Influence of Patient Anatomy on Intraoperative Radiation Exposure and Operation Time during Standard EVAR
Abstract
1. Introduction
2. Materials and Methods
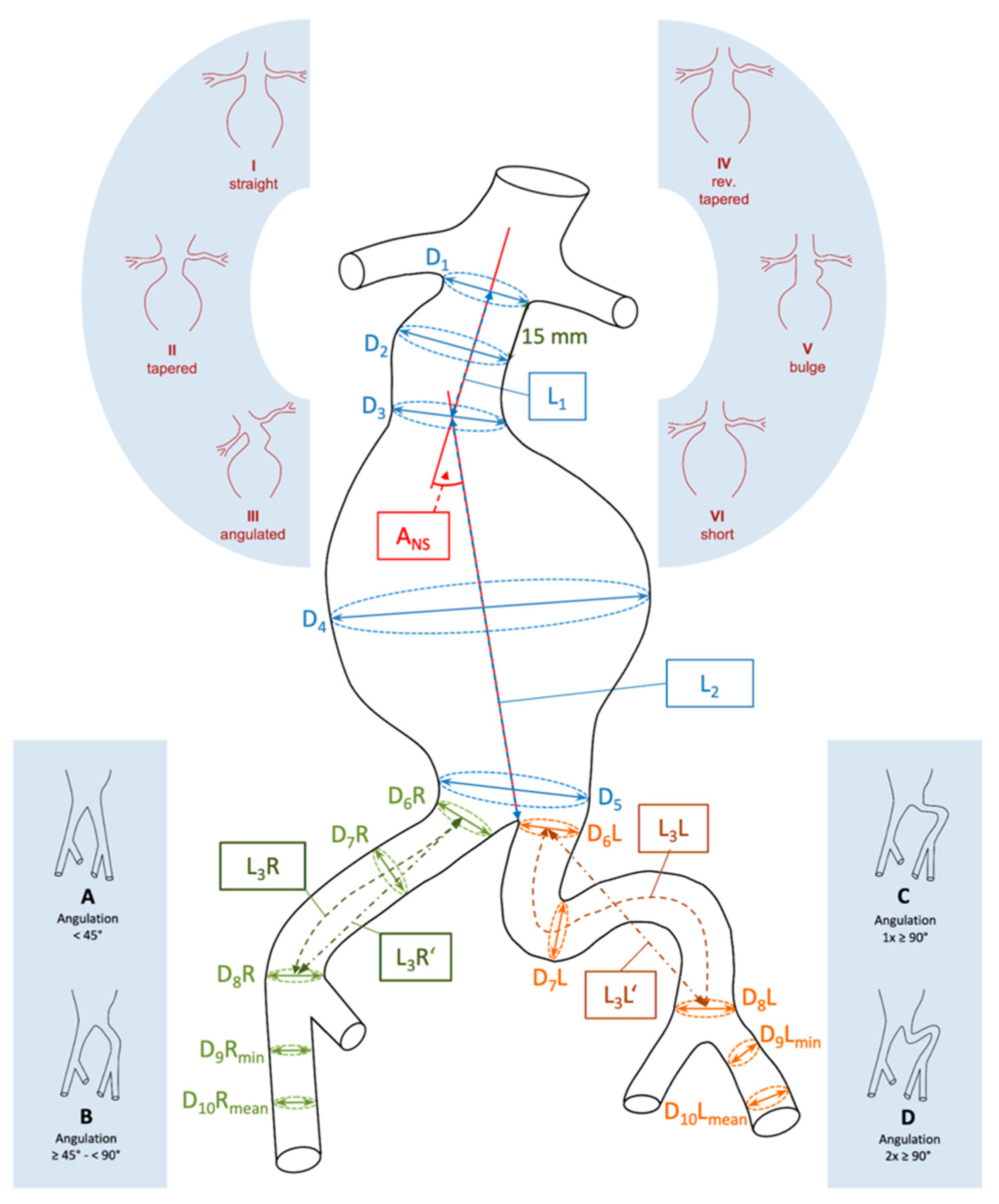
2.1. EVAR Planning
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Intra- and Inter-Observer Variability
3.3. Factors Influencing Radiation Exposure
3.4. Factors Influencing Procedure Time
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmitz-Rixen, T.; Steffen, M.; Böckler, D.; Grundmann, R.T. Treatment of abdominal aortic aneurysms (AAA) 2019: Registry report from the German Institute for Vascular Public Health Research (DIGG) of the German Society for Vascular Surgery and Vascular Medicine (DGG). Gefasschirurgie 2020, 26, 41–48. [Google Scholar] [CrossRef]
- Speziale, F.; Sirignano, P.; Setacci, F.; Menna, D.; Capoccia, L.; Mansour, W.; Galzerano, G.; Setacci, C. Immediate and two-year outcomes after EVAR in ‘on-label’ and ‘off-label’ neck anatomies using different commercially available devices. Analysis of the experience of two Italian vascular centers. Ann. Vasc. Surg. 2014, 28, 1892–1900. [Google Scholar] [CrossRef]
- Georgakarakos, E.; Papatheodorou, N.; Argyriou, C.; Tasopoulou, K.M.; Doukas, D.; Georgiadis, G.S. An update on the ovation abdominal stent graft for the treatment of abdominal aortic aneurysms: Current evidence and future perspectives. Expert. Rev. Med. Devices 2020, 17, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.F.G.; Ultee, K.; van Rijn, M.J.; Pinto, J.P.; Raa, S.T.; Gonçalves, F.B.; Hoeks, S.E.; Verhagen, H.J.M. Anatomic predictors for late mortality after standard endovascular aneurysm repair. J. Vasc. Surg. 2019, 69, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.; Ferreira, V.M.D.; Loureiro, L.; Gonçalves, J.; Oliveira, P.; Almeida, R. Radiation exposure in endovascular infra-renal aortic aneurysm repair and factors that influence it. Braz. J. Cardiovasc. Surg. 2016, 31, 415–421. [Google Scholar] [CrossRef]
- Kim, J.B.; Lee, J.; Park, K. Radiation hazards to vascular surgeon and scrub nurse in mobile fluoroscopy equipped hybrid vascular room. Ann. Surg. Treat. Res. 2017, 92, 156–163. [Google Scholar] [CrossRef]
- Matsu Matsumoto, T.; Tanaka, S.; Okadome, J.; Kyuragi, R.; Fukunaga, R.; Kawakubo, E.; Itoh, H.; Okazaki, J.; Shirabe, K.; Fukuda, A.; et al. Midterm outcomes of endovascular repair for abdominal aortic aneurysms with the on-label use compared with the off-label use of an endoprosthesis. Surg. Today 2015, 45, 880–885. [Google Scholar] [CrossRef]
- Sobocinski, J.; Chenorhokian, H.; Maurel, B.; Midulla, M.; Hertault, A.; Le Roux, M.; Azzaoui, R.; Haulon, S. The benefits of EVAR planning using a 3D workstation. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Rolls, A.E.; Riga, C.V.; Rudarakanchana, N.; Lee, S.L.; Albayati, M.; Hamady, M.; Bicknell, C.D.; Cheshire, N.J. Planning for EVAR: The role of modern software. J. Cardiovasc. Surg. 2014, 55, 418–423. [Google Scholar]
- Strøm, M.; Lönn, L.; Bech, B.; Schroeder, T.V.; Konge, L. Assessment of competence in EVAR stent graft sizing and selection. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 844–852. [Google Scholar] [CrossRef][Green Version]
- Knöps, E.; van Schaik, J.; van der Bogt, K.E.A.; Veger, H.T.C.; Putter, H.; Waasdorp, E.J.; van der Vorst, J.R. Stent graft sizing for endovascular abdominal aneurysm repair using open source image processing software. Ann. Vasc. Surg. 2021, 71, 411–418. [Google Scholar] [CrossRef] [PubMed]
- White, G.H.; Yu, W.; May, J.; Chaufour, X.; Stephen, M.S. Endoleak as a complication of endoluminal grafting of abdominal aortic aneurysms: Classification, incidence, diagnosis, and management. J. Endovasc. Surg. 1997, 4, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Obuchowski, N.A.; Bullen, J.A. Receiver operating characteristic (ROC) curves: Review of methods with applications in diagnostic medicine. Phys. Med. Biol. 2018, 63, 07TR01. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s choice—European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef]
- Oliveira-Pinto, J.; Oliveira, N.; Bastos-Gonçalves, F.; Hoeks, S.; Van Rijn, M.J.; Raa, S.T.; Mansilha, A.; Verhagen, H.J. Long-term results of outside ‘instructions for use’ EVAR. J. Cardiovasc. Surg. 2017, 58, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Joh, J.H.; Park, H.C. Measuring of abdominal aortic aneurysm with three-dimensional computed tomography reconstruction before endovascular aortic aneurysm repair. Vasc. Spec. Int. 2017, 33, 27–32. [Google Scholar] [CrossRef]
- Kaladji, A.; Lucas, A.; Kervio, G.; Haigron, P.; Cardon, A. Sizing for endovascular aneurysm repair: Clinical evaluation of a new automated three-dimensional software. Ann. Vasc. Surg. 2010, 24, 912. [Google Scholar] [CrossRef]
- Gurkan, S.; Gur, O.; Sahin, A.; Donbaloglu, M. The impact of obesity on perioperative and postoperative outcomes after elective endovascular abdominal aortic aneurysm repair. Vascular 2023, 31, 211–218. [Google Scholar] [CrossRef]
- Sen, I.; Tenorio, E.R.; Pitcher, G.; Mix, D.; Marcondes, G.B.M.; Lima, G.B.B.; Ozbek, P.; Oderich, G.S. Effect of obesity on radiation exposure, quality of life scores, and outcomes of fenestrated-branched endovascular aortic repair of pararenal and thoracoabdominal aortic aneurysms. J. Vasc. Surg. 2021, 73, 1156–1166.e2. [Google Scholar] [CrossRef]
- Badger, S.A.; Jones, C.; Boyd, C.S.; Soong, C.V. Determinants of radiation exposure during EVAR. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 320–325. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kakkos, S.K.; Efthymiou, F.O.; Metaxas, V.I.; Dimitroukas, C.P.; Panayiotakis, G.S. Factors affecting radiation exposure in endovascular repair of abdominal aortic aneurysms: A pilot study. Int. Angiol. 2021, 40, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Torsello, G.; Troisi, N.; Donas, K.P.; Austermann, M. Evaluation of the endurant stent graft under instructions for use vs off-label conditions for endovascular aortic aneurysm repair. J. Vasc. Surg. 2011, 54, 300–306. [Google Scholar] [CrossRef][Green Version]
- Beckerman, W.E.; Tadros, R.O.; Faries, P.L.; Torres, M.; Wengerter, S.P.; Vouyouka, A.G.; Lookstein, R.A.; Marin, M.L. No major difference in outcomes for endovascular aneurysm repair stent grafts placed outside of instructions for use. J. Vasc. Surg. 2016, 64, 63–74.e2. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, M.S.; Peters, A.S.; Meisenbacher, K.; Böckler, D. Challenging access in endovascular repair of infrarenal aortic aneurysms. J. Cardiovasc. Surg. 2014, 55, 75–83. [Google Scholar]
| Parameter | Gender | Mean ± Standard Deviation (SD) | Median (Min.; Max.) | 95% Confidence Interval (CI) | p |
|---|---|---|---|---|---|
| Age (years) | Male | 73.8 ± 8.3 | 84 (57; 90) | (71.9; 75.6) | <0.05 |
| Female | 69.4 ± 6.2 | 68 (60; 77) | (63.7; 75.1) | ||
| Body mass index (BMI) (kg/m2) | Male | 27.5 ± 4 | 26.9 (17.6; 39.2) | (26.6; 28.3) | >0.05 |
| Female | 27.6 ± 6.9 | 26.6 (19.2; 38.4) | (21.1; 34) |
| Parameter | Dose Area Product | Procedure Time | ||||
|---|---|---|---|---|---|---|
| Noncritical (n = 75) | Critical (n = 14) | p | Noncritical (n = 80) | Critical (n = 9) | p | |
| Open access inguinal on both sides | 55 (73.3%) | 14 (100%) | <0.05 | 60 (75%) | 9 (100%) | >0.05 |
| Percutaneous access inguinal on both sides | 11 (14.7%) | 0 (0%) | >0.05 | 11 (13.8%) | 0 (0%) | >0.05 |
| Open access/percutaneous access contralateral | 9 (12.0%) | 0 (0%) | >0.05 | 9 (11.3%) | 0 (0%) | >0.05 |
| Additional brachial/axillar vascular access | 4 (5.3%) | 4 (28.6%) | <0.05 | 4 (5.0%) | 4 (44.4%) | <0.05 |
| Femoral TEA on one side | 3 (4.0%) | 0 (0%) | >0.05 | 2 (2.5%) | 1 (11.1%) | >0.05 |
| Femoral TEA on both sides | 1 (1.3%) | 1 (7.1%) | >0.05 | 1 (1.3%) | 1 (11.1%) | >0.05 |
| Angioplasty of access vessels | 9 (12.0%) | 2 (14.3%) | >0.05 | 10 (12.5%) | 2 (22.2%) | >0.05 |
| Conversion from endovascular aortic repair (EVAR) to open aortic repair (OAR) | 2 (2.7%) | 0 (0%) | >0.05 | 0 (0%) | 2 (22.2%) | <0.05 |
| Access-related bleeding | 1 (1.3%) | 0 (0%) | >0.05 | 0 (0%) | 1 (11.1%) | >0.05 |
| (A) | ||||
|---|---|---|---|---|
| Parameter | Mean ± SD | Median (Min.; Max.) | 95% CI | |
| Neck-sac angulation | 35.4 ± 20.3 | 33 (2; 84) | (31.6; 40.46) | |
| D1 | 22.8 ± 3.2 | 23 (17; 32) | (22.2; 23.5) | |
| D2 | 24 ± 3.6 | 24 (17; 33) | (23.2; 24.8) | |
| D3 | 26.4 ± 4.1 | 27 (17; 34) | (25.3; 27.3) | |
| D4 | 57.1 ± 12 | 55 (41; 110) | (55.2; 59.9) | |
| D5 | 27.2 ± 9.6 | 25 (15; 68) | (25.2; 29.2) | |
| D6 right | 14 ± 3.1 | 14 (8; 25) | (13.3; 14.6) | |
| D6 left | 13.3 ± 2.6 | 13 (6; 20) | (12.8; 13.8) | |
| D7 right | 14.7 ± 4.4 | 14 (7; 40) | (13.8; 15.6) | |
| D7 left | 13.4 ± 2.8 | 13 (8; 22) | (12.9; 14.0) | |
| D8 right | 13.7 ± 3.2 | 14 (7; 23) | (13.0; 14.3) | |
| D8 left | 13.7 ± 3.1 | 13 (7; 26) | (13.0; 14.3) | |
| D9 right | 8.2 ± 1.5 | 8 (5; 12) | (7.9; 8.5) | |
| D9 left | 8.2 ± 1.6 | 8 (4; 12) | (7.9; 8.5) | |
| D10 right | 9.1 ± 1.5 | 9 (6; 13) | (8.8; 9.4) | |
| D10 left | 9.2 ± 1.5 | 9 (5; 13) | (8.8; 9.5) | |
| D11 right | 9.7 ± 1.7 | 10 (7; 14) | (9.4; 10.1) | |
| D11 left | 9.8 ± 1.7 | 10 (7; 14) | (9.4; 10.1) | |
| L1 | 38.1 ± 15 | 36 (13; 83) | (35.0; 41.2) | |
| L2 | 128.4 ± 15.3 | 129 (93; 181) | (125.2; 131,6) | |
| L3 right | 70 ± 18.1 | 67 (36; 121) | (66.2; 73.8) | |
| L3 left | 75 ± 18.3 | 72 (41; 142) | (71.1; 78.8) | |
| (B) | ||||
| Parameter | n | % | ||
| Tortuosity of infrarenal aortic aneurysm | 1.0–1.04 | 41 | 45.6 | |
| 1.05–1.09 | 22 | 24.4 | ||
| ≥1.1 | 27 | 30.0 | ||
| Tortuosity of common iliac artery | 1.0–1.04 | Right | 18 | 20.0 |
| Left | 13 | 14.4 | ||
| 1.05–1.09 | Right | 28 | 31.1 | |
| Left | 33 | 36.7 | ||
| ≥1.1 | Right | 44 | 48.9 | |
| Left | 44 | 48.9 | ||
| Angulation of common iliac artery | <45° | Right | 31 | 34.4 |
| Left | 37 | 41.1 | ||
| 45–90° | Right | 40 | 44.4 | |
| Left | 23 | 25.6 | ||
| >90° | Right | 14 | 15.6 | |
| Left | 18 | 20.0 | ||
| 2 × 90° | Right | 5 | 5.6 | |
| Left | 12 | 13.3 | ||
| Neck configuration | Straight | 35 | 38.9 | |
| Tapered | 1 | 1.1 | ||
| Angulated | 44 | 48.9 | ||
| Reverse tapered | 3 | 3.3 | ||
| Bulging | 6 | 6.7 | ||
| Short | 1 | 1.1 | ||
| Parameter | Mean ± SD | Median (Min.; Max.) | 95% CI |
|---|---|---|---|
| Intra-observer reliability for the first observer | 0.86 ± 0.12 | 0.91 (0.4; 0.97) | (0.81; 0.91) |
| Intra-observer reliability for the second observer | 0.88 ± 0.07 | 0.89 (0.71; 0.98) | (0.86; 0.91) |
| Inter-observer reliability for the first sizing round | 0.88 ± 0.08 | 0.89 (0.61; 0.98) | (0.85; 0.91) |
| Inter-observer reliability for the second sizing round | 0.88 ± 0.09 | 0.9 (0.46; 0.96) | (0.84; 0.91) |
| Parameter | Mean ± SD | Median (Min.; Max.) | 95% CI |
|---|---|---|---|
| Procedure time (min) | 147.6 ± 71.6 | 124.5 (60; 465) | (132.6; 162.6) |
| Dose area product (Gycm2) | 23 ± 21 | 14.2 (2.2; 92.8) | (18.5; 27.4) |
| Radiation time (s) | 1699.7 ± 1193.2 | 1347 (448; 7927) | (1446.9; 1952.5) |
| Amount of contrast medium (mL) | 89.4 ± 57.1 | 75 (20; 400) | (76.8; 101.9) |
| Parameter | Dose Area Product | Procedure Time | ||
|---|---|---|---|---|
| ρ | p | ρ | p | |
| BMI | 0.37 | <0.01 | 0.1 | >0.05 |
| Radiation time | 0.48 | <0.001 | 0.69 | <0.001 |
| Amount of contrast medium | 0.5 | <0.001 | 0.25 | <0.05 |
| Type of endograft | −0.3 | <0.01 | 0.06 | >0.05 |
| Neck-sac angulation | 0.1 | >0.05 | 0.18 | <0.05 |
| Neck diameter | −0.11 | >0.05 | −0.05 | >0.05 |
| Maximum aneurysm diameter | 0.18 | >0.05 | 0.3 | <0.01 |
| Common iliac artery diameter right | 0.24 | <0.05 | 0.16 | >0.05 |
| Common iliac artery diameter left | 0.06 | >0.05 | 0.1 | >0.05 |
| Minimum external iliac artery diameter right | 0.14 | >0.05 | −0.01 | >0.05 |
| Minimum external iliac artery diameter left | 0.11 | >0.05 | 0.1 | >0.05 |
| Common femoral artery diameter right | 0.25 | <0.05 | 0.12 | >0.05 |
| Common femoral artery diameter left | 0.23 | <0.05 | 0.21 | >0.05 |
| Tortuosity of infrarenal aortic aneurysm | 0.08 | >0.05 | 0.24 | <0.05 |
| Tortuosity of common iliac artery right | 0.02 | >0.05 | 0.21 | <0.05 |
| Tortuosity of common iliac artery left | 0.15 | >0.05 | 0.38 | <0.001 |
| Angulation of common iliac artery right | 0.07 | >0.05 | 0.33 | <0.01 |
| Angulation of common iliac artery left | 0.25 | <0.05 | 0.5 | <0.001 |
| Parameter | Odds Ratio (OR) | 95% CI | p |
|---|---|---|---|
| BMI | 1.177 | (1.032; 1.354) | <0.05 |
| Radiation time | 1.002 | (1.001; 1.003) | <0.05 |
| Amount of contrast medium | 1.007 | (0.998; 1.017) | >0.05 |
| Diameter of common iliac artery right | 0.996 | (0.852; 1.121) | >0.05 |
| Diameter of common femoral artery right | 1.182 | (0.845; 1.668) | >0.05 |
| Diameter of common femoral artery left | 1.014 | (0.709; 1.424) | >0.05 |
| Parameter | OR | 95% CI | p |
|---|---|---|---|
| BMI | 1.183 | (1.015; 1.389) | <0.05 |
| Radiation time | 1.002 | (1.001; 1.003) | <0.05 |
| Amount of contrast medium | 1.007 | (0.996; 1.017) | >0.05 |
| Neck-sac angulation | 1.051 | (1.016; 1.095) | <0.05 |
| Tortuosity of infrarenal aortic aneurysm | 1.124 | (1.041; 1.230) | <0.05 |
| Maximum aneurysm diameter | 1.054 | (1.007; 1.109) | <0.05 |
| Tortuosity of common iliac artery right | 1.004 | (0.951; 1.045) | >0.05 |
| Tortuosity of common iliac artery left | 1.028 | (1.002; 1.054) | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derwich, W.; Barb, A.; Vogl, T.; Oikonomou, K.; Gray, D. Influence of Patient Anatomy on Intraoperative Radiation Exposure and Operation Time during Standard EVAR. J. Clin. Med. 2023, 12, 5851. https://doi.org/10.3390/jcm12185851
Derwich W, Barb A, Vogl T, Oikonomou K, Gray D. Influence of Patient Anatomy on Intraoperative Radiation Exposure and Operation Time during Standard EVAR. Journal of Clinical Medicine. 2023; 12(18):5851. https://doi.org/10.3390/jcm12185851
Chicago/Turabian StyleDerwich, Wojciech, Alexandru Barb, Thomas Vogl, Kyriakos Oikonomou, and Daphne Gray. 2023. "Influence of Patient Anatomy on Intraoperative Radiation Exposure and Operation Time during Standard EVAR" Journal of Clinical Medicine 12, no. 18: 5851. https://doi.org/10.3390/jcm12185851
APA StyleDerwich, W., Barb, A., Vogl, T., Oikonomou, K., & Gray, D. (2023). Influence of Patient Anatomy on Intraoperative Radiation Exposure and Operation Time during Standard EVAR. Journal of Clinical Medicine, 12(18), 5851. https://doi.org/10.3390/jcm12185851






