén, S.S.; Niiranen, T.J.; Kantola, I.M.; Jula, A.M. White-coat and masked hypertension as risk factors for progression to sustained hypertension: The Finn-Home study. J. Hypertens. 2016, 34, 54–60. [Google Scholar] [CrossRef] [PubMed]


| Age (Years) | BMI (Kg/m2) | Systolic BP Office (mmHg) | Systolic ABPM (mmHg) | Diastolic BP Office (mmHg) | ||
|---|---|---|---|---|---|---|
| N | Valid | 1023 | 1023 | 1023 | 1023 | 1023 |
| Missing | 0 | 0 | 0 | 0 | 0 | |
| Mean | 54.17 | 25.01 | 130.68 | 119.09 | 81.02 | |
| Median | 54.00 | 24.40 | 129.00 | 117 | 80.50 | |
| Mode 1 | 55 | 24.50 | 121.50 a | 116 | 78.50 | |
| Std. Deviation | 13.61 | 4.40 | 17.05 | 11.84 | 11.86 | |
| Minimum | 21 | 16.30 | 46.0 | 90 | 29.50 | |
| Std. Deviation | 93 | 53.20 | 210.50 | 179 | 119.00 | |
| Percentiles | 25 | 46.00 | 21.90 | 119.50 | 111 | 73.50 |
| 50 | 54.00 | 24.40 | 129.00 | 117 | 80.50 | |
| 75 | 63.00 | 27.40 | 141.00 | 126 | 88.00 | |
| Distolic ABPM (mmHg) | Ratio (Night/Day) Hourly Natriuria | Ratio (Night/Day) Hourly Urinary Volume | Creatinine Excretion Night (mmol) | Creatinine Excretion Day (mmol) | ||
| N | Valid | 1023 | 1023 | 1023 | 1023 | 1023 |
| Missing | 0 | 0 | 0 | 0 | 0 | |
| Mean | 74.16 | 1.08 | 0.64 | 5.75 | 8.96 | |
| Median | 73 | 0.87 | 0.43 | 4.10 | 7.00 | |
| Mode 1 | 72 | 0.91 | 0.50 | 3.15 a | 4.60 | |
| Std. Deviation | 8.77 | 2.12 | 2.29 | 21.82 | 19.35 | |
| Minimum | 50 | 0.12 | 0.04 | 0.48 | 1.20 | |
| Std. Deviation | 110 | 58.59 | 60.35 | 511.00 | 603.00 | |
| Percentiles | 25 | 68 | 0.64 | 0.28 | 3.15 | 4.60 |
| 50 | 73 | 0.87 | 0.43 | 4.10 | 7.00 | |
| 75 | 80 | 1.17 | 0.63 | 5.46 | 10.90 | |
| Parameter | Value |
|---|---|
| Total N | 1018 |
| Mann-Whitney U | 127,891.500 |
| Wilcoxon W | 186,544.500 |
| Test Statistic | 127,891.500 |
| Standard Error | 4430.145 |
| Standardized Test Statistic | 2.775 |
| Asymptotic Sig. (2-sided test) | 0.006 |
| Parameter | Value |
|---|---|
| Total N | 1020 |
| Mann-Whitney U | 123,113.500 |
| Wilcoxon W | 181,766.500 |
| Test Statistic | 123,113.500 |
| Standard Error | 4441.709 |
| Standardized Test Statistic | 1.615 |
| Asymptotic Sig. (2-sided test) | 0.106 |
| Intercept | Standard Error | Wald | Chi-Square | p-Value | Odds Ratio | ||
|---|---|---|---|---|---|---|---|
| Step 1 a | age | 0.025 | 0.005 | 21.585 | 1 | <0.001 | 1.026 |
| MAP 24 h | 0.038 | 0.008 | 20.826 | 1 | <0.001 | 0.963 | |
| BMI | 0.141 | 0.018 | 59.383 | 1 | <0.001 | 1.151 | |
| Constant | −2.070 | 0.730 | 8.041 | 1 | 0.005 | 0.126 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moretti, F.; Gianini, J.; Del Giorno, R.; Gabutti, L. Circadian Urinary Excretion of Water, and Not Salt, Is Affected by the White Coat Effect. J. Clin. Med. 2023, 12, 5725. https://doi.org/10.3390/jcm12175725
Moretti F, Gianini J, Del Giorno R, Gabutti L. Circadian Urinary Excretion of Water, and Not Salt, Is Affected by the White Coat Effect. Journal of Clinical Medicine. 2023; 12(17):5725. https://doi.org/10.3390/jcm12175725
Chicago/Turabian StyleMoretti, Fabio, Jvan Gianini, Rosaria Del Giorno, and Luca Gabutti. 2023. "Circadian Urinary Excretion of Water, and Not Salt, Is Affected by the White Coat Effect" Journal of Clinical Medicine 12, no. 17: 5725. https://doi.org/10.3390/jcm12175725
APA StyleMoretti, F., Gianini, J., Del Giorno, R., & Gabutti, L. (2023). Circadian Urinary Excretion of Water, and Not Salt, Is Affected by the White Coat Effect. Journal of Clinical Medicine, 12(17), 5725. https://doi.org/10.3390/jcm12175725





