Preliminary Evidence to Support a De-Escalated Cochlear Implant Programming Paradigm for New Adult Recipients: A Systematic Review
Abstract
:1. Introduction
2. Material and Methods
2.1. Search Strategy
2.2. Study Identification
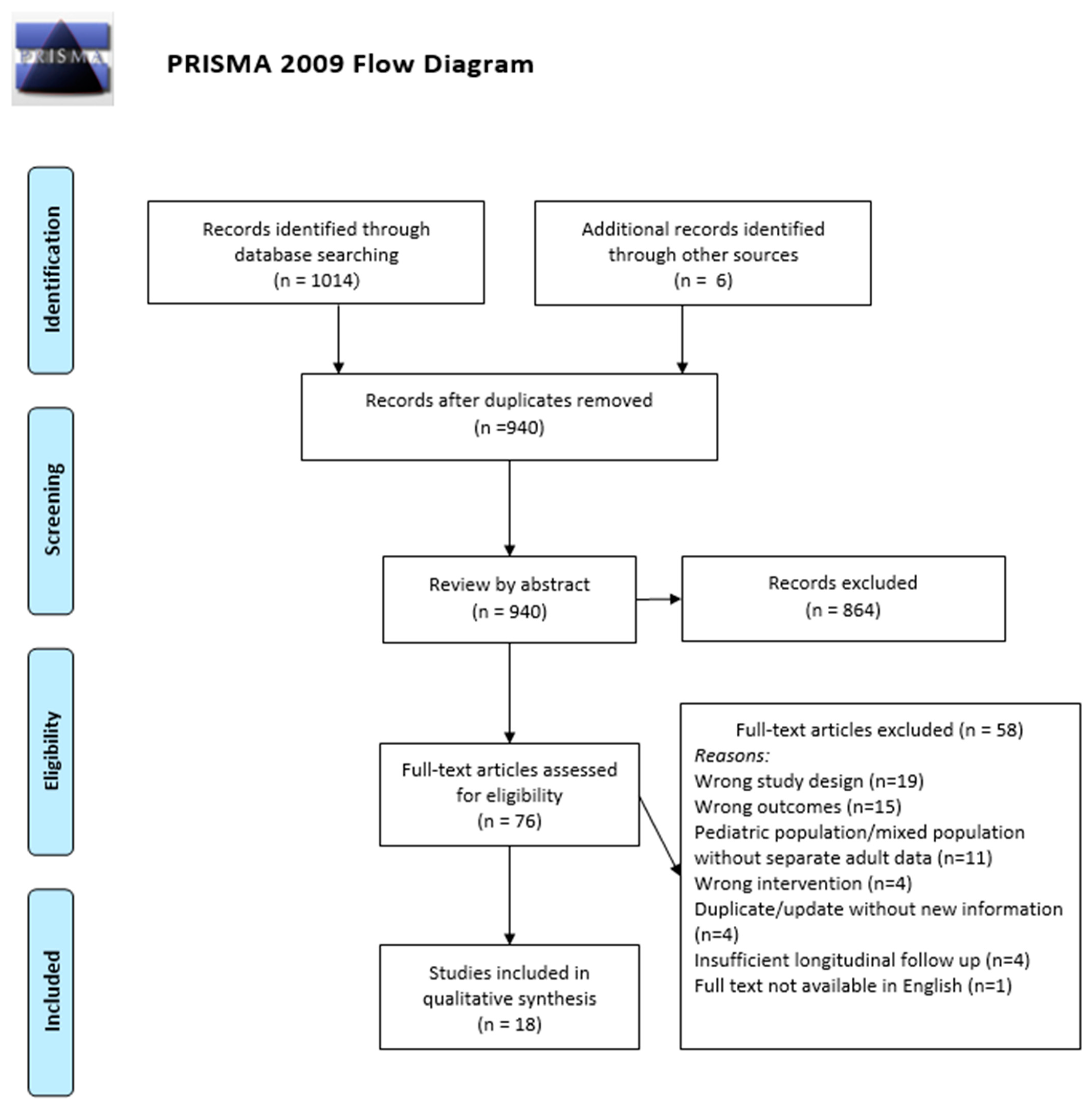
2.3. Study Screening and Selection
2.4. Data Extraction
3. Results
3.1. Study Characteristics and Populations
3.2. Speech Outcomes over Time
3.3. Programming Parameters
3.4. Evidence-Based Programming Trials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, F.; Ni, W.; Li, W.; Li, H. Cochlear Implantation and Rehabilitation. Adv. Exp. Med. Biol. 2019, 1130, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.L. Cochlear Implantation in Adults. N. Engl. J. Med. 2020, 382, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, W.H.; Bradham, T.S. Cochlear implant programming. Otolaryngol. Clin. N. Am. 2012, 45, 111–127. [Google Scholar] [CrossRef]
- Vaerenberg, B.; Smits, C.; De Ceulaer, G.; Zir, E.; Harman, S.; Jaspers, N.; Tam, Y.; Dillon, M.; Wesarg, T.; Martin-Bonniot, D.; et al. Cochlear implant programming: A global survey on the state of the art. Sci. World J. 2014, 2014, 501738. [Google Scholar] [CrossRef]
- Slager, H.K.; Jensen, J.; Kozlowski, K.; Teagle, H.; Park, L.R.; Biever, A.; Mears, M. Remote Programming of Cochlear Implants. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2019, 40, e260–e266. [Google Scholar] [CrossRef]
- Nassiri, A.M.; Holcomb, M.A.; Perkins, E.L.; Bucker, A.L.; Prentiss, S.M.; Welch, C.M.; Andresen, N.S.; Valenzuela, C.V.; Wick, C.C.; Angeli, S.I.; et al. Catchment Profile of Large Cochlear Implant Centers in the United States. Otolaryngol.-Head Neck Surg. 2022, 167, 545–551. [Google Scholar] [CrossRef]
- Nassiri, A.M.; Marinelli, J.P.; Sorkin, D.L.; Carlson, M.L. Barriers to Adult Cochlear Implant Care in the United States: An Analysis of Health Care Delivery. Semin. Hear. 2021, 42, 311–320. [Google Scholar] [CrossRef]
- Grisel, J.; Miller, S.; Schafer, E.C. A Novel Performance-Based Paradigm of Care for Cochlear Implant Follow-Up. Laryngoscope 2022, 132 (Suppl. S1), S1–S10. [Google Scholar] [CrossRef]
- Wathour, J.; Govaerts, P.J.; Deggouj, N. Variability of fitting parameters across cochlear implant centres. Eur. Arch. Otorhinolaryngol. 2021, 278, 4671–4679. [Google Scholar] [CrossRef]
- Dornhoffer, J. An Otologist’s Experience as a Cochlear Implant Patient-The Power of Neuroplasticity. JAMA Otolaryngol.-Head Neck Surg. 2019, 145, 401–402. [Google Scholar] [CrossRef]
- Porps, S.L.; Bennett, D.M.; Gilden, J.; Ravelo, K.; Buck, B.; Reinhart, P.; Hong, R.S. Effects of an evidence-based model for cochlear implant aftercare delivery on clinical efficiency and patient outcomes. Cochlear Implants Int. 2023, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.A.; Forrest, J.L. Enhancing your practice through evidence-based decision making: PICO, learning how to ask good questions. J. Evid. Based Dent. Pract. 2001, 1, 136–141. [Google Scholar] [CrossRef]
- Aimoni, C.; Ciorba, A.; Hatzopoulos, S.; Ramacciotti, G.; Mazzoli, M.; Bianchini, C.; Rosignoli, M.; Skarżyński, H.; Skarżyński, P.H. Cochlear Implants in Subjects Over Age 65: Quality of Life and Audiological Outcomes. Med. Sci. Monit. 2016, 22, 3035–3042. [Google Scholar] [CrossRef]
- Bruschke, S.; Baumann, U.; Stöver, T. Long-Term Follow-Up of Early Cochlear Implant Device Activation. Audiol. Neurootol. 2021, 26, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Caswell-Midwinter, B.; Doney, E.M.; Arjmandi, M.K.; Jahn, K.N.; Herrmann, B.S.; Arenberg, J.G. The Relationship between Impedance, Programming and Word Recognition in a Large Clinical Dataset of Cochlear Implant Recipients. Trends Hear. 2022, 26, 23312165211060983. [Google Scholar] [CrossRef]
- Domville-Lewis, C.; Santa Maria, P.L.; Upson, G.; Chester-Browne, R.; Atlas, M.D. Psychophysical Map Stability in Bilateral Sequential Cochlear Implantation: Comparing Current Audiology Methods to a New Statistical Definition. Ear Hear. 2015, 36, 497–504. [Google Scholar] [CrossRef]
- Frijns, J.H.; Briaire, J.J.; de Laat, J.A.; Grote, J.J. Initial evaluation of the Clarion CII cochlear implant: Speech perception and neural response imaging. Ear Hear. 2002, 23, 184–197. [Google Scholar] [CrossRef]
- Gajadeera, E.A.; Galvin, K.L.; Dowell, R.C.; Busby, P.A. Investigation of Electrical Stimulation Levels Over 8 to 10 Years Postimplantation for a Large Cohort of Adults Using Cochlear Implants. Ear Hear. 2017, 38, 736–745. [Google Scholar] [CrossRef]
- Gajadeera, E.A.; Galvin, K.L.; Dowell, R.C.; Busby, P.A. The Change in Electrical Stimulation Levels during 24 Months Postimplantation for a Large Cohort of Adults Using the Nucleus® Cochlear Implant. Ear Hear. 2017, 38, 357–367. [Google Scholar] [CrossRef]
- Hilly, O.; Hwang, E.; Smith, L.; Shipp, D.; Nedzelski, J.M.; Chen, J.M.; Lin, V.W. Cochlear implantation in elderly patients: Stability of outcome over time. J. Laryngol. Otol. 2016, 130, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.L.; Vander Werff, K.R.; Brown, C.J.; Abbas, P.J.; Kelsay, D.M.; Teagle, H.F.; Lowder, M.W. A longitudinal study of electrode impedance, the electrically evoked compound action potential, and behavioral measures in nucleus 24 cochlear implant users. Ear Hear. 2001, 22, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Mosca, F.; Grassia, R.; Leone, C.A. Longitudinal variations in fitting parameters for adult cochlear implant recipients. Acta Otorhinolaryngol. Ital. 2014, 34, 111–116. [Google Scholar]
- Ruffin, C.V.; Tyler, R.S.; Witt, S.A.; Dunn, C.C.; Gantz, B.J.; Rubinstein, J.T. Long-term performance of Clarion 1.0 cochlear implant users. Laryngoscope 2007, 117, 1183–1190. [Google Scholar] [CrossRef]
- Zwolan, T.A.; Presley, R.; Chenier, L.; Buck, B. Investigation of an Outcomes-Driven, Computer-Assisted Approach to CI Fitting in Newly Implanted Patients. Ear Hear. 2021, 42, 558–564. [Google Scholar] [CrossRef]
- Zwolan, T.; Kileny, P.R.; Smith, S.; Mills, D.; Koch, D.; Osberger, M.J. Adult cochlear implant patient performance with evolving electrode technology. Otol. Neurotol. 2001, 22, 844–849. [Google Scholar] [CrossRef]
- Clark, E.; Burkett, K.; Stanko-Lopp, D. Let Evidence Guide Every New Decision (LEGEND): An evidence evaluation system for point-of-care clinicians and guideline development teams. J. Eval. Clin. Pract. 2009, 15, 1054–1060. [Google Scholar] [CrossRef]
- Lenarz, M.; Sönmez, H.; Joseph, G.; Büchner, A.; Lenarz, T. Effect of gender on the hearing performance of adult cochlear implant patients. Laryngoscope 2012, 122, 1126–1129. [Google Scholar] [CrossRef]
- Kelsall, D.; Lupo, J.; Biever, A. Longitudinal outcomes of cochlear implantation and bimodal hearing in a large group of adults: A multicenter clinical study. Am. J. Otolaryngol. 2021, 42, 102773. [Google Scholar] [CrossRef]
- Tillman, T.W.; Carhart, R. An expanded test for speech discrimination utilizing CNC monosyllabic words. Northwestern University Auditory Test No. 6. SAM-TR-66-55. Tech. Rep. SAM-TR 1966, 1–12. [Google Scholar] [CrossRef]
- Hey, M.; Brademann, G.; Ambrosch, P. The Freiburg monosyllable word test in postoperative cochlear implant diagnostics. Hno 2016, 64, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Spahr, A.J.; Dorman, M.F.; Litvak, L.M.; Van Wie, S.; Gifford, R.H.; Loizou, P.C.; Loiselle, L.M.; Oakes, T.; Cook, S. Development and validation of the AzBio sentence lists. Ear Hear. 2012, 33, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Hochmair-Desoyer, I.; Schulz, E.; Moser, L.; Schmidt, M. The HSM sentence test as a tool for evaluating the speech understanding in noise of cochlear implant users. Am. J. Otol. 1997, 18, S83. [Google Scholar]
- Nilsson, M.; Soli, S.D.; Sullivan, J.A. Development of the Hearing in Noise Test for the measurement of speech reception thresholds in quiet and in noise. J. Acoust. Soc. Am. 1994, 95, 1085–1099. [Google Scholar] [CrossRef]
- Dornhoffer, J.R.; Reddy, P.; Ma, C.; Schvartz-Leyzac, K.C.; Dubno, J.R.; McRackan, T.R. Use of Auditory Training and Its Influence on Early Cochlear Implant Outcomes in Adults. Otol. Neurotol. 2022, 43, e165–e173. [Google Scholar] [CrossRef]
- Fu, Q.J.; Galvin, J.J., 3rd. Perceptual learning and auditory training in cochlear implant recipients. Trends Amplif. 2007, 11, 193–205. [Google Scholar] [CrossRef]
- Shapiro, S.B.; Lipschitz, N.; Kemper, N.; Abdelrehim, L.; Hammer, T.; Wenstrup, L.; Breen, J.T.; Grisel, J.J.; Samy, R.N. Real-World Compliance With Follow-up in 2,554 Cochlear Implant Recipients: An Analysis of the HERMES Database. Otol. Neurotol. 2021, 42, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Winchester, A.; Kay-Rivest, E.; Friedmann, D.R.; McMenomey, S.O.; Shapiro, W.H.; Roland, J.T., Jr.; Waltzman, S.; Jethanamest, D. HiRes ultra series cochlear implant field recall: Failure rates and early outcomes. Cochlear Implants Int. 2022, 24, 87–94. [Google Scholar] [CrossRef]
- McHugh, C.I.; Swedenborg, B.K.; Chen, J.X.; Jung, D.H.; Mankarious, L.A.; Quesnel, A.M.; Cohen, M.S.; Arenberg, J.G.; Franck, K.H.; Santos, F. Voluntary Field Recall of Advanced Bionics HiRes Cochlear Implants: A Single-Institution Experience. Otol. Neurotol. 2022, 43, e1094–e1099. [Google Scholar] [CrossRef]
- Van den Borre, E.; Denys, S.; van Wieringen, A.; Wouters, J. The digit triplet test: A scoping review. Int. J. Audiol. 2021, 60, 946–963. [Google Scholar] [CrossRef]
- Carter, J.M.; Killan, C.F.; Ridgwell, J.J. Telehealth rehabilitation for adults with cochlear implants in response to the COVID-19 pandemic: Platform selection and case studies. Cochlear Implants Int. 2022, 23, 43–51. [Google Scholar] [CrossRef] [PubMed]
| Participant | New cochlear implant recipient, implanted as an adult (≥18 years) |
| Intervention | Any de-escalated or evidence-based scheduling of follow-up and/or programming; or any examination of follow-up, programming, or outcomes over the first 6 months or more post-activation to inform the above |
| Control | Natural or observational studies did not require a control; studies examining specific modifications in follow-up or scheduling were compared to normal control or historical implant data |
| Outcome | Subjective implant function, objective speech testing, quality of life, cost/time savings, and/or programming parameters |
| Study | Randomized controlled trials, non-randomized controlled trials, cohort studies with control groups, and repeated measure studies (including single sample case studies) |
| Study Details | Implanted Patient Population | Implant Details | LEGEND Grading | ||||
|---|---|---|---|---|---|---|---|
| Author Year | Study Design | N | Age in Years (Avg (Range)) | Gender (% Female) | UL/BL | Company (% Cohort) | |
| Aimoni C 2016 [14] | Case-control comparing elderly and nonelderly outcomes; indirect repeated measures data is available on outcomes over time | 57 | Elderly group: 77 (65–86) Nonelderly group 50 (40–49) | 53% | 100% UL | Cochlear (NA) Advanced Bionics (NA) MED-EL (NA) | 4b |
| Bruschke S 2021 [15] | Controlled cohort comparing early and normal date of activation; indirect repeated measures data is available on outcomes over time | 127 | Early activation: 63 (22–88) Normal activation: 61 (27–81) | 50% | 91% UL 9% BL | Cochlear (65%) Advanced Bionics (6%) MED-EL (39%) | 4b |
| Caswell-Midwinter B 2022 [16] | Repeated measure analysis of a large database | 88 * | 63 (24–93) | NA | 89% UL 11% BL | Advanced Bionics (100%) | 4a |
| Domville-Lewis C 2015 [17] | Repeated measure analysis of stability of CI maps | 33 | First implant, 48 (1–79) Second implant 51 (2–81) | 57% | 100% BL | Cochlear (100%) | 4a |
| Frijns JH 2002 [18] | Repeated measure analysis of CI outcomes | 10 | 44 (14–62) | 70% | 100% UL | Advanced Bionics (100%) | 4b |
| Gajadeera E 2017 [19] | Repeated measure analysis of T and C levels | 128 | 59 (19–85) | NA | NA | Cochlear (100%) | 4a |
| Gajadeera E 2017 [20] | Repeated measure analysis of T and C levels | 680 | 59 (19–93) | NA | NA | Cochlear (100%) | 4a |
| Grisel J 2022 [8] | Repeated measure analysis of HERMES CI database | 804 | Database: NA (18–109) | NA | 100% UL | Cochlear (80%) Advanced Bionics (11%) MED-EL (9%) | 4a |
| Hilly O 2016 [21] | Controlled cohort comparing elderly and nonelderly CI recipients; indirect repeated measures data is available on outcomes over time | 87 | 32–≤ 60 years 33–61–70 years 22–>70 years | 62% | 90% UL 10% BL | Cochlear (36%) Advanced Bionics (59%) MED-EL (5%) † | 4b |
| Hughes ML 2001 [22] | Repeated measures analysis of T and C levels | 35 | 53 (29–77) | NA | 83% UL 17% BL | Cochlear (100%) | 4a |
| Kelsall D 2021 [29] | Repeated measures analysis of CI outcomes | 100 | 67 (23–93) | 37% | 100% UL | Cochlear (100%) | 4a |
| Lenarz M 2012 [28] | Controlled cohort comparing male and female CI outcomes; indirect repeated measures data is available on outcomes over time | 638 | 5 (no range provided) | 56% | 100% UL | NA | 4b |
| Mosca F 2014 [23] | Repeated measures analysis of CI fitting parameters | 26 | no average (18–58) | 39% | 100% UL | Cochlear (100%) | 4a |
| Porps SL 2023 [11] | Repeated measures analysis of CI outcomes and satisfaction with a streamlined programming strategy | 17 | 62 (24–80) | NA | 94% UL 6% BL | Cochlear (100%) | 4a |
| Ruffin CV 2007 [24] | Repeated measures analysis of CI outcomes | 31 | 51 (25–74) | 58% | 100% UL | Cochlear (100%) | 4b |
| Wathour J 2021 [9] | Cross-sectional comparison of fitting practices between 4 CI centers | 97 | Center 1: 55 Center 2: 54 Center 3: 51 Center 4: 58 | NA | NA | Cochlear (100%) | 4a |
| Zwolan TA 2021 [25] | Repeated measures analysis of CI outcomes after fitting with computer-assistance | 31 | 63 (23–90) | 48% | 100% UL | Cochlear (100%) | 4a |
| Zwolan TA 2001 [26] | Repeated measures analysis of CI outcomes and comparison between pre-curved and straight electrodes with electrode positioning systems; indirect repeated measures data is available on outcomes over time | 112 | First cohort: 54 ± 16 Second cohort: 61 ± 16 | NA | 100% UL | Advanced Bionics (100%) | 4b |
| Author Year | Speech Outcome Measure(s) over Time | Results |
|---|---|---|
| Aimoni C 2016 [14] | CI pure tone thresholds | Significant improvements from preop to 1 month, no significant improvement from 1 month to 12 months. |
| Speech perception performance category | Significant improvements in perception category from preop to 1 month. Significant improvement in category allocation from 1 month to 12 months. | |
| Bruschke S 2021 [15] | Multisyllabic word score | Significant improvement from preop/activation to 3 months; no significant change from 3 months to 6 or 12 months. |
| Monosyllabic word score | Significant improvement from preop/activation to 3 months; no significant change from 3 months to 6 or 12 months. | |
| Caswell-Midwinter B 2022 [16] | Time to word recognition score plateau (CNC word) | Median time plateau score of 2.9 months [IQR = 0.9–9.0 months], Median plateau score of 61.2% [IQR = 46.8–71.3%], |
| Frijns JH 2002 [18] | Word recognition testing with CVC word lists-phonemes reported | Score plateau at 3 months: 80% phoneme, 62% word. Avg last available score: 84% phoneme, 66% word. † |
| Speech tracking (words per minute) | Peak at 3 months (66 words per minute), slight drop of at 6 months and no data beyond 6 months. | |
| Grisel J 2022 [8] | CNC word | Individually, significant improvements were seen at each interval up to 12 months, with the largest changes between preimplantation and 1 month, and 1 month and 3 months. Highest CNC word score: 76.7% achieved between 3 and 12 months after activation. |
| Hilly O 2016 [21] | HINT score stability * | No patients with deterioration > 20% after 1 year. 13.6% of patients older than 70 showed continued improvement. |
| Kelsall D 2021 [29] | CNC word | Significant improvement from preimplantation to 3, 6, and 12 months. Significant improvement between intervals; greatest interval of improvement from preimplant to 3 months (41.8%) with lesser improvement from 3 to 6 months (4.6%) and 6 to 12 months (3.4%). |
| AzBio sentences in +10 SNR | Significant improvement from preimplantation to 3, 6, and 12 months. Significant improvement between 3 months and 6 and 12 months; greatest interval of improvement from preimplant to 3 months (19.1%) with lesser improvement from 3 to 6 months (8.8%) and 6 to 12 months (3.2%). | |
| AzBio sentences in +5 SNR | Significant improvement from preimplantation to 6, and 12 months (3 months data not collected). No significant improvement between 6 and 12 months; greatest interval of improvement from preimplant to 6 months (10.9%) with lesser improvement from 6 to 12 months (3.2%). | |
| Lenarz M 2012 [28] | Freiburger monosyllabic Test | No statistical analysis on repeated measures over time; qualitative analysis shows largest increased from implantation to 3 months with small gradual increase to 1 year with stable scores up to 5 years. |
| Speech tracking test | No statistical analysis on repeated measures over time; qualitative analysis shows largest increased from implantation to 3 months with stable scores up to 5 years. | |
| HSM sentence test in quiet | No statistical analysis on repeated measures over time; qualitative analysis shows largest increased from implantation to 3 months with small gradual increase to 2 years with stable scores up to 5 years. | |
| HSM sentence test in −10 SNR | No statistical analysis on repeated measures over time; qualitative analysis shows the largest increased from implantation to 3 months with small gradual increase to 2 years with stable scores up to 5 years. | |
| Porps SL 2023 [11] | CNC word | Significant improvement from preimplantation to both 3 and 6 months post activation with significant improvement between 3 and 6 months; greatest interval of improvement from preimplant to 3 months (53.3%) with lesser improvement from 3 to 6 months (9%). |
| AzBio sentence test in +10 SNR | Significant improvement from preimplantation to 3 and 6 months post activation with no significant difference between 3 and 6 months; greatest interval of improvement from preimplant to 3 months (31.2%) with lesser improvement from 3 to 6 months (5.3%). | |
| Ruffin CV 2007 [24] | Speech recognition with a combination of NU-6 and CNC word lists | Most significant growth in performance noted in first 9 months; time to maximum score ranged from 9 months to 120 months for the whole cohort. |
| Analysis of performance after 24 months shows no significant change beyond this timepoint. | ||
| Wathour J 2021 [9] | CI pure tone thresholds | No longitudinal analysis; thresholds at 1 year post activation without significant difference between centers-despite programming differences. |
| Speech recognition testing | No longitudinal analysis: scores provided for 2 centers but disparate tests prevent comparison. | |
| Zwolan TA 2021 [25] | CNC word | Significant improvements from preimplantation to 3 and 6 months; no significant difference between 3 and 6 months |
| AzBio | No preoperative measures; no significant difference between 3 and 6 months. | |
| Zwolan TA 2001 [26] | CNC words CID sentences HINT in Quiet HINT +10 SNR | Significant improvement from preimplantation to 1 month; continued improvement from 1 to 3 months and 3 to 6 months but no significant difference. ‡ |
| Author (Year) | Programming Parameter(s) over Time | Results |
|---|---|---|
| Caswell-Midwinter B 2022 [16] | Association of initial T- and C-level with time to performance plateau | Programming parameters were not significantly associated with time to plateau |
| Domville-Lewis C 2015 [17] | Time to CI map stability * | Mean days to stability: 77.6 ± 47.4 for first implant, 57.8 ± 28.2 for second implant, and 67.7 ± 39.9 for all implants |
| Time to CI map stability † | Mean days to stability: 87.3 ± 53.9 for the first implant, 50.6 ± 24.6 for the second implant, and 69.0 ± 45.5 for all implants | |
| Gajadeera E 2017 [19] | Mean T-level of all cochlear segments | 18% of patients showed a significant trend in change over time from 6 months to 8–10 years; however, these trends were equally likely in either direction. |
| Mean C-level of all cochlear segments | 24% of patients showed a significant trend in change over time from 6 months to 8–10 years; however, these trends were equally likely in either direction. | |
| At least 75% of patients showed less than 6% change in C and T levels over from 6 months to 8–10 years as a function of dynamic range | ||
| Gajadeera E 2017 [20] | Mean T-level of all cochlear segments | Current level at 2 months significantly lower than compared to all time points up to 24 months; consecutive time points did not differ significantly after the 3-month time period |
| Mean C-level of all cochlear segments | Current level at 2 and 3 months significantly lower than compared to all time points up to 24 months and current level at 24 months was significant higher compared to 6 and 12 months; consecutive time points did not differ significantly after the 6-month time period | |
| Hughes ML 2001 [22] | MAP C-level | Significant improvement in the first 12 months; average increase in 11.8 programming units (30% of average dynamic range) over the first year. ‡ |
| MAP T-level | No significant change from initial stimulation to 24 months. § | |
| Mosca F 2014 [23] | Average T-level | Significant change from preimplantation to 1 month and 1 month to 3 months; no significant change from 3 month to 6 or 12 months. |
| Average C-level | Significant change from preimplantation to 1 month and 1 month to 3 months; no significant change from 3 month to 6 or 12 months. | |
| Wathour J 2021 [9] | Average T-level | Significant different between centers at each time point; levels are stable after 3 months with low difference within centers after this point; no statistical analysis on levels over time but authors describe patients reaching steady state at 3 months for 1 center, 6 months for 1 center, and by 1 year for 2 centers. |
| Average C-level | Significant different between centers at each time point; levels are stable after 3 months with low difference within centers after this point; no statistical analysis on levels over time but authors describe patients reaching steady state at 3 months for 1 center, 6 months for 1 center, and by 1 year for 2 centers. | |
| Maintenance of default manufacturer parameters at 1 year | Parameters that rarely changed from default values were programming strategy, stimulation mode, rate, pulse width, and loudness growth. | |
| Parameters that were more often changed from default values were T-SPL, C-SPL, and maxima. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dornhoffer, J.R.; Khandalavala, K.R.; Zwolan, T.A.; Carlson, M.L. Preliminary Evidence to Support a De-Escalated Cochlear Implant Programming Paradigm for New Adult Recipients: A Systematic Review. J. Clin. Med. 2023, 12, 5774. https://doi.org/10.3390/jcm12185774
Dornhoffer JR, Khandalavala KR, Zwolan TA, Carlson ML. Preliminary Evidence to Support a De-Escalated Cochlear Implant Programming Paradigm for New Adult Recipients: A Systematic Review. Journal of Clinical Medicine. 2023; 12(18):5774. https://doi.org/10.3390/jcm12185774
Chicago/Turabian StyleDornhoffer, James R., Karl R. Khandalavala, Teresa A. Zwolan, and Matthew L. Carlson. 2023. "Preliminary Evidence to Support a De-Escalated Cochlear Implant Programming Paradigm for New Adult Recipients: A Systematic Review" Journal of Clinical Medicine 12, no. 18: 5774. https://doi.org/10.3390/jcm12185774
APA StyleDornhoffer, J. R., Khandalavala, K. R., Zwolan, T. A., & Carlson, M. L. (2023). Preliminary Evidence to Support a De-Escalated Cochlear Implant Programming Paradigm for New Adult Recipients: A Systematic Review. Journal of Clinical Medicine, 12(18), 5774. https://doi.org/10.3390/jcm12185774






