Cortical Mechanisms Underlying Immersive Interactive Virtual Walking Treatment for Amelioration of Neuropathic Pain after Spinal Cord Injury: Findings from a Preliminary Investigation of Thalamic Inhibitory Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Regulatory Approvals
2.4. Procedures
2.5. Immersive Interactive Virtual Walking Interface
2.6. Measures
2.6.1. Chronic Pain Measures
Pain Intensity
Pain Disability Index
Pain Interference
Neuropathic Pain Scale
2.7. MR Spectroscopy Measures
GABA-Edited MEscher–GArwood Point RESolved Spectroscopy (MEGA-PRESS) Spectra
2.8. Analysis
2.8.1. GABA-Edited MEGA-PRESS Spectral Analysis
2.8.2. Spectral Quality Assessment
2.8.3. Data Analysis
3. Results
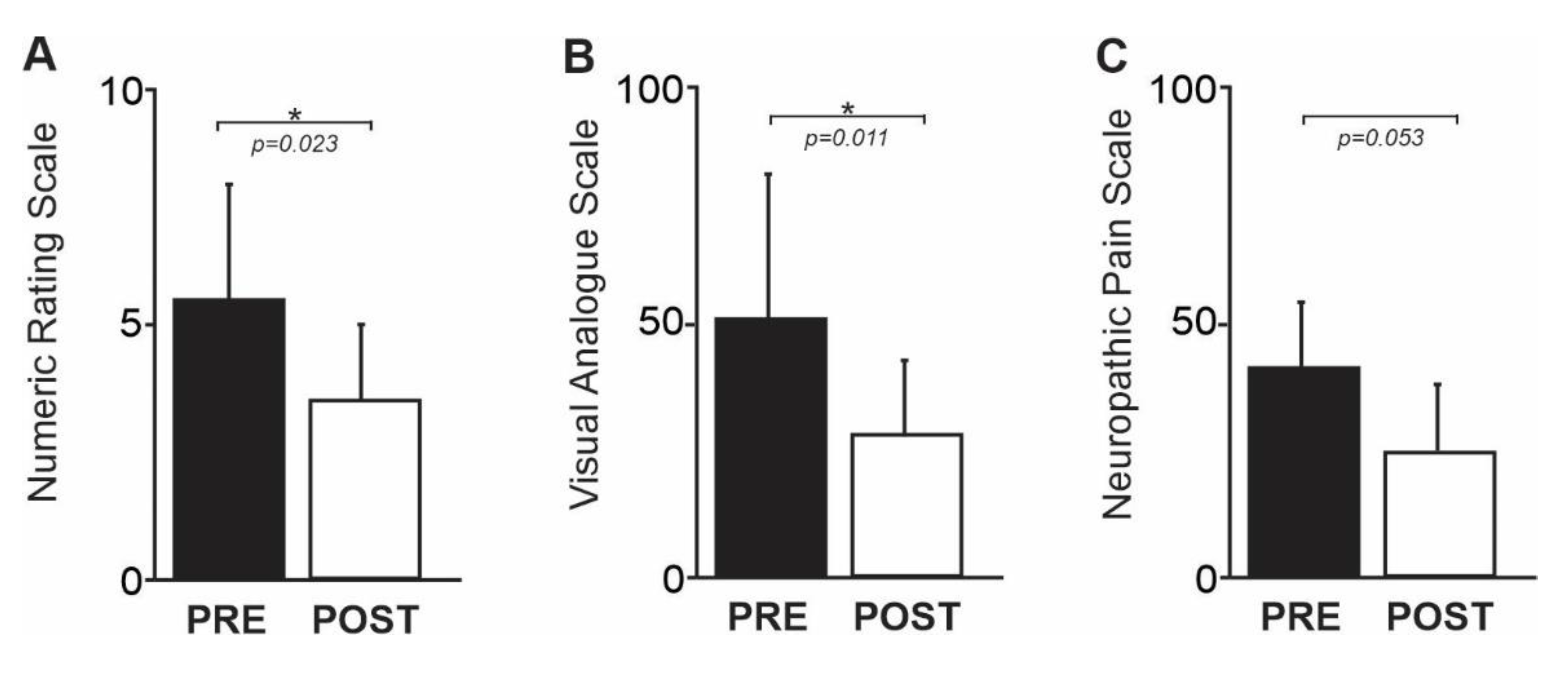
3.1. Pain Intensity
3.2. GABA-Edited MEGA-PRESS Spectroscopy
3.3. Spectral Quality Assessment
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moseley, G.L. Using visual illusion to reduce at-level neuropathic pain in paraplegia. Pain 2007, 130, 294–298. [Google Scholar] [PubMed]
- Finnerup, N.B. Pain in patients with spinal cord injury. PAIN® 2013, 154, S71–S76. [Google Scholar] [PubMed]
- Siddall, P. Management of neuropathic pain following spinal cord injury: Now and in the future. Spinal Cord 2009, 47, 352–359. [Google Scholar]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Baastrup, C.; Finnerup, N.B. Pharmacological management of neuropathic pain following spinal cord injury. CNS Drugs 2008, 22, 455–475. [Google Scholar] [PubMed]
- Mehta, S.; McIntyre, A.; Janzen, S.; Loh, E.; Teasell, R.; Spinal Cord Injury Rehabilitation Evidence Team. Systematic review of pharmacologic treatments of pain after spinal cord injury: An update. Arch. Phys. Med. Rehabil. 2016, 97, 1381–1391.e1. [Google Scholar]
- Snedecor, S.; Sudharshan, L.; Cappelleri, J.C.; Sadosky, A.; Desai, P.; Jalundhwala, Y.J.; Botteman, M. Systematic review and comparison of pharmacologic therapies for neuropathic pain associated with spinal cord injury. J. Pain Res. 2013, 6, 539. [Google Scholar] [PubMed]
- Finnerup, N.B.; Haroutounian, S.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpaa, M.; Jensen, T.S.; Kamerman, P.R.; McNicol, E.; Moore, A.; et al. Neuropathic pain clinical trials: Factors associated with decreases in estimated drug efficacy. Pain 2018, 159, 2339–2346. [Google Scholar] [CrossRef]
- Hagen, E.M.; Rekand, T. Management of neuropathic pain associated with spinal cord injury. Pain Ther. 2015, 4, 51–65. [Google Scholar]
- Ahn, S.-H.; Park, H.-W.; Lee, B.-S.; Moon, H.-W.; Jang, S.-H.; Sakong, J.; Bae, J.-H. Gabapentin effect on neuropathic pain compared among patients with spinal cord injury and different durations of symptoms. Spine 2003, 28, 341–346. [Google Scholar]
- Cardenas, D.D.; Nieshoff, E.C.; Suda, K.; Goto, S.; Sanin, L.; Kaneko, T.; Sporn, J.; Parsons, B.; Soulsby, M.; Yang, R.; et al. A randomized trial of pregabalin in patients with neuropathic pain due to spinal cord injury. Neurology 2013, 80, 533–539. [Google Scholar] [CrossRef]
- Boldt, I.; Eriks-Hoogland, I.; Brinkhof, M.W.; de Bie, R.; Joggi, D.; von Elm, E. Non-pharmacological interventions for chronic pain in people with spinal cord injury. Cochrane Database Syst. Rev. 2014, CD009177. [Google Scholar] [CrossRef]
- Trost, Z.; Anam, M.; Seward, J.; Shum, C.; Rumble, D.; Sturgeon, J.; Mark, V.; Chen, Y.Y.; Mitchell, L.; Cowan, R.; et al. Immersive Interactive Virtual Walking Reduces Neuropathic Pain in Spinal Cord Injury: Findings from a Preliminary Investigation of Feasibility and Clinical Efficacy. Pain 2022, 163, 350–361. [Google Scholar]
- Eick, J.; Richardson, E.J. Cortical activation during visual illusory walking in persons with spinal cord injury: A pilot study. Arch. Phys. Med. Rehabil. 2015, 96, 750–753. [Google Scholar]
- Jordan, M.; Richardson, E.J. Effects of Virtual Walking Treatment on Spinal Cord Injury–Related Neuropathic Pain: Pilot Results and Trends Related to Location of Pain and at-level Neuronal Hypersensitivity. Am. J. Phys. Med. Rehabil. 2016, 95, 390–396. [Google Scholar]
- Soler, M.D.; Kumru, H.; Pelayo, R.; Vidal, J.; Tormos, J.M.; Fregni, F.; Navarro, X.; Pascual-Leone, A. Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury. Brain 2010, 133, 2565–2577. [Google Scholar] [PubMed]
- Richardson, E.J.; McKinley, E.C.; Rahman, A.; Klebine, P.; Redden, D.T.; Richards, J.S. Effects of virtual walking on spinal cord injury-related neuropathic pain: A randomized, controlled trial. Rehabil. Psychol. 2019, 64, 13. [Google Scholar] [PubMed]
- Kobashi, N.; Holper, L.; Scholkmann, F.; Kiper, D.; Eng, K. Enhancement of motor imagery-related cortical activation during first-person observation measured by functional near-infrared spectroscopy. Eur. J. Neurosci. 2012, 35, 1513–1521. [Google Scholar] [PubMed]
- Jackson, P.L.; Meltzoff, A.N.; Decety, J. Neural circuits involved in imitation and perspective-taking. Neuroimage 2006, 31, 429–439. [Google Scholar] [PubMed]
- Gustin, S.; Wrigley, P.; Siddall, P.; Henderson, L. Brain anatomy changes associated with persistent neuropathic pain following spinal cord injury. Cereb. Cortex 2010, 20, 1409–1419. [Google Scholar]
- Gustin, S.M.; Wrigley, P.J.; Henderson, L.A.; Siddall, P.J. Brain circuitry underlying pain in response to imagined movement in people with spinal cord injury. PAIN® 2010, 148, 438–445. [Google Scholar]
- Gustin, S.M.; Wrigley, P.J.; Youssef, A.M.; McIndoe, L.; Wilcox, S.L.; Rae, C.D.; Edden, R.; Siddall, P.J.; Henderson, L.A. Thalamic activity and biochemical changes in individuals with neuropathic pain after spinal cord injury. PAIN® 2014, 155, 1027–1036. [Google Scholar] [CrossRef]
- Wrigley, P.J.; Press, S.R.; Gustin, S.M.; Macefield, V.G.; Gandevia, S.C.; Cousins, M.J.; Middleton, J.W.; Henderson, L.A.; Siddall, P. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. PAIN® 2009, 141, 52–59. [Google Scholar] [CrossRef]
- Kramer, P.R.; Stinson, C.; Umorin, M.; Deng, M.; Rao, M.; Bellinger, L.L.; Yee, M.B.; Kinchington, P.R. Lateral thalamic control of nociceptive response after whisker pad injection of varicella zoster virus. Neuroscience 2017, 356, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, R.-X.; Zhang, Y.; Wang, J.; Liu, F.-Y.; Cai, J.; Liao, F.-F.; Xu, F.-Q.; Yi, M.; Wan, Y. Reduced GABAergic transmission in the ventrobasal thalamus contributes to thermal hyperalgesia in chronic inflammatory pain. Sci. Rep. 2017, 7, 41439. [Google Scholar] [CrossRef]
- Wang, C.; Hao, H.; He, K.; An, Y.; Pu, Z.; Gamper, N.; Zhang, H.; Du, X. Neuropathic Injury–Induced Plasticity of GABAergic System in Peripheral Sensory Ganglia. Front. Pharmacol. 2021, 12, 702218. [Google Scholar] [CrossRef] [PubMed]
- Bryce, T.N.; Budh, C.N.; Cardenas, D.D.; Dijkers, M.; Felix, E.R.; Finnerup, N.B.; Kennedy, P.; Lundeberg, T.; Richards, J.S.; Rintala, D.H. Pain after spinal cord injury: An evidence-based review for clinical practice and research: Report of the National Institute on Disability and Rehabilitation Research Spinal Cord Injury Measures meeting. J. Spinal Cord Med. 2007, 30, 421–440. [Google Scholar] [CrossRef] [PubMed]
- Karcioglu, O.; Topacoglu, H.; Dikme, O.; Dikme, O. A systematic review of the pain scales in adults: Which to use? Am. J. Emerg. Med. 2018, 36, 707–714. [Google Scholar] [CrossRef]
- Williamson, A.; Hoggart, B. Pain: A review of three commonly used pain rating scales. J. Clin. Nurs. 2005, 14, 798–804. [Google Scholar] [CrossRef]
- Acerra, N.; Souvlis, T.; Moseley, G. Does mirror-box therapy improve sensory and motor changes in the early post-stroke population? A randomised controlled trial. Aust. J. Physiother. 2005, 51, S7. [Google Scholar]
- Biering-Sørensen, F.; Alai, S.; Anderson, K.; Charlifue, S.; Chen, Y.; DeVivo, M.; Flanders, A.E.; Jones, L.; Kleitman, N.; Lans, A. Common data elements for spinal cord injury clinical research: A National Institute for Neurological Disorders and Stroke project. Spinal Cord 2015, 53, 265–277. [Google Scholar] [CrossRef]
- Ferreira-Valente, M.A.; Pais-Ribeiro, J.L.; Jensen, M.P. Validity of four pain intensity rating scales. Pain 2011, 152, 2399–2404. [Google Scholar] [CrossRef] [PubMed]
- Widerström-Noga, E.; Biering-Sørensen, F.; Bryce, T.; Cardenas, D.; Finnerup, N.; Jensen, M.; Richards, J.; Siddall, P. The international spinal cord injury pain basic data set (version 2.0). Spinal Cord 2014, 52, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, S.F. Research diagnostic criteria for temporomandibular disorders: Review, criteria, examinations and specifications, critique. J. Craniomandib. Disord. 1992, 6, 301–355. [Google Scholar]
- Collins, S.L.; Moore, A.R.; McQuay, H.J. The visual analogue pain intensity scale: What is moderate pain in millimetres? Pain 1997, 72, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Tait, R.; Pollard, C.A.; Margolis, R.; Duckro, P.N.; Krause, S.J. The Pain Disability Index: Psychometric and validity data. Arch. Phys. Med. Rehabil. 1987, 68, 438–441. [Google Scholar] [PubMed]
- Amtmann, D.; Cook, K.F.; Jensen, M.P.; Chen, W.H.; Choi, S.; Revicki, D.; Cella, D.; Rothrock, N.; Keefe, F.; Callahan, L.; et al. Development of a PROMIS item bank to measure pain interference. Pain 2010, 150, 173–182. [Google Scholar] [CrossRef]
- Galer, B.S.; Jensen, M.P. Development and preliminary validation of a pain measure specific to neuropathic pain: The Neuropathic Pain Scale. Neurology 1997, 48, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Spreafico, R.; Frassoni, C.; Arcelli, P.; De Biasi, S. GABAergic interneurons in the somatosensory thalamus of the guinea-pig: A light and ultrastructural immunocytochemical investigation. Neuroscience 1994, 59, 961–973. [Google Scholar] [CrossRef]
- Choi, I.Y.; Andronesi, O.C.; Barker, P.; Bogner, W.; Edden, R.A.; Kaiser, L.G.; Lee, P.; Marjańska, M.; Terpstra, M.; de Graaf, R.A. Spectral editing in 1H magnetic resonance spectroscopy: Experts’ consensus recommendations. NMR Biomed. 2020, 34, e4411. [Google Scholar] [CrossRef]
- Wilson, M.; Andronesi, O.; Barker, P.B.; Bartha, R.; Bizzi, A.; Bolan, P.J.; Brindle, K.M.; Choi, I.Y.; Cudalbu, C.; Dydak, U. Methodological consensus on clinical proton MRS of the brain: Review and recommendations. Magn. Reson. Med. 2019, 82, 527–550. [Google Scholar] [CrossRef]
- Kang, D.; Hesam-Shariati, N.; McAuley, J.H.; Alam, M.; Trost, Z.; Rae, C.D.; Gustin, S.M. Disruption to normal excitatory and inhibitory function within the medial prefrontal cortex in people with chronic pain. Eur. J. Pain 2021, 25, 2242–2256. [Google Scholar] [CrossRef]
- Naylor, B.; Hesam-Shariati, N.; McAuley, J.H.; Boag, S.; Newton-John, T.; Rae, C.D.; Gustin, S.M. Reduced glutamate in the medial prefrontal cortex is associated with emotional and cognitive dysregulation in people with chronic pain. Front. Neurol. 2019, 10, 1110. [Google Scholar] [CrossRef]
- Henderson, L.A.; Peck, C.C.; Petersen, E.T.; Rae, C.D.; Youssef, A.M.; Reeves, J.M.; Wilcox, S.L.; Akhter, R.; Murray, G.M.; Gustin, S.M. Chronic pain: Lost inhibition? J. Neurosci. 2013, 33, 7574–7582. [Google Scholar] [CrossRef] [PubMed]
- Trost, Z.; France, C.; Anam, M.; Shum, C. Virtual reality approaches to pain: Toward a state of the science. Pain 2021, 162, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Boesch, E.; Bellan, V.; Moseley, G.L.; Stanton, T.R. The effect of bodily illusions on clinical pain: A systematic review and meta-analysis. Pain 2016, 157, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Garrett, B.; Taverner, T.; Masinde, W.; Gromala, D.; Shaw, C.; Negraeff, M. A rapid evidence assessment of immersive virtual reality as an adjunct therapy in acute pain management in clinical practice. Clin. J. Pain 2014, 30, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Thieme, H.; Morkisch, N.; Rietz, C.; Dohle, C.; Borgetto, B. The efficacy of movement representation techniques for treatment of limb pain—A systematic review and meta-analysis. J. Pain 2016, 17, 167–180. [Google Scholar] [CrossRef]
- Lotze, M.; Grodd, W.; Birbaumer, N.; Erb, M.; Huse, E.; Flor, H. Does use of a myoelectric prosthesis prevent cortical reorganization and phantom limb pain? Nat. Neurosci. 1999, 2, 501–502. [Google Scholar] [CrossRef]
- Cai, K.; Nanga, R.P.; Lamprou, L.; Schinstine, C.; Elliott, M.; Hariharan, H.; Reddy, R.; Epperson, C.N. The impact of gabapentin administration on brain GABA and glutamate concentrations: A 7T 1 H-MRS study. Neuropsychopharmacology 2012, 37, 2764–2771. [Google Scholar] [CrossRef]


| Code | Sex | Age | ASIA ISNCSCI Grade | Level of Injury | Years Since Injury | Pain Duration (Years) | Pain Location | Pain Level | Pain Medication |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 23 | A | T7 | 5 | 5 | Bilateral back, feet | Below | None |
| 2 | M | 35 | A | T7 | 15 | 15 | Bilateral feet, shins | Below | None |
| 3 | M | 36 | A | T12 | 10 | 10 | Left buttocks, left lower back | Below | None |
| 4 | M | 48 | A | T12 | 6 | 6 | Bilateral toes | Below | Baclofen, gabapentin |
| 5 | M | 48 | A | T1 | 14 | 14 | Bilateral buttocks, feet | Below | None |
| 6 | M | 56 | A | T10 | 11 | 11 | Bilateral toes, upper legs | Below | Baclofen, gabapentin |
| 7 | M | 70 | A | T11–12 | 4 | 4 | Bilateral abdomen, legs | Below | None |
| Code | Pain Duration (Years) | GABA/Cr (ppm) PRE Therapy | GABA/Cr (ppm) POST Therapy | NRS PRE Therapy | NRS POST Therapy | VAS PRE Therapy | VAS POST Therapy | NPS PRE Therapy | NPS POST Therapy |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 0.25 | 0.31 | 6 | 2 | 30 | 8 | 24 | 11 |
| 2 | 15 | 0.21 | 0.27 | 9 | 6 | 85 | 48 | 63 | 15 |
| 3 | 10 | 0.24 | 0.36 | 8 | 5 | 80 | 13 | 55 | 56 |
| 4 | 6 | 0.28 | 0.26 | 3 | 3 | 77 | 50 | 27 | 10 |
| 5 | 14 | 0.21 | 0.27 | 6 | 3 | 55 | 15 | 46 | 21 |
| 6 | 11 | 0.24 | 0.29 | 4 | 1 | 3 | 1 | 36 | 28 |
| 7 | 4 | 0.22 | 0.31 | 3 | 4 | 32 | 21 | 33 | 34 |
| Mean (±SD) | 9.3 ± 4.4 | 0.24 ± 0.022 | 0.30 ± 0.034 | 5.6 ± 2.4 | 3.4 ± 1.7 | 52 ± 31 | 22 ± 19 | 40.6 ± 15 | 25 ± 16 |
| Variable 1 | Variable 2 | Correlation Coefficient (r) | Significance Level (p) |
|---|---|---|---|
| Change in GABA/creatine ratios from pre- to post-intervention | Change in pain intensity (NRS) from pre- to post-intervention | −0.282 | 0.3 |
| Change in GABA/creatine ratios from pre- to post-intervention | Change in pain intensity (VAS) from pre- to post-intervention | 0.417 | 0.2 |
| Pre-intervention GABA/creatine ratios | Pre-intervention NRS ratings | −0.2 | 0.3 |
| Pre-intervention GABA/creatine ratios | Pain duration | −0.04 | 0.2 |
| Pre-intervention GABA/creatine ratios | Pre-intervention VAS ratings | 0.5 | 0.1 |
| Post-intervention GABA/creatine ratios | Post-intervention NRS ratings | 0.6 | 0.1 |
| Post-intervention GABA/creatine ratios | Post-intervention NRS ratings | −0.2 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gustin, S.M.; Bolding, M.; Willoughby, W.; Anam, M.; Shum, C.; Rumble, D.; Mark, V.W.; Mitchell, L.; Cowan, R.E.; Richardson, E.; et al. Cortical Mechanisms Underlying Immersive Interactive Virtual Walking Treatment for Amelioration of Neuropathic Pain after Spinal Cord Injury: Findings from a Preliminary Investigation of Thalamic Inhibitory Function. J. Clin. Med. 2023, 12, 5743. https://doi.org/10.3390/jcm12175743
Gustin SM, Bolding M, Willoughby W, Anam M, Shum C, Rumble D, Mark VW, Mitchell L, Cowan RE, Richardson E, et al. Cortical Mechanisms Underlying Immersive Interactive Virtual Walking Treatment for Amelioration of Neuropathic Pain after Spinal Cord Injury: Findings from a Preliminary Investigation of Thalamic Inhibitory Function. Journal of Clinical Medicine. 2023; 12(17):5743. https://doi.org/10.3390/jcm12175743
Chicago/Turabian StyleGustin, Sylvia M., Mark Bolding, William Willoughby, Monima Anam, Corey Shum, Deanna Rumble, Victor W. Mark, Lucie Mitchell, Rachel E. Cowan, Elizabeth Richardson, and et al. 2023. "Cortical Mechanisms Underlying Immersive Interactive Virtual Walking Treatment for Amelioration of Neuropathic Pain after Spinal Cord Injury: Findings from a Preliminary Investigation of Thalamic Inhibitory Function" Journal of Clinical Medicine 12, no. 17: 5743. https://doi.org/10.3390/jcm12175743
APA StyleGustin, S. M., Bolding, M., Willoughby, W., Anam, M., Shum, C., Rumble, D., Mark, V. W., Mitchell, L., Cowan, R. E., Richardson, E., Richards, S., & Trost, Z. (2023). Cortical Mechanisms Underlying Immersive Interactive Virtual Walking Treatment for Amelioration of Neuropathic Pain after Spinal Cord Injury: Findings from a Preliminary Investigation of Thalamic Inhibitory Function. Journal of Clinical Medicine, 12(17), 5743. https://doi.org/10.3390/jcm12175743






