A Comparison of Immersive vs. Non-Immersive Virtual Reality Exercises for the Upper Limb: A Functional Near-Infrared Spectroscopy Pilot Study with Healthy Participants
Abstract
:1. Introduction
- (a)
- The immersive VR and the non-immersive desktop (nVR) conditions;
- (b)
- Both of these conditions compared to the baseline (B) condition.
2. Materials and Methods
2.1. Study Design and Participants
2.2. VR Setup
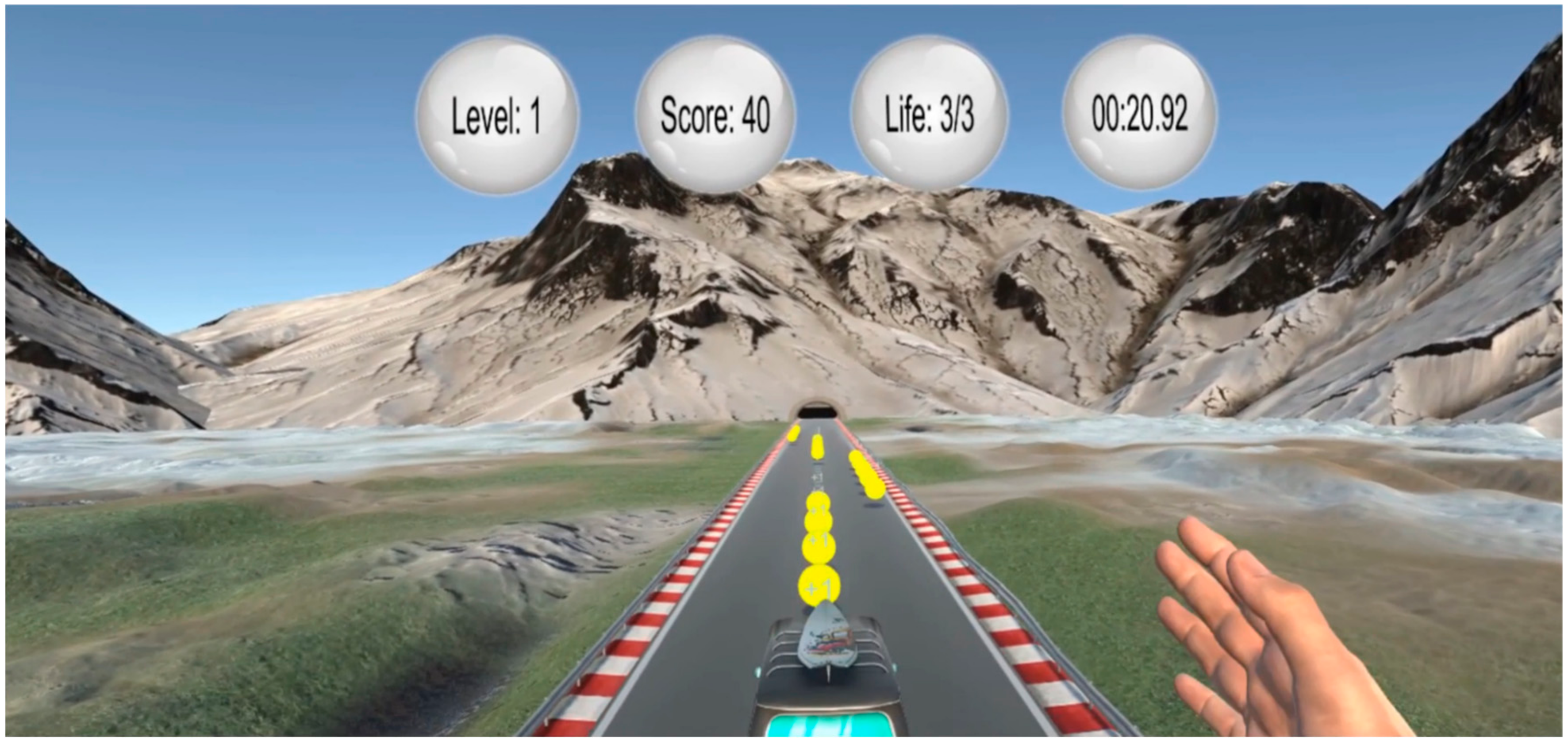
2.3. VR Paradigm—Bus Riding
2.4. Data Acquisition
2.5. fNIRS Preprocessing and Analysis
3. Results
3.1. Immersive (VR) vs. Baseline (B)
3.2. Non-Immersive (nVR) vs. Baseline (B)
3.3. Immersive (VR) vs. Non-Immersive (nVR)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slater, M.; Sanchez-Vives, M.V. Enhancing Our Lives with Immersive Virtual Reality. Front. Robot. AI 2016, 3, 74. [Google Scholar] [CrossRef]
- Mekbib, D.B.; Han, J.; Zhang, L.; Fang, S.; Jiang, H.; Zhu, J.; Roe, A.W.; Xu, D. Virtual reality therapy for upper limb rehabilitation in patients with stroke: A meta-analysis of randomized clinical trials. Brain Inj. 2020, 34, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Shi, P.; Fan, M.; Liang, S.; Li, S.; Yu, H. Effects of passive and active training modes of upper-limb rehabilitation robot on cortical activation: A functional near-infrared spectroscopy study. NeuroReport 2021, 32, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Kiper, P.; Szczudlik, A.; Agostini, M.; Opara, J.; Nowobilski, R.; Ventura, L.; Tonin, P.; Turolla, A. Virtual Reality for Upper Limb Rehabilitation in Subacute and Chronic Stroke: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2018, 99, 834–842.e4. [Google Scholar] [CrossRef]
- Hao, J.; Xie, H.; Harp, K.; Chen, Z.; Siu, K.-C. Effects of Virtual Reality Intervention on Neural Plasticity in Stroke Rehabilitation: A Systematic Review. Arch. Phys. Med. Rehabil. 2022, 103, 523–541. [Google Scholar] [CrossRef]
- Hao, J.; He, Z.; Yu, X.; Remis, A. Comparison of Immersive and Non-Immersive Virtual Reality for Upper Extremity Functional Recovery in Patients with Stroke: A Systematic Review and Network Meta-Analysis; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar] [CrossRef]
- Checa, D.; Bustillo, A. A review of immersive virtual reality serious games to enhance learning and training. Multimed. Tools Appl. 2020, 79, 5501–5527. [Google Scholar] [CrossRef]
- Ventura, S.; Brivio, E.; Riva, G.; Baños, R.M. Immersive Versus Non-immersive Experience: Exploring the Feasibility of Memory Assessment Through 360° Technology. Front. Psychol. 2019, 10, 2509. [Google Scholar] [CrossRef]
- Paes, D.; Irizarry, J.; Pujoni, D. An evidence of cognitive benefits from immersive design review: Comparing three-dimensional perception and presence between immersive and non-immersive virtual environments. Autom. Constr. 2021, 130, 103849. [Google Scholar] [CrossRef]
- Pallavicini, F.; Pepe, A.; Minissi, M.E. Gaming in Virtual Reality: What Changes in Terms of Usability, Emotional Response and Sense of Presence Compared to Non-Immersive Video Games? Simul. Gaming 2019, 50, 136–159. [Google Scholar] [CrossRef]
- Jin, M.; Pei, J.; Bai, Z.; Zhang, J.; He, T.; Xu, X.; Zhu, F.; Yu, D.; Zhang, Z. Effects of virtual reality in improving upper extremity function after stroke: A systematic review and meta-analysis of randomized controlled trials. Clin. Rehabil. 2022, 36, 573–596. [Google Scholar] [CrossRef]
- Herold, F.; Wiegel, P.; Scholkmann, F.; Müller, N.G. Applications of Functional Near-Infrared Spectroscopy (fNIRS) Neuroimaging in Exercise–Cognition Science: A Systematic, Methodology-Focused Review. J. Clin. Med. 2018, 7, 466. [Google Scholar] [CrossRef] [PubMed]
- Kurkin, S.; Badarin, A.; Grubov, V.; Maksimenko, V.; Hramov, A. The oxygen saturation in the primary motor cortex during a single hand movement: Functional near-infrared spectroscopy (fNIRS) study. Eur. Phys. J. Plus 2021, 136, 548. [Google Scholar] [CrossRef]
- Herold, F.; Behrendt, T.; Törpel, A.; Hamacher, D.; Müller, N.G.; Schega, L. Cortical hemodynamics as a function of handgrip strength and cognitive performance: A cross-sectional fNIRS study in younger adults. BMC Neurosci. 2021, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Nishiyori, R.; Harris, M.; Baur, K.; Meehan, S. Changes in cortical hemodynamics with the emergence of skilled motor ability in infants: An fNIRS study. Brain Res. 2021, 1772, 147666. [Google Scholar] [CrossRef]
- Prôa, R.; Balardin, J.; de Faria, D.D.; Paulo, A.M.; Sato, J.R.; Baltazar, C.A.; Borges, V.; Silva, S.M.C.A.; Ferraz, H.B.; Aguiar, P.d.C. Motor Cortex Activation During Writing in Focal Upper-Limb Dystonia: An fNIRS Study. Neurorehabilit. Neural Repair 2021, 35, 729–737. [Google Scholar] [CrossRef]
- Arun, K.M.; Smitha, K.A.; Sylaja, P.N.; Kesavadas, C. Identifying Resting-State Functional Connectivity Changes in the Motor Cortex Using fNIRS During Recovery from Stroke. Brain Topogr. 2020, 33, 710–719. [Google Scholar] [CrossRef]
- Delorme, M.; Vergotte, G.; Perrey, S.; Froger, J.; Laffont, I. Time course of sensorimotor cortex reorganization during upper extremity task accompanying motor recovery early after stroke: An fNIRS study. Restor. Neurol. Neurosci. 2019, 37, 207–218. [Google Scholar] [CrossRef]
- Seidel-Marzi, O.; Hähner, S.; Ragert, P.; Carius, D. Task-Related Hemodynamic Response Alterations During Slacklining: An fNIRS Study in Advanced Slackliners. Front. Neuroergonom. 2021, 2, 4. [Google Scholar] [CrossRef]
- Jurcak, V.; Tsuzuki, D.; Dan, I. 10/20, 10/10, and 10/5 systems revisited: Their validity as relative head-surface-based positioning systems. NeuroImage 2007, 34, 1600–1611. [Google Scholar] [CrossRef]
- Huppert, T.J.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. HomER: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009, 48, D280. [Google Scholar] [CrossRef]
- Fishburn, F.A.; Ludlum, R.S.; Vaidya, C.J.; Medvedev, A.V. Temporal Derivative Distribution Repair (TDDR): A motion correction method for fNIRS. NeuroImage 2019, 184, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Pinti, P.; Scholkmann, F.; Hamilton, A.; Burgess, P.; Tachtsidis, I. Current Status and Issues Regarding Pre-processing of fNIRS Neuroimaging Data: An Investigation of Diverse Signal Filtering Methods Within a General Linear Model Framework. Front. Hum. Neurosci. 2019, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, L.; Cooper, R.J.; Yücel, M.A.; Perdue, K.L.; Greve, D.N.; Boas, D.A. Short separation channel location impacts the performance of short channel regression in NIRS. NeuroImage 2012, 59, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Dan, I. Exploring the false discovery rate in multichannel NIRS. NeuroImage 2006, 33, 542–549. [Google Scholar] [CrossRef]
- Li, C.; Xu, Y.; He, L.; Zhu, Y.; Kuang, S.; Sun, L. Research on fNIRS Recognition Method of Upper Limb Movement Intention. Electronics 2021, 10, 1239. [Google Scholar] [CrossRef]
- Sattar, N.Y.; Kausar, Z.; Usama, S.A.; Farooq, U.; Shah, M.F.; Muhammad, S.; Khan, R.; Badran, M. fNIRS-Based Upper Limb Motion Intention Recognition Using an Artificial Neural Network for Transhumeral Amputees. Sensors 2022, 22, 726. [Google Scholar] [CrossRef]
- Rea, M.; Rana, M.; Lugato, N.; Terekhin, P.; Gizzi, L.; Brötz, D.; Fallgatter, A.; Birbaumer, N.; Sitaram, R.; Caria, A. Lower Limb Movement Preparation in Chronic Stroke. Neurorehabilit. Neural Repair 2014, 28, 564–575. [Google Scholar] [CrossRef]
- Morioka, S.; Yamada, M.; Komori, T. Frontal Lobe Activity during the Performance of Spatial Tasks: fNIRS Study. J. Phys. Ther. Sci. 2008, 20, 135–139. [Google Scholar] [CrossRef]
- Xu, G.; Huo, C.; Yin, J.; Li, W.; Xie, H.; Li, X.; Li, Z.; Wang, Y.; Wang, D. Effective brain network analysis in unilateral and bilateral upper limb exercise training in subjects with stroke. Med. Phys. 2022, 49, 3333–3346. [Google Scholar] [CrossRef]
- Abtahi, M.; Amiri, A.M.; Byrd, D.; Mankodiya, K. Hand Motion Detection in fNIRS Neuroimaging Data. Healthcare 2017, 5, 20. [Google Scholar] [CrossRef]
- Jalalvandi, M.; Alam, N.R.; Sharini, H. Optical Imaging of Brain Motor Cortex Activation During Wrist Movement Using Functional Near-Infrared Spectroscopy (fNIRS). Arch. Neurosci. 2019, 6. [Google Scholar] [CrossRef]
- Batula, A.M.; Mark, J.A.; Kim, Y.E.; Ayaz, H. Comparison of Brain Activation during Motor Imagery and Motor Movement Using fNIRS. Comput. Intell. Neurosci. 2017, 2017, 5491296. [Google Scholar] [CrossRef] [PubMed]
- Khaksari, K.; Smith, E.G.; Miguel, H.O.; Zeytinoglu, S.; Fox, N.; Gandjbakhche, A.H. An fNIRS Study of Brain Lateralization During Observation and Execution of a Fine Motor Task. Front. Hum. Neurosci. 2022, 15, 798870. [Google Scholar] [CrossRef]
- Plechatá, A.; Sahula, V.; Fayette, D.; Fajnerová, I. Age-Related Differences with Immersive and Non-immersive Virtual Reality in Memory Assessment. Front. Psychol. 2019, 10, 1330. [Google Scholar] [CrossRef]
- Patsaki, I.; Dimitriadi, N.; Despoti, A.; Tzoumi, D.; Leventakis, N.; Roussou, G.; Papathanasiou, A.; Nanas, S.; Karatzanos, E. The effectiveness of immersive virtual reality in physical recovery of stroke patients: A systematic review. Front. Syst. Neurosci. 2022, 16, 880447. [Google Scholar] [CrossRef] [PubMed]
- Baceviciute, S.; Terkildsen, T.; Makransky, G. Remediating learning from non-immersive to immersive media: Using EEG to investigate the effects of environmental embeddedness on reading in Virtual Reality. Comput. Educ. 2021, 164, 104122. [Google Scholar] [CrossRef]

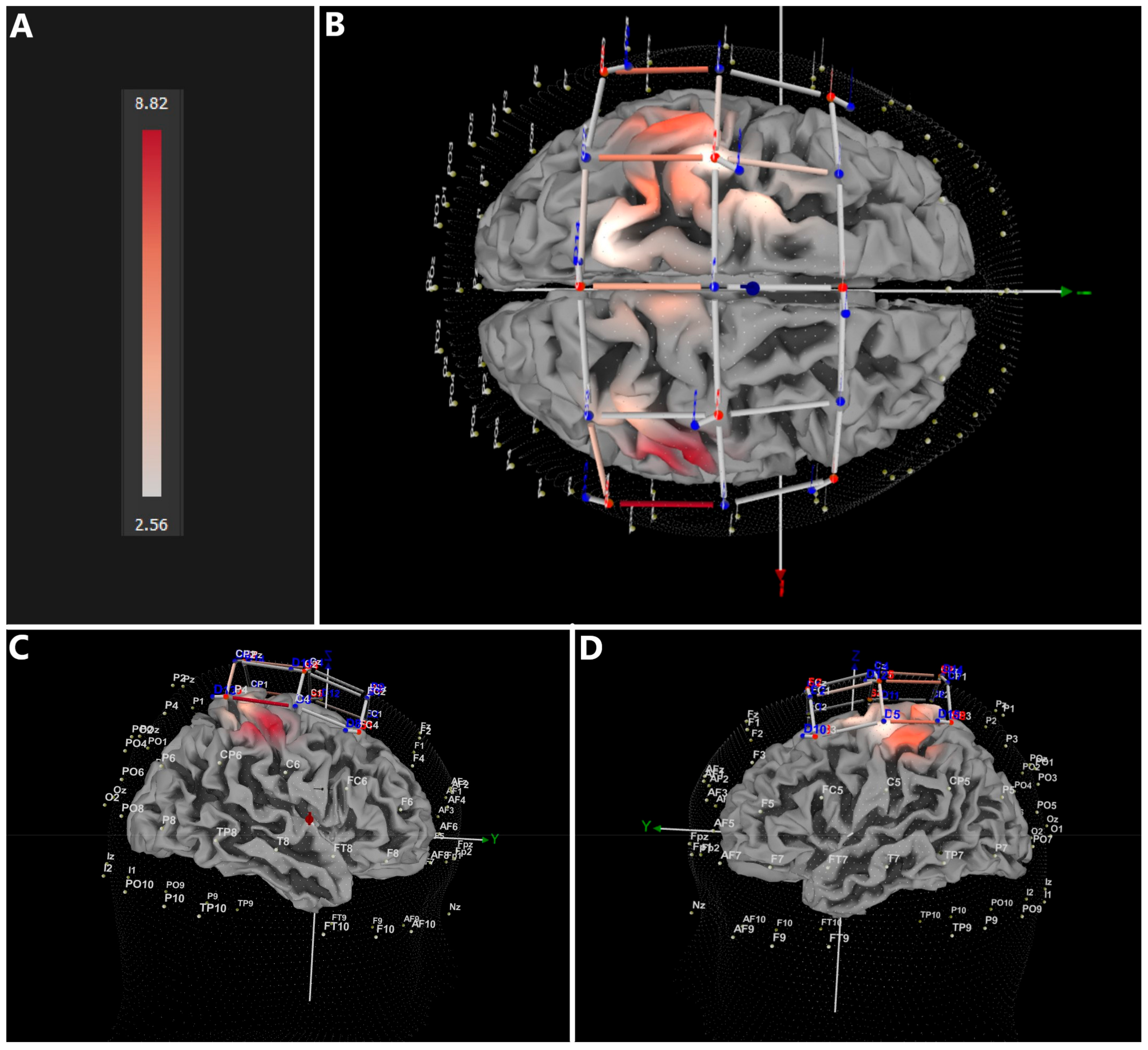

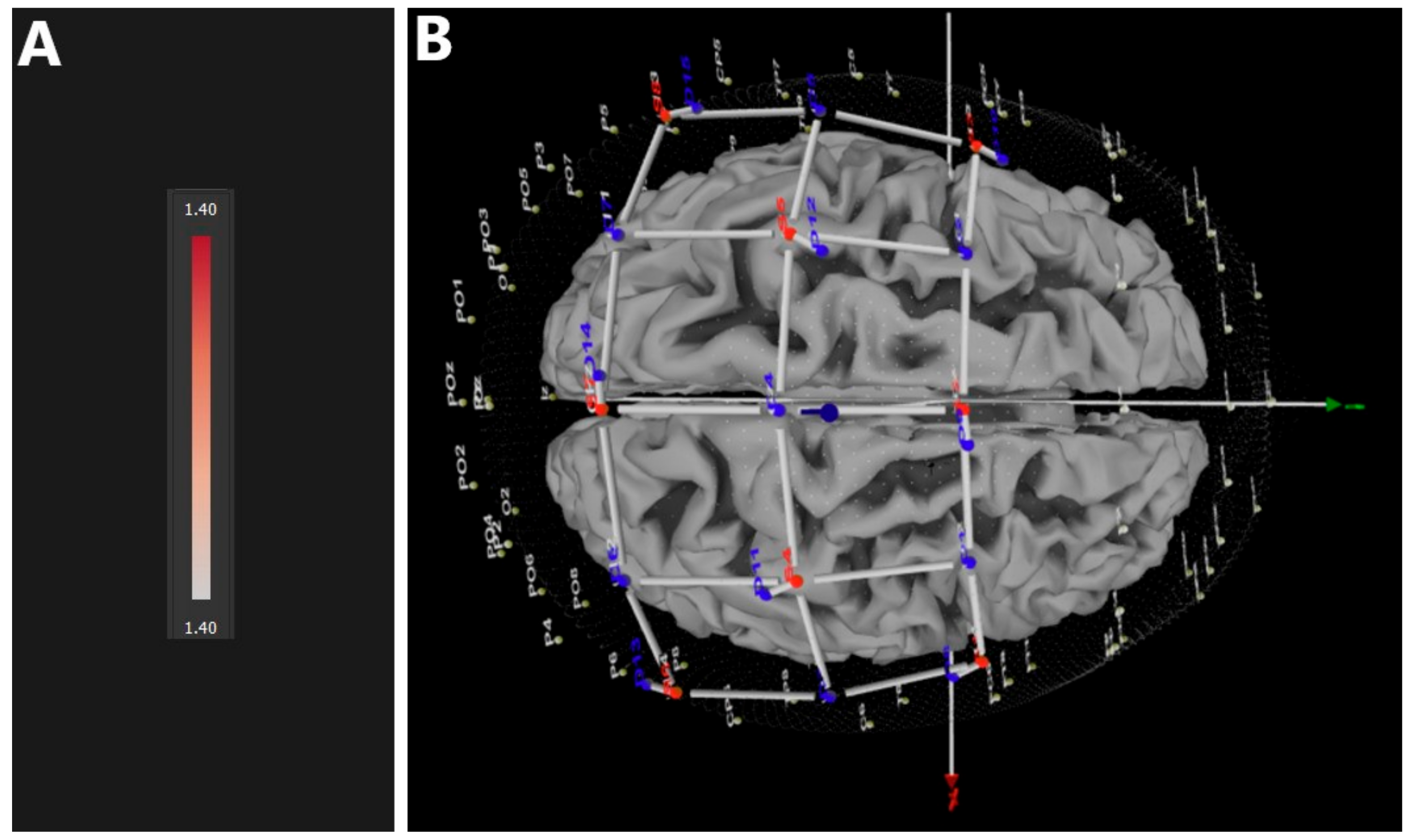
| Comparison | Confidence Interval (CI) of the Difference in HbO2 | |
|---|---|---|
| Min | Max | |
| VR vs. B | 2.560 | 8.820 |
| nVR vs. B | 3.802 | 9.796 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dordevic, M.; Maile, O.; Das, A.; Kundu, S.; Haun, C.; Baier, B.; Müller, N.G. A Comparison of Immersive vs. Non-Immersive Virtual Reality Exercises for the Upper Limb: A Functional Near-Infrared Spectroscopy Pilot Study with Healthy Participants. J. Clin. Med. 2023, 12, 5781. https://doi.org/10.3390/jcm12185781
Dordevic M, Maile O, Das A, Kundu S, Haun C, Baier B, Müller NG. A Comparison of Immersive vs. Non-Immersive Virtual Reality Exercises for the Upper Limb: A Functional Near-Infrared Spectroscopy Pilot Study with Healthy Participants. Journal of Clinical Medicine. 2023; 12(18):5781. https://doi.org/10.3390/jcm12185781
Chicago/Turabian StyleDordevic, Milos, Olga Maile, Anustup Das, Sumit Kundu, Carolin Haun, Bernhard Baier, and Notger G. Müller. 2023. "A Comparison of Immersive vs. Non-Immersive Virtual Reality Exercises for the Upper Limb: A Functional Near-Infrared Spectroscopy Pilot Study with Healthy Participants" Journal of Clinical Medicine 12, no. 18: 5781. https://doi.org/10.3390/jcm12185781
APA StyleDordevic, M., Maile, O., Das, A., Kundu, S., Haun, C., Baier, B., & Müller, N. G. (2023). A Comparison of Immersive vs. Non-Immersive Virtual Reality Exercises for the Upper Limb: A Functional Near-Infrared Spectroscopy Pilot Study with Healthy Participants. Journal of Clinical Medicine, 12(18), 5781. https://doi.org/10.3390/jcm12185781






